Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(1):140-148. doi:10.7150/jca.89715 This issue Cite
Research Paper
Prevalence of cervical precancers or cancers in women with ASC-H/HSIL cytology according to Aptima HPV (AHPV) assay-detected HPV genotypes and age
1. Departments of Pathology, International Peace Maternity and Child Health Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200030, China.
2. Department of Pathology, Zhejiang University School of Medicine Women's Hospital, Hangzhou, Zhejiang Province, 310006, China.
3. Department of Gynecology, Zhejiang University School of Medicine Women's Hospital, Hangzhou, Zhejiang Province, 310006, China.
4. Department of Pathology, University of Texas Southwestern Medical Center, Dallas, TX, 75390, USA.
5. Department of Pathology, Parkland Hospital, Dallas, TX, 75235, USA.
* Q.L. and L.C. contributed equally.
Received 2023-8-31; Accepted 2023-10-23; Published 2024-1-1
Abstract
Objective: Aimed to potentially risk-stratify patients with different cervical cytology diagnoses, by HPV genotypes and/or age, we have conducted a series of studies to examine the prevalence of cervical precancers and cancers for women with different cytology diagnoses. This paper will be focusing on patients with ASC-H/HSIL cytology.
Methods: In total, 1183 patients aged 20-78 years with atypical squamous cells, cannot rule out HSIL (ASC-H)/HSIL by cytology underwent AHPV assay and cervical biopsy in a developed region in southern China were included in this study.
Results: Overall, 59.2% women with ASC-H/HSIL cytology had cervical intraepithelial neoplasia (CIN)2/3 lesions while 1.6% had adenocarcinoma in situ (AIS) lesions. Compared to other groups, HPV-16+ group (80.8%) showed a significantly higher prevalence of CIN2/3 than other genotype+ groups (p<0.0001). Further, HPV-16+ (9.3%) or HPV-18/45+ (6.3%) group showed a significantly higher prevalence of squamous cell carcinoma (SCC) than other genotype+ groups (p<0.0001). The prevalence of AIS glandular lesions in HPV-18/45+ group (13.8%) is significantly higher than other genotype groups (p<0.0001). When stratified by age, younger group showed a significantly higher prevalence of CIN2/3 (p=0.009) while older group presented an obvious higher prevalence of SCC (p<0.0001).
Conclusions: In this patient population, among women with ASC-H/HSIL cytology, HPV positive groups are at significantly higher risk of CIN2/3 compared to HPV negative group. Specifically, prevalence of CIN2/3 and SCC is significantly higher in HPV-16+ group while AIS lesions are more prevalent among HPV-18/45+ patients. In addition, younger group showed a significantly higher prevalence of CIN2/3 while older group presented an obvious higher prevalence of SCC.
Keywords: risk stratification, E6/E7 mRNA genotyping, ASC-H, HSIL, hrHPV
Introduction
Cervical cancer is a major gynecological malignancy that typically develops from the progression of cervical precancerous lesions. In most cases, chronic infection with high-risk types human papillomavirus (hrHPV) is necessary for the cervical precancerous lesion and cancer development [1, 2]. Although cervical cytology screening programs are widely used, they have shown relatively low sensitivity and inter-observer consistency [3-5]. Consequently, co-testing, which combines cervical cytology and hrHPV testing, has been recommended as the screening regimen by American Society for Colposcopy and Cervical Pathology (ASCCP) [6]. Current HPV detection methods detect HPV DNA and/or HPV mRNA [7-10]. For example, the Aptima HPV (AHPV) assay magnifies E6/E7 mRNA transcripts that are linked to persistent HPV infections, and this method has a better specificity for detecting high-grade squamous intraepithelial lesions (HSIL+) than DNA-based assays [11]. Therefore, AHPV testing is considered better suited as the primary cervical cancer screening method [12].
HSIL (High-grade squamous intraepithelial lesion) exhibits pronounced nuclear abnormalities, such as enlarged irregular nuclei, significant hyperchromasia with coarse and uneven chromatin, and increased nuclear-cytoplasmic ratio. ASC-H (Atypical squamous cells - cannot exclude high-grade squamous intraepithelial lesion), on the other hand, displays similar atypical cytological features but to a lesser degree qualitatively and/or quantitatively. From a molecular perspective, HSIL is strongly associated with human papillomavirus (HPV) infection. Common molecular alterations observed in HSIL cells encompass the overexpression of viral proteins E6/E7 and enhanced expression of p16INK4a protein [13]. Techniques like immunohistochemistry can be employed to detect these molecular features. To summarize, ASC-H and HSIL present distinct morphological characteristics in cytological images, with HSIL demonstrating more prominent nuclear abnormalities. While most cervical cytology abnormalities are not severe and will not develop into cancer or its precursors, high-grade cervical cytology abnormalities, most of which were positive for hrHPV, have a higher risk of precancerous and cancerous cervical lesions [14], and these patients often undergo immediate treatment [6]. However, different genotypes of hrHPV carry different risks of developing high-grade lesions [15]. In our previous studies, we demonstrated the benefit of risk-stratifying patients with NILM and ASC-US cytology based on hrHPV genotype for optimizing patient management [16, 17]. However, there is limited research on the association between ASC-H/HSIL cytology and hrHPV infection using Aptima assays, particularly on a large scale in China. Therefore, the aim of this study was to investigate the prevalence and the distribution of hrHPV among women with ASC-H/HSIL cytology, to evaluate the immediate histological risk in women with ASC-H/HSIL cytology stratified by age and HPV genotypes based on Aptima assays, and to assess the applicability of the ASCCP guidelines in the population of women from developed southern China.
Materials and Methods
Population study
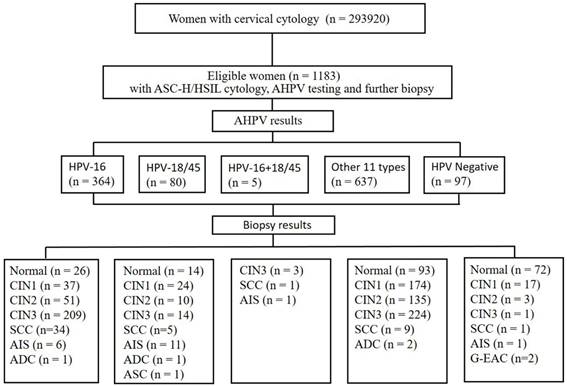
In total, 293920 women with cytology results were recorded in our pathology database between September 2016 and May 2020 at the Zhejiang University School of Medicine Women's Hospital. As demonstrated in Figure 1, a total of 1183 women aged between 20 and 78 years who were previously diagnosed as ASC-H/HSIL cytology with concurrent hrHPV results and underwent follow-up biopsy according to the American Society of Colposcopy & Cervical Pathology (ASCCP) guidelines [18, 19]. Inclusion Criteria: Females with a history of sexual activity who are non-pregnant; all subjects with a clear diagnosis of cervical biopsy pathology; all subjects underwent AHPV testing and TCT testing, with results indicating ASC-H/HSIL; subjects with no autoimmune disorders. Exclusion Criteria: subjects with incomplete clinical data or unreliable follow-up; subjects with history of hysterectomy or malignant tumors; subjects with history of other malignancies or pelvic radiotherapy/chemotherapy. The colposcopists were informed of the cytology and AHPV results before the colposcopy visit. The permission was obtained from the ethics committee of the Zhejiang University School of Medicine Women's Hospital.
Liquid-based cytology and AHPV testing
Specimen collection was performed by physicians and preserved in PreservCyt collection medium (Hologic Inc., Marlborough, MA) according to the manufacturer's instructions. The pap smear slides were completed by the Sakura Tissue-Tek Automated Slide Stainer (Sakura Finteck USA, Torrance, California, USA), and all cytology slides were diagnosed by cytopathologists based on the 2014 Bethesda system. Remaining LBC samples were used for the AHPV assay (Hologic, Inc., San Diego, CA) following the manufacturer's instructions. The AHPV assay screened 14 hrHPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) and further typed HPV 16 and 18/45.
Colposcopy, cervical biopsy and follow-up histopathological diagnoses in patients
The cervical tissue samples obtained during colposcopy were paraffin embedded and sectioned, and the slides were dewaxed and stained with hematoxylin and eosin. Finally, the slides were read by two senior pathologists in the pathology department. The histopathological results included three general groups: (1) Benign; (2) CIN1; (3) CIN2/3; (4) dysplastic cervical glandular lesion including AIS and ADC. CIN2/3 and AIS lesions were regarded as cervical precancers. p16/Ki-67 immunohistochemical staining assays were used to verify CIN2/3. If different sites or multiple biopsies, the highest pathological grade is recorded as the final diagnosis.
Statistical analysis
The database was established by Excel and the data were statistically analyzed by SPSS 23.0 software (IBM). Pearson χ2 or Fisher exact test was used to compare the distribution of cervical precancers or cancers in different hrHPV types and age groups, with the histopathological results used as the gold standard. A p<0.05 was considered statistically significant.
Results
Demographic characteristics of women with ASC-H/HSIL cytology
This study included a total of 1183 cases with cytological ASC-H/HSIL positivity and immediate histological examination results. Among them, 807 cases (68.2%) were classified as ASC-H and 376 cases (31.8%) were classified as HSIL. As shown in Table 1, the women enrolled were 20-78 years (mean age 41.6 ± 11.0 years). Additionally, the average age of ASC-H patients (41.6 years) was similar with HSIL patients (41.6 years) (p = 0.93). The prevalence of hrHPV infection in ASC-H/HSIL patients was 91.8% (1086/1183). The hrHPV positivity rate in HSIL patients (97.6%) was significantly higher compared to ASC-H patients (89.1%) (p < 0.0001). The detection rates of precancerous lesions and cancer in ASC-H/HSIL women were 54.9% (650/1183) and 6.4% (76/1183), respectively. Among them, HSIL women had significantly higher immediate pathological severity compared to ASC-H women (p < 0.0001).
Demographic characteristics of patients with ASC-H and HSIL cytology.
| Parameters | ASC-H (n=807) | HSIL (n=376) | ASC-H/HSIL (n=1183) | P |
|---|---|---|---|---|
| Age (year) | 41.63±11.17 | 41.57±10.72 | 41.61±11.02 | 0.93 |
| hrHPV infection | <0.0001* | |||
| Positive | 719/807 | 367/376 | 1086/1183 | |
| Negative | 88/807 | 9/376 | 97/1183 | |
| Histopathological outcome | <0.0001* | |||
| Benign | 388/807 | 69/376 | 457/1183 | |
| Premalignancy | 373/807 | 277/376 | 650/1183 | |
| Malignancy | 46/807 | 30/376 | 76/1183 |
Note: *p<0.05. hrHPV, high-risk human papillomavirus; HSIL, high grade squamous intraepithelial lesion; ASC-H, atypical squamous cells - cannot rule out HSIL.
Flow chart of selection criteria of participants. ASC-H, atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CIN, cervical intraepithelial neoplasia grade; ADC, adenocarcinoma; AIS, adenocarcinoma in situ; ASC, adenosquamous carcinoma; SCC, squamous cell carcinoma; G-EAC, gastric-type endocervical adenocarcinoma.
Specific HPV genotype prevalence per histological diagnosis among women with AGC
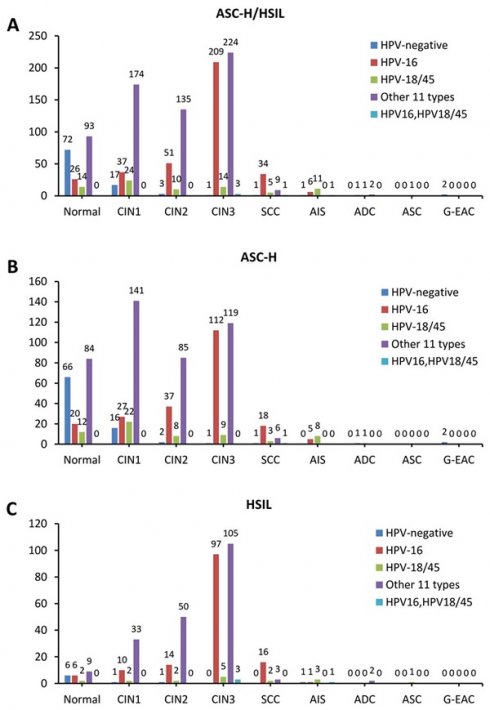
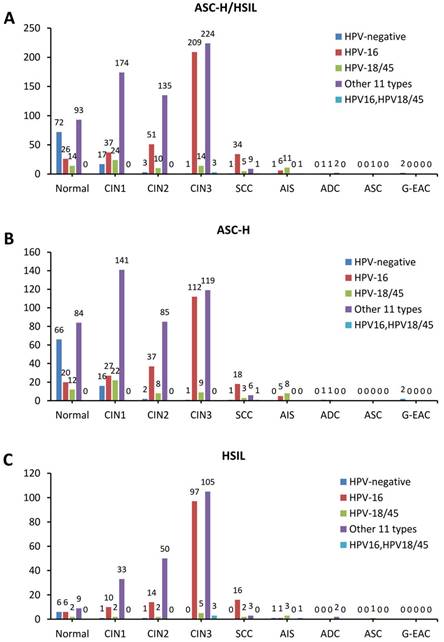
In histopathological results, 17.3% (205/1183) were diagnosed as benign, 21.3% (252/1183) were CIN1, 16.8% (199/1183) were CIN2, 38.1% (451/1183) were CIN3, 4.2% (50/1183) were SCC, 0.5% (6/1183) were ADC, 1.6% (19/1183) were AIS, and 0.1% (1/1183) were adenosquamous carcinoma (ASC). Among all the AHPV results, it is shown that 8.2% (97/1183) were HPV negative, 30.8% (364/1183) were HPV-16 positive, 6.8% (80/1183) were HPV-18/45 positive, 0.4% (5/1183) were HPV-16, 18/45 dual positive and 53.8% (637/1183) were positive for 1 of the other 11 types of hrHPV. Detailed data is presented in Table 2.
The prevalence of CIN2/3 in hrHPV-negative group was 5.2% (5/97), in HPV-16 positive group was 80.8% (294/364), in HPV-18/45 positive group was 36.3% (29/80), in the other 11 types hrHPV positive group was 57.8% (368/637), and in HPV-16, HPV-18/45 dual positive group was 80% (4/5). Immediate histopathological results showed that CIN2/3 prevalence is significantly higher in HPV-16 positive group and HPV-16, 18/45 dual positive than other groups by Pearson χ2 test (p<0.0001). Similarly, the SCC prevalence in HPV-16 positive group was 9.3% (34/364) and in HPV-18/45 positive group was 6.3% (5/80), which was significantly higher than that in other groups (p<0.0001). When it comes to glandular lesions, we found that AIS prevalence in HPV-18/45 positive group was 13.8% (11/80), which was significantly higher than that in other groups (p<0.0001) (Table 3).
Comparison of precancer and cancer in different age groups among women with ASC-H/HSIL cytology
Among different age groups, prevalence of the hrHPV types and histopathological results were further compared. Among the 1183 ASC-H/HSIL cases with following histopathological results, 1.6% (19/1183) patients were younger than 25 years, in which 36.8% (7/19) were HPV-16 positive, 42.1% (8/19) were positive for the other 11 types of hrHPV, and 21.1% (4/19) were negative for hrHPV. 47.8% (565/1183) patients were in group aged 25-39 years, among which 8.0% (45/565) were negative for hrHPV, 33.3% (188/565) were HPV-16 positive, 6.7% (38/565) were HPV-18/45 positive, 0.7% (4/565) were HPV-16/HPV-18/45 dual positive, and 51.3% (290/565) were positive for the other 11 types of hrHPV. In the 577 (48.8%, 577/1183) patients in group aged 40-65 years, 8.1% (47/577) were hrHPV negative, 28.2% (163/577) were HPV-16 positive, 7.1% (41/577) were HPV-18/45 positive, 0.2% (1/577) were HPV-16/HPV-18/45 dual positive, and 56.3% (325/577) were positive for 1 of the other 11 types of hrHPV. 1.9% (22/1183) patients were aged over 65 years old, among which 4.5% (1/22) were HPV negative, 27.3% (6/22) were HPV-16 positive, 4.5% (1/22) were HPV-18/45 positive, and 63.6% (14/22) were positive for the other 11 types of hrHPV. Immediate histopathological results showed that CIN2/3 prevalence varied in different age groups: the prevalence of CIN2/3 in patients younger than 25 years is 68.4% (13/19), in patients aged 25-39 years is 63.9% (361/565), in patients aged 40-65 years is 54.4% (314/577), and in patients aged over 65 years old is 54.5% (12/22). When we chose 40 years or 50 years as the age cutoffs for further analysis, the prevalence of CIN2/3 was significantly higher in younger group than that in older group (p<0.0001), while SCC prevalence is significantly higher in older age groups than in younger (p<0.0001). However, there were no significant differences for glandular lesions among different age groups. Detailed analysis is included in tables 4 and 5.
Discussion
In this study, our objective was to investigate the prevalence of cervical precancers or cancers (HSIL, AIS, SCC and adenocarcinoma) for women with ASC-H/HSIL cytology stratified by AHPV assay-detected HPV genotypes or age in a developed region in southern China. In total, 59.2% women with ASC-H/HSIL cytology had cervical intraepithelial neoplasia (CIN)2/3 lesions while 1.6% had adenocarcinoma in situ (AIS) lesions.
Distribution of HPV infection patterns among the women with ASC-H/HSIL cytology.
| hrHPV genotype | Normal | CIN1 | CIN2 | CIN3 | SCC | ADC | AIS | ASC | Total |
|---|---|---|---|---|---|---|---|---|---|
| HPV negative | 72 (74.2%) | 17 (17.5%) | 3 (3.1%) | 1 (1%) | 1 (1%) | 2 (2.1%) | 1 (1%) | 0 | 97 (8.2%) |
| HPV16 | 26 (7.1%) | 37 (10.2%) | 51 (14.0%) | 209 (57.4%) | 34 (9.3%) | 1 (0.3%) | 6 (1.6%) | 0 | 364 (30.8%) |
| HPV-18/45 | 14 (17.5%) | 24 (30%) | 10 (12.5%) | 14 (17.5%) | 5 (6.3%) | 1 (1.3%) | 11 (13.8%) | 1 (1.3%) | 80 (6.8%) |
| Other 11 types | 93 (14.6%) | 174 (27.3%) | 135 (21.2%) | 224 (35.2%) | 9 (1.4%) | 2 (0.3%) | 0 | 0 | 637 (53.8%) |
| HPV-16, 18/45 | 0 | 0 | 0 | 3 (60%) | 1 (20%) | 0 | 1 (20%) | 0 | 5 (0.4%) |
| Total | 205 (17.3%) | 252 (21.3%) | 199 (16.8%) | 451 (38.1%) | 50 (4.2%) | 6 (0.5%) | 19 (1.6%) | 1 (0.1%) | 1183 |
hrHPV, high-risk human papillomavirus; CIN, cervical intraepithelial neoplasia; SCC, squamous carcinoma; ASC, adenosquamous carcinoma; AIS, adenocarcinoma in situ; ADC, adenocarcinoma.
The prevalence of precancer and cancer among women with ASC-H/HSIL.
| Squamous lesions | Glandular lesions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| hrHPV genotype | Total | CIN2/3 | P value | SCC | P value | AIS | P value | ADC | P value |
| HPV negative | 97 | 5 (5.2%) | <0.0001* | 1 (1.0%) | <0.0001* | 1 (1.0%) | <0.0001* | 2 (2.1%) | 0.367 |
| HPV16 | 364 | 294 (80.8%) | 34 (9.3%) | 6 (1.6%) | 1 (0.3%) | ||||
| HPV18/45 | 80 | 29 (36.3%) | 5 (6.3%) | 11(13.8%) | 1 (1.3%) | ||||
| Other 11 types | 637 | 368 (57.8%) | 9 (1.4%) | 0 | 2 (0.3%) | ||||
| HPV16, 18/45 | 5 | 4 (80%) | 1 (20%) | 1 (20%) | 0 | ||||
| Total | 1183 | 700 (59.2%) | 50 (4.2%) | 19 (1.6%) | 6 (0.5%) | ||||
Note: *p<0.05. hrHPV, high-risk human papillomavirus; CIN, cervical intraepithelial neoplasia; HSIL, high grade squamous intraepithelial lesion; ASC-H, atypical squamous cells, cannot rule out HSIL; AIS, adenocarcinoma in situ; ADC, adenocarcinoma.
Age-stratified immediate histopathological correlation among women with ASC-H/HSIL.
| Squamous lesions | Glandular lesions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Total | CIN2/3 | P value | SCC | P value | AIS | P value | ADC | P value |
| <25 | 19 (1.6%) | 13 (68.4%) | 0.009* | 0 | <0.0001* | 0 | 0.739 | 0 | 0.058 |
| 25-39 | 565 (47.8%) | 361 (63.9%) | 12 (2.1%) | 11 (1.9%) | 2 (0.4%) | ||||
| 40-65 | 577 (48.8%) | 314 (54.4%) | 37 (6.4%) | 8 (1.4%) | 3 (0.5%) | ||||
| >65 | 22 (1.9%) | 12 (54.5%) | 1 (4.5%) | 0 | 1 (4.5%) | ||||
| Total | 1183 | 700 (59.2%) | 50 (4.2%) | 19 (1.6%) | 6 (0.5%) | ||||
Note: *p<0.05. hrHPV, high-risk human papillomavirus; CIN, cervical intraepithelial neoplasia; HSIL, high grade squamous intraepithelial lesion; ASC-H, atypical squamous cells, cannot rule out HSIL; AIS, adenocarcinoma in situ; ADC, adenocarcinoma.
Immediate risk of high- grade squamous lesions/glandular lesions between older and younger group with ASC-H/HSIL.
| Squamous lesions | Glandular lesions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Total | CIN2/3 | P value | SCC | P value | AIS | P value | ADC | P value |
| 25-year cut-off | |||||||||
| <25 years | 19 (1.6%) | 13 (68.4%) | 0.486 | 0 | 0.728 | 0 | 1 | 0 | 0.754 |
| ≥25 years | 1164 (98.4%) | 687 (59.0%) | 50 (4.3%) | 19 (1.6%) | 6 (0.5%) | ||||
| 40-year cut-off | |||||||||
| <40 years | 584 (49.4%) | 374 (64.0%) | 0.001* | 12 (2.1%) | <0.0001* | 11 (1.9%) | 0.495 | 2 (0.3%) | 0.431 |
| ≥40 years | 599 (50.6%) | 326 (54.4%) | 38 (6.3%) | 8 (1.3%) | 4 (0.7%) | ||||
| 50-year cut-off | |||||||||
| <50 years | 888 (75.1%) | 554 (62.4%) | <0.0001* | 26 (2.9%) | <0.0001* | 16 (1.8%) | 0.353 | 4 (0.5%) | 0.634 |
| ≥50 years | 295 (24.9%) | 146 (49.5%) | 24 (8.1%) | 3 (1.0%) | 2 (0.7%) | ||||
Note: *p<0.05. hrHPV, high-risk human papillomavirus; CIN, cervical intraepithelial neoplasia; HSIL, high grade squamous intraepithelial lesion; ASC-H, atypical squamous cells, cannot rule out HSIL; AIS, adenocarcinoma in situ; ADC, adenocarcinoma.
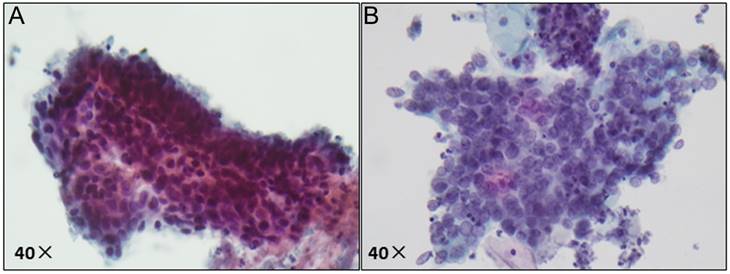
Pathological image of ASC-H/HSIL by liquid-based cytology. (A) ASC-H displays non-canonical cytological features, including nuclear enlargement, hyperchromasia, and increased nuclear-cytoplasmic ratio; (B) HSIL exhibits more pronounced nuclear abnormalities, such as enlarged nuclei, hyperchromasia with coarse and uneven chromatin, and increased nuclear-cytoplasmic ratio.
The stacked column chart showing the proportions of different HPV subtypes in the histological outcomes of women with ASC-H/HSIL cytology. The distribution of HPV genotypes in ASC-H/HSIL women (a), ASC-H women (b), and HSIL women (c). ASC-H, atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CIN, cervical intraepithelial neoplasia grade; ADC, adenocarcinoma; AIS, adenocarcinoma in situ; ASC, adenosquamous carcinoma; SCC, squamous cell carcinoma; G-EAC, gastric-type endocervical adenocarcinoma.
One 2015 large sample study from China on HSIL and hrHPV infection showed that the CIN2+ rate in women with HSIL cytology within 6 months was 82.6% (1994/2414) [20]. Another recent study reported that the following CIN2+ rates in cases with abnormal cytology: ASC-US (18.58%), ASC-H (53.76%), LSIL (16.62%), HSIL (82.07%), SCC/AC (100.00%), AGC (63.77%) [21]. A study conducted in a South Korean population demonstrated an 83.5% CIN2+ rate in HSIL cytology [22]. In our study, the prevalence of CIN2/3 in cases with ASC-H and HSIL cytology were 49.8% and 79.2%, respectively, which was lower than the rates reported in the aforementioned studies. Factors contributing to this difference may include variations in examination methods, screening populations (economically developed region vs. developing region) and the increasing prevalence of cervical cancer screening in recent years. A study conducted in Shanghai, a region with a similar economic status to our study population, showed that 71.5% of patients with HSIL cytology were diagnosed with CIN2+ lesions, including 3.2% with invasive squamous cell carcinoma (SCC) [23]. Additionally, they conducted another study revealing a CIN2+ rate of 76.8% in hrHPV mRNA-positive cases [15]. Similarly, our study observed a CIN2+ rate of 66.1% in hrHPV mRNA-positive cases.
Our study represents the first analysis of HSIL cytology and AHPV results based on a large sample in China. Among the 1183 cases with ASC-H/HSIL cytology, hrHPV was detected in 91.8% of cases (1086/1183). The HPV-negative rate was 8.2% (97/1183) for women with HSIL cytology, which coincides with that previously reported, which showed an ~5% to 8% hrHPV-negative HSIL cytology rate [24, 25]. The results from immediate histopathological examinations demonstrated a significantly higher prevalence of CIN2/3 in the hrHPV positive group compared to the hrHPV negative group (p<0.0001). However, we observed a CIN2+ rate of 8.2% (8/97) among the 97 cases with HSIL/HPV negative results, which is significantly lower than another study conducted from Shanghai (53.3%) in 2020[23]. A blinded second cytology test for HSIL/HPV-negative cases exhibited the probability that some HSIL interpretations in hrHPV-negative cases might be actually false positives [25]. The findings from this study provide additional evidence to support the notion that combining cytology and hrHPV testing offers enhanced safety advantages, reducing the risk of undetected significant lesions. Furthermore, individuals positive for HPV-16 or HPV-16, 18/45 dual positive had a significantly elevated risk of developing CIN2/3 compared to other groups (p<0.0001).
Given the rising incidence of ADC among young females in recent times, there has been increased focus on the assessment of glandular cells in cervical smears [26]. This study encompassed 19 cases of AIS. Several studies have reported that LBC smears showed a high sensitivity for the detection of cervical ADC [27], possibly due to the morphologic characteristics of the samples [28]. Nevertheless, other studies have demonstrated that a co-testing strategy may be advantageous in the detection of cervical carcinoma, particularly ADC or its precursor lesions [29-31]. It is worth noting that among the 97 patients with ASC-H/HSIL cytology but negative hrHPV results in our study, 5 cases (5.2%) were diagnosed as CIN2+ squamous lesions, and 3 cases (3.1%) were diagnosed as AIS+ glandular lesions upon histological follow-up. This further demonstrates the suitability of co-testing as an effective screening method for cervical cancer, given its low rate of missed diagnoses in both cancer and precancerous lesions. Different from the previous studies, our studies distinguished between squamous and glandular lesions. As is known, the distribution of hrHPV genotypes varies among distinct histological subcategories of cervical malignancies [32]. HPV16 is the dominant genotype in squamous cell carcinomas (SCCs),32 whereas HPV18 frequently, though not always, emerges as the predominant genotype in AIS [33, 34]. In our study, we compared the prevalence of AIS among different hrHPV genotype groups. The prevalence of AIS lesions in HPV-18/45 positive group was 13.8%, while it was 1.6% and 1.0% in HPV-16 positive and hrHPV-negative group respectively (p<0.0001). These findings support the notion that women with HPV 18/45+ are at higher risk of cervical ADC.
In a study conducted by Wang et [28], it was found that the incidence of high-grade squamous intraepithelial lesion (HSIL+) in younger women (≤30 years) was significantly higher compared to older women (51-60 years) [35]. This finding was supported by another independent study which also reported a comparable risk of HSIL+ in older women relative to their younger counterparts [36]. In our study, the rate of CIN2/3 decreased significantly with increasing age, while the rate of SCC increased. This finding is consistent with a large sample study conducted by Kaiser Permanente Northern California (KPNC) [24]. One possible explanation for this phenomenon is that as cervical cancer screening becomes more prevalent in developed areas of China, the number of screening tests disproportionately increases among older patients, leading to a decrease in the CIN2/3 rate. However, as age increases, the risk of developing cancer accumulates, resulting in a higher SCC reporting rate among older people. Hence, the data suggest that age is a significant risk factor for the progression from cervical precancerous lesions to cancer, with perimenopausal and postmenopausal women with HSIL cytology showing a higher risk of developing cancer.
To summarize, the study concluded that the incidence of CIN2/3 was significantly higher among the hrHPV-positive group in comparison to the hrHPV-negative group of women in developed southern China with ASC-H/HSIL cytology. Specifically, the prevalence of CIN2/3 and SCC is significantly higher in HPV-16+ group, while AIS lesions are more prevalent among HPV-18/45+ patients. Furthermore, the younger age group showed a significantly higher prevalence of CIN2/3, while the older age group presented an obvious higher prevalence of SCC. Our data demonstrate that AHPV testing is an effective method for HPV phenotyping. These results are compatible with international findings and support the applicability of the ASCCP guideline in developed southern China.
Data availability
The datasets generated during and/or analyses during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethics committee approval was obtained from the Zhejiang University School of Medicine Women's Hospital. The ethics committee waived the requirement for informed written consent, as the study was a retrospective study and there was no additional risk to patients. All data were anonymized to maintain patient privacy.
Author contributions
All authors contributed to the study conception and design. F.Z. had full access to all the data in the study and taken responsibility for the integrity of the data and accuracy of the data analysis. Q.L., L.C., M.Y., X.Z., and X.Z. involved in drafting the manuscript and revising it critically for important intellectual content; F.Z. and S.N. reviewed and edited the manuscript. All authors read and approved the final manuscript. All researchers listed as authors are independent from the funders, and all final decisions about the research were made without constraint by the investigators.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV. et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-27
2. Walboomers J, Jacobs MV, Manos MM, Bosch FX, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. Journal of Pathology. 1999;189:12-9
3. Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:687-97 W214-5
4. Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111:167-77
5. Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA. et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;8:CD008587
6. Salazar KL, Duhon DJ, Olsen R, Thrall M. A review of the FDA-approved molecular testing platforms for human papillomavirus. Journal of the American Society of Cytopathology. 2019;8:284-92
7. Wang J, Du Y, Dong J, Zhou Y, Wang P, Zhang X. et al. Clinical significance of genotyping for human papillomavirus (HPV) 16 18/45 combined with cytology in cervical exfoliated cells in HPV oncogenic mRNA-positive women. Gynecologic oncology. 2019;153:34-40
8. Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. Journal of clinical microbiology. 2011;49:1071-6
9. Wu R, Belinson SE, Du H, Na W, Qu X, Wu R. et al. Human papillomavirus messenger RNA assay for cervical cancer screening: the Shenzhen Cervical Cancer Screening Trial I. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2010;20:1411-4
10. Stoler MH, Wright TC Jr, Cuzick J, Dockter J, Reid JL, Getman D. et al. APTIMA HPV assay performance in women with atypical squamous cells of undetermined significance cytology results. American journal of obstetrics and gynecology. 2013;208:144.e1-8
11. Haedicke J, Iftner T. A review of the clinical performance of the Aptima HPV assay. J Clin Virol. 2016;76(Suppl 1):S40-s8
12. Cuzick J, Cadman L, Mesher D, Austin J, Ashdown-Barr L, Ho L. et al. Comparing the performance of six human papillomavirus tests in a screening population. British journal of cancer. 2013;108:908-13
13. Zhu Y, Ren C, Yang L, Zhang X, Liu L, Wang Z. Performance of p16/Ki67 immunostaining, HPV E6/E7 mRNA testing, and HPV DNA assay to detect high-grade cervical dysplasia in women with ASCUS. BMC cancer. 2019;19:271
14. Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T. et al. Five-year risks of CIN 3+ and cervical cancer among women with HPV-positive and HPV-negative high-grade Pap results. Journal of lower genital tract disease. 2013;17:S50-5
15. Li A, Li J, Austin RM, Wang T, Ashman D, Zhang H. et al. Aptima HPV messenger RNA testing and histopathologic follow-up in women with HSIL cytology: A study emphasizing additional review of HPV-negative cases. Cancer cytopathology. 2021;129:622-31
16. Liu Q, Zhou X, Zhang X, Strickland AL, Zheng W, Chen H. et al. HPV Genotype Specific and Age Stratified Immediate Prevalence of Cervical Precancers and Cancers in Women with NILM/hrHPV+: A Single Center Retrospective Study of 26,228 Cases. Cancer management and research. 2021;13:6869-77
17. Liu Q, Zhang T, Chen L, Zhou X, Zhang X, Zheng W. et al. Correlation of immediate prevalence of cervical precancers and cancers with HPV genotype and age in women with ASC-US/hrHPV+: a retrospective analysis of 2292 cases. Journal of clinical pathology. 2023
18. Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M. et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstetrics and gynecology. 2013;121:829-46
19. Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F. et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. Journal of lower genital tract disease. 2020;24:102-31
20. Zheng B, Austin RM, Liang X, Li Z, Chen C, Yan S. et al. PPV of an HSIL cervical cytology result in China's largest CAP-certified laboratory. Journal of the American Society of Cytopathology. 2015;4:84-9
21. Xu H, Chen Y, Zhao C, Wang Q, Tang M, Zhang W. et al. Bethesda system reporting rates, histological follow-up and HPV genotypes distribution of abnormal cytology in Anhui province of China: Analysis of 93,168 cervical liquid-based cytology test reports. Diagnostic cytopathology. 2023
22. Kim SI, Kim SJ, Suh DH, Kim K, No JH, Kim YB. Pathologic discrepancies between colposcopy-directed biopsy and loop electrosurgical excision procedure of the uterine cervix in women with cytologic high-grade squamous intraepithelial lesions. Journal of gynecologic oncology. 2020;31:e13
23. Ashman D, Zhang H, Li J, Austin M, Wang T, Pradhan D. et al. HPV detection rates and histopathologic follow-up of patients with HSIL cytology in a large academic women's hospital laboratory. Journal of the American Society of Cytopathology. 2020;9:550-5
24. Castle PE, Fetterman B, Thomas Cox J, Shaber R, Poitras N, Lorey T. et al. The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstetrics and gynecology. 2010;116:76-84
25. Gao FF, Austin RM, Zhao C. Histopathologic follow-up and human papillomavirus DNA test results in 290 patients with high-grade squamous intraepithelial lesion Papanicolaou test results. Cancer cytopathology. 2011;119:377-86
26. Kumar N, Gupta R, Gupta S. Glandular cell abnormalities in cervical cytology: What has changed in this decade and what has not? European journal of obstetrics, gynecology, and reproductive biology. 2019;240:68-73
27. Kirschner B, Simonsen K, Junge J. Comparison of conventional Papanicolaou smear and SurePath liquid-based cytology in the Copenhagen population screening programme for cervical cancer. Cytopathology: official journal of the British Society for Clinical Cytology. 2006;17:187-94
28. Burnley C, Dudding N, Parker M, Parsons P, Whitaker CJ, Young W. Glandular neoplasia and borderline endocervical reporting rates before and after conversion to the SurePath(TM) liquid-based cytology (LBC) system. Diagnostic cytopathology. 2011;39:869-74
29. Kituncharoen S, Tantbirojn P, Niruthisard S. Comparison of Unsatisfactory Rates and Detection of Abnormal Cervical Cytology Between Conventional Papanicolaou Smear and Liquid-Based Cytology (Sure Path®). Asian Pacific journal of cancer prevention: APJCP. 2015;16:8491-4
30. Budak M, Senturk MB, Kaya C, Akgol S, Bademkiran MH, Tahaoğlu AE. et al. A comparative study of conventional and liquid-based cervical cytology. Ginekologia polska. 2016;87:190-3
31. Fischer G, Cormier K. Glandular Cell Abnormalities on SurePath Preparations: A Retrospective Review with Cytology-Histology Correlations. Acta cytologica. 2018;62:423-9
32. Zhou F, Chen H, Li M, Strickland AL, Zheng W, Zhang X. The Prognostic Values of HPV Genotypes and Tumor PD-L1 Expression in Patients With HPV-associated Endocervical Adenocarcinoma. The American journal of surgical pathology. 2022;46:300-8
33. Andersson S, Rylander E, Larson B, Sigurdardottir S, Backlund I, Sällström J. et al. Types of human papillomavirus revealed in cervical adenocarcinomas after DNA sequencing. Oncology reports. 2003;10:175-9
34. Bulk S, Berkhof J, Bulkmans NW, Zielinski GD, Rozendaal L, van Kemenade FJ. et al. Preferential risk of HPV16 for squamous cell carcinoma and of HPV18 for adenocarcinoma of the cervix compared to women with normal cytology in The Netherlands. British journal of cancer. 2006;94:171-5
35. Wang Z, Gu Y, Wang H, Chen J, Zheng Y, Cui B. et al. Distribution of cervical lesions in high-risk HPV (hr-HPV) positive women with ASC-US: a retrospective single-center study in China. Virology journal. 2020;17:185
36. Rodríguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME. et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. Journal of the National Cancer Institute. 2010;102:315-24
Author contact
![]() Corresponding authors: Feng Zhou, Department of Pathology, International Peace Maternity and Child Health Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China, 200030. Email: fungchewedu.cn; Shuang Niu, Department of Pathology, University of Texas Southwestern Medical Center; Dallas, 5323 Harry Hines Blvd. Dallas, TX, USA 75390. Email: Shuang.Niuedu.
Corresponding authors: Feng Zhou, Department of Pathology, International Peace Maternity and Child Health Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China, 200030. Email: fungchewedu.cn; Shuang Niu, Department of Pathology, University of Texas Southwestern Medical Center; Dallas, 5323 Harry Hines Blvd. Dallas, TX, USA 75390. Email: Shuang.Niuedu.

 Global reach, higher impact
Global reach, higher impact