Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(1):168-177. doi:10.7150/jca.26600 This issue Cite
Research Paper
Modern Radiation Further Improves Survival in Non-Small Cell Lung Cancer: An Analysis of 288,670 Patients
1. Department of Radiation Oncology, Indiana University School of Medicine, Indianapolis, IN;
2. Department of Radiation Oncology, University of Michigan, Ann Arbor, MI;
3. Epidemiology Research Core, Metropolitan Detroit Cancer Surveillance System, Surveillance, Epidemiology and End Results (SEER) Program, Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, MI;
4. Department of Radiation Oncology, Dartmouth-Hitchcock Medical Center, Lebanon, NH;
5. Department of Radiation Oncology, Seidman Comprehensive Cancer Center, Case Western Reserve University, Cleveland, OH.
Received 2018-6-15; Accepted 2018-7-19; Published 2019-1-1
Abstract
Background: Radiation therapy plays an increasingly important role in the treatment of patients with non-small-cell lung cancer (NSCLC). The purpose of the present study is to assess the survival outcomes of radiotherapy treatment compared to other treatment modalities and to determine the potential role of advanced technologies in radiotherapy on improving survival.
Methods: We used cancer incidence and survival data from the Surveillance, Epidemiology, and End Results database linked to U.S. Census data to compare survival outcomes of 288,670 patients with stage I-IV NSCLC treated between 1999 and 2008. The primary endpoint was overall survival.
Results: Among the 288,670 patients diagnosed with stage I-IV NSCLC, 92,374 (32%) patients received radiotherapy—almost double the number receiving surgery (51,961, 18%). Compared to other treatment groups and across all stages of NSCLC, patients treated with radiotherapy showed greater median and overall survival than patients without radiation treatment (p < 0.0001). Radiotherapy had effectively improved overall survival regardless of age, gender, and histological categorization. Radiotherapy treatment received during the recent time period 2004 - 2008 is correlated with enhanced survival compared to the earlier time period 1999 - 2003.
Conclusion: Radiation therapy was correlated with increased overall survival for all patients with primary NSCLC across stages. Combined surgery and radiotherapy treatment also correlates with improved survival, signaling the value of bimodal or multimodal treatments. Population-based increases in overall survival were seen in the recent time period, suggesting the potential role of advanced radiotherapeutic technologies in enhancing survival outcomes for lung cancer patients.
Keywords: non-small cell lung cancer (NSCLC), radiotherapy, treatments, overall survival
Introduction
Lung cancer is a leading cause of cancer-related death in the United States, with non-small-cell lung cancer (NSCLC) accounting for approximately 85% of cases [1]. Over 60% of patients with NSCLC require radiotherapy at least once during their course of disease [2, 3], and radiotherapy plays an increasingly pivotal role in local tumor control and survival outcomes [4-8].
Recent years have witnessed significant advances in radiotherapeutic technology [9], with the increasing utilization of stereotactic techniques, intensity modulated radiation therapy, 4-dimensional treatment planning, and image guidance for the treatment of stage-specific NSCLC [3, 10-14]. Aided by novel radiotherapeutic technologies, radiotherapy has made remarkable progress in achieving high local tumor control particularly in the treatment of inoperable NSCLC [15, 16]. More recently, targeted therapy and immunotherapy in combination with radiotherapy has shown promise in improving treatment outcomes and survival [3, 17-22].
In light of these developments, we hypothesized that radiotherapy treatment, facilitated by technological advances in radiation imaging, planning, and delivery, is correlated with enhanced survival outcomes. We sought to test this hypothesis through a comprehensive retrospective study of the Surveillance, Epidemiology, and End Results (SEER) data to provide insight into patterns of care and the relationship between survival and radiotherapy treatment between the years 1999 and 2008.
Methods and Materials
Data Source and Study Population
The study analyzed the SEER 17 Registry (1999-2008) from the National Cancer Institute, the comprehensive source of cancer incidence and survival data in the United States. Within SEER, we identified 288,670 patients with pathologically confirmed NSCLC between 1999 and 2008. The specific histologic types selected were adenocarcinoma, squamous cell carcinoma, large-cell carcinoma, carcinoid, and others. The use of radiotherapy, typically within 6 months of initial diagnosis, was abstracted from local tumor registries and reported to SEER. The following information was obtained and analyzed: age at diagnosis, gender, race, marital status, histology subtypes, the 5th and 6th editions of American Joint Cancer Committee (AJCC)/TNM staging category [23], SEER stage, and treatment type (radiotherapy, surgery, combined radiotherapy and surgery, or neither radiotherapy nor surgery). There was no difference in AJCC staging groups between 5th and 6th editions. Information about the use of adjuvant chemotherapy was not available within the SEER data and therefore was not included in our analysis.
Statistical Analysis
The primary endpoint of this study was overall survival (OS). The Pearson χ2 test was used to determine unadjusted associations between radiotherapy treatment and categorical variables of interest, including age, gender, stage distribution, and histology. OS was defined as the time between diagnosis and death. The Kaplan-Meier survival estimates method was used for generating the survival curves, and the log-rank test was used to assess the differences between the survival curves. Cases were also divided into two 5 year-of-diagnosis periods (1999-2003 and 2004-2008) for analysis of temporal trends. Results were considered statistically significant when P value <0.05. All P values were two-sided.
Results
Patient Characteristics
Using the SEER database, we identified a total of 288,670 patients diagnosed with stage I-IV NSCLC between 1999 and 2008. Baseline patient, tumor, and treatment-related characteristics were provided in Table 1. The male to female ratio was 1.19:1. Regarding race, 238,147 (82.5%) patients were white, and 31,100 (10.8%) black. 99,495 (34.5%) patients were aged <65 years, while 189,175 (65.5%) patients were aged ≥65 years. 147,399 (51.1%) patients were married, and 129,918 (45%) unmarried. In terms of AJCC stage, 53,764 (18.6%) patients were stage I, 10,937 (3.8%) stage II, 74,570 (25.8%) stage III, and 117,228 (40.6%) stage IV. Using the SEER staging system, 51,802 (18%) patients had localized tumor, while 69,650 (24.1%) had regional tumor. In terms of histology, 98,141 (34%) had adenocarcinoma, 60,057 (20.8%) had squamous cell carcinoma, 11,669 (4.1%) had large-cell carcinoma, 115,219 (39.9%) had others, and 3,584 (1.2%) had carcinoid (Table 1).
Overall Survival and Its Associations with Patient Characteristics
The median OS for all patients (n=288,670) was 8 months. The 1-year, 2-year, 3-year, 5-year OS were 40%, 26.3%, 20.2%, and 14.5%, respectively (Table 1; Figure 1).
Gender, race, age, marital status, AJCC stage, SEER stage, and histology were all significantly associated with survival outcomes (Table 1). Young white married female patients with lower stage NSCLC had a higher median and OS. 17.2% of females had a 5-year OS, compared to 12.2% of males (P<0.0001). Patients aged <65 years had a 5-year OS of 19.6% (median survival, 11 months), while patients aged ≥65 years have a 5-year OS of 11.7% (median survival, 7 months) (P<0.0001). Stage I NSCLC had highest proportion of 5-year OS at 45.2%, with a median survival of 49 months (P<0.0001). Stage IV had the lowest proportion of 5-year OS at 2.4%, with a median survival of 4 months (P<0.0001). Five-year OS was more favorable for localized (43.5%, median survival, 45 months) than regional (19.9%, median survival, 15 months) tumors (P<0.0001).
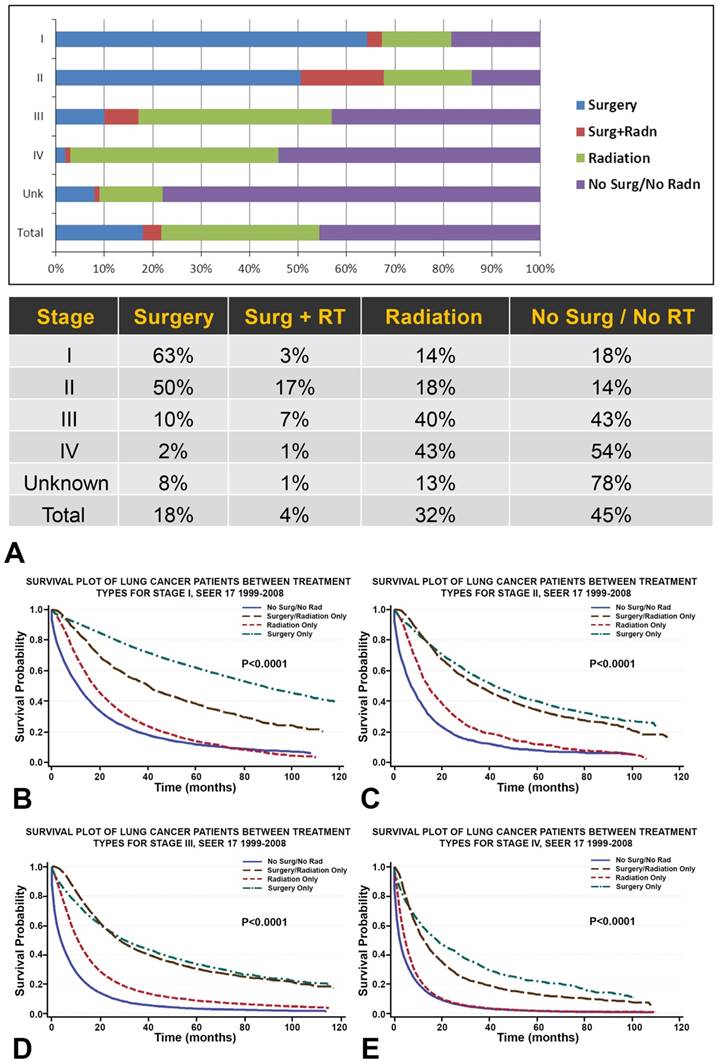
A. Among all primary NSCLC patients (n=288,670), 18% of patients received surgery, 4% combined surgery and radiotherapy, 32% radiotherapy, and 45% neither surgery nor radiotherapy. Overall, the proportion of NSCLC patients receiving radiation (32%) is almost double that receiving surgery (18%). B-E. Survival plots for NSCLC patients (n=288,670) between treatment types show that, across all stages, NSCLC treated with radiotherapy is associated with greater OS than NSCLC treated without surgery or radiation. While surgical treatment is correlated with the greatest OS across all stages of NSCLC, the survival distribution of combined surgery and radiation treatment begins to match that of surgery for patients with stage II/III NSCLC.
Patient characteristics and survival.
| Characteristics | # Cases | % Pts | MS ( 95% CI ) | % of Overall Survival (95% CI) | P Value | |||
|---|---|---|---|---|---|---|---|---|
| months | 1 Year | 2 Year | 3 Year | 5 Year | ||||
| Gender | <0.0001 | |||||||
| Male | 156,721 | 54.3 | 7 (na, na) | 36.9 (36.6, 37.1) | 23.1 (22.9, 23.3) | 17.3 (17.1, 17.5) | 12.2 (12.0, 12.4) | |
| Female | 131,949 | 45.7 | 10 (9,10) | 43.8 (43.6, 44.1) | 30.2 (30.0, 30.5) | 23.8 (23.5, 24.0) | 17.2 (17.0, 17.5) | |
| Race | <0.0001 | |||||||
| White | 238,147 | 82.5 | 8 (na, na) | 40.1 (39.9, 40.3) | 26.6 (26.4, 26.8) | 20.5 (20.3, 20.6) | 14.8 (14.6, 14.9) | |
| Black | 31,100 | 10.8 | 7 (7, 8) | 36.8 (36.2, 37.3) | 22.3 (21.8, 22.8) | 16.8 (16.3, 17.2) | 11.7 (11.2, 12.1) | |
| Other/Unk | 19,423 | 6.7 | 10 (na,na) | 44.7 (44.0, 45.5) | 29.8 (29.1, 30.5) | 22.8 (22.1, 23.4) | 15.5 (14.9, 16.1) | |
| Age | <0.0001 | |||||||
| <65 | 99,495 | 34.5 | 11 (na, na) | 47.0 (46.7, 47.3) | 32.0 (31.7, 32.3) | 25.7 (25.4, 26.0) | 19.6 (19.4, 19.9) | |
| ≥65 | 189,175 | 65.5 | 7 (na, na) | 36.4 (36.2, 36.6) | 23.3 (23.1, 23.5) | 17.4 (17.2, 17.6) | 11.7 (11.5, 11.9) | |
| Marital Status | <0.0001 | |||||||
| Married | 147,399 | 51.1 | 10 (na, na) | 43.8 (43.6, 44.1) | 29.5 (29.2, 29.7) | 23.1 (22.8, 23.3) | 16.9 (16.7, 17.1) | |
| Not Married | 129,918 | 45.0 | 7 (na, na) | 35.8(35.6, 36.1) | 22.8 (22.6, 23.1) | 17.1 (16.8, 17.3) | 11.8 (11.6, 12.0) | |
| Unknown | 11,353 | 3.9 | 8 (7, 8) | 39.1 (38.1, 40.0) | 25.8 (24.9, 26.7) | 19.6 (18.8, 20.4) | 13.9 (13.2, 14.7) | |
| AJCC Stage | <0.0001 | |||||||
| Stage I | 53,764 | 18.6 | 49 (48, 50) | 78.7 (78.3, 79.0) | 65.7 (65.3, 66.2) | 56.9 (56.4, 57.3) | 45.2 (44.7, 45.8) | |
| Stage II | 10,937 | 3.8 | 27 (26, 29) | 71.0 (70.1, 71.9) | 53.3 (52.3, 54.3) | 42.3 (41.2, 43.3) | 29.8 (28.7, 30.9) | |
| Stage III | 74,570 | 25.8 | 9 (9, 10) | 41.7 (41.3, 42.1) | 24.0 (23.7, 24.3) | 16.6 (16.3, 16.9) | 10.6 (10.4, 10.9) | |
| Stage IV | 117,228 | 40.6 | 4 (na, na) | 19.7 (19.5, 20.0) | 8.5 (8.3, 8.7) | 4.9 (4.7, 5.0) | 2.4 (2.3, 2.5) | |
| SEER Stage | <0.0001 | |||||||
| Localized | 51,802 | 18.0 | 45 (44, 46) | 76.7 (76.5, 77.2) | 63.5 (63.0, 63.9) | 54.9 (54.4, 55.4) | 43.5 (43.0, 44.0) | |
| Regional | 69,650 | 24.1 | 15 (15, 16) | 55.7 (55.3, 56.1) | 37.5 (37.1, 37.9) | 28.5 (28.1, 28.9) | 19.9 (19.5, 20.2) | |
| Histology | <0.0001 | |||||||
| Adenocarcinoma | 98,141 | 34.0 | 10 (na, na) | 45.5 (45.1, 45.8) | 31.0 (30.7, 31.3) | 24.1 (23.8, 24.4) | 17.0 (16.7, 17.3) | |
| Squamous Ca | 60,057 | 20.8 | 10 (na, na) | 44.9 (44.5, 45.3) | 29.0 (28.6, 29.4) | 21.9 (21.5, 22.2) | 15.6 (15.3, 16.0) | |
| Large Cell Ca | 11,669 | 4.1 | 8 (7,8) | 36.5 (35.6, 37.4) | 22.5 (21.7, 23.3) | 17.4 (16.7, 18.2) | 12.7 (12.0, 13.4) | |
| Carcinoid | 3,584 | 1.2 | N/A | 93.8 (93.0, 94.6) | 90.6 (89.6, 91.6) | 87.8 (86.4, 88.8) | 82.6 (81.1, 84.1) | |
| Others | 115,219 | 39.9 | 6 (na, na) | 31.7 (31.4, 31.9) | 19.5 (19.2, 19.7) | 14.4 (14.2, 14.6) | 10.0 (9.8, 10.2) | |
| All Patients | 288,670 | 100 | 8 (na, na) | 40.0 (39.9, 40.2) | 26.3 (26.2, 26.5) | 20.2 (20.1, 20.4) | 14.5 (14.3, 14.7) | |
Note: MS=median survival, pt= patients, CI= confidence interval, ca= carcinoma, N/A= an interval that was too narrow to be computable.
Treatment Modality and Survival
Among all primary NSCLC patients, 18% of patients received surgery, 4% combined surgery and radiotherapy, 32% radiotherapy, and 45% neither surgery nor radiotherapy (Figure 1, Table 2A, Table 2B and Table 2C). Overall, the proportion of NSCLC patients receiving radiation (32%) was almost double that receiving surgery (18%) (Figure 1, Table 2A, Table 2B and Table 2C). The majority of patients with stage I/II NSCLC received surgery, whereas patients with stage III/IV NSCLC were more likely to receive radiation therapy. Combined surgery and radiotherapy was used most frequently when treating stage II NSCLC (Figure 1, Table 2A, Table 2B and Table 2C).
Survival plots for NSCLC patients between treatment types were shown in Figure 1. NSCLC treated with radiotherapy alone was associated with greater OS than NSCLC treated without surgery or radiation (Figure 1). While surgical treatment was correlated with the greatest OS across all stages of NSCLC, the survival distribution of combined surgery and radiation treatment begins to match that of surgery for patients with stage II/III NSCLC.
The Influence of Radiation Therapy on Overall Survival
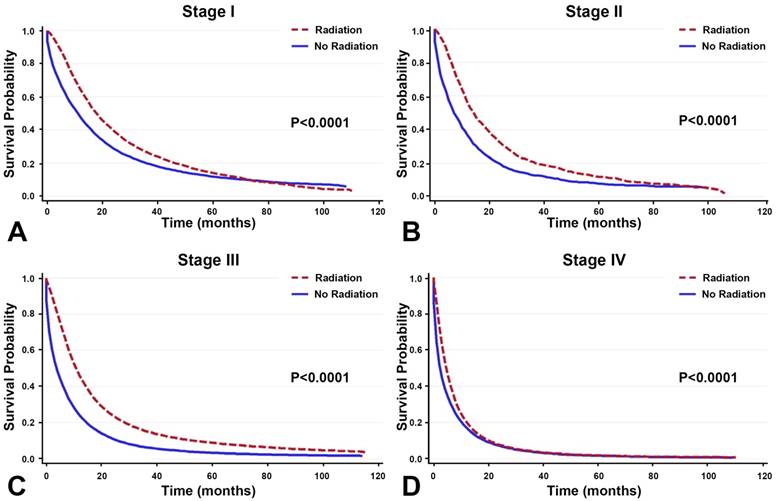
In the patients who did not receive surgery, radiotherapy improved survival across all stages of NSCLC. The overall median survival gain was 4 months, with the most pronounced gains found in stage II/III lung cancer patients (Table 3). The median survival gain for stage I, II, III, and IV was 6 months, 8 months, 7 months, and 2 months, respectively. Moreover, in all patients with NSCLC, treatment with radiotherapy improved OS more than treatment without (Figure 2).
Radiotherapy had effectively improved OS regardless of age, gender, and histological categorization (Table 4).
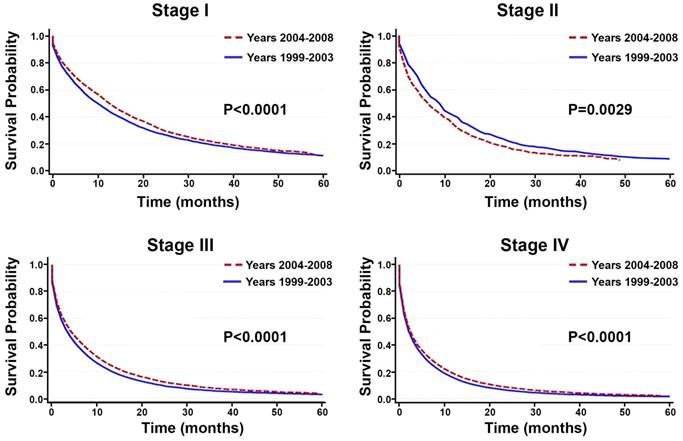
Comparison of Overall Survival Between Time Periods
Between the time periods of 1999-2003 and 2004-2008, OS improved by 2% (Table 4, Figure 3). This improvement in survival outcomes is significant for patients with stage I, stage III, and stage IV NSCLC (P<0.0001, Figure 3). Among patients receiving radiotherapy (n=93,633), treatment received during the between 2004-2008 was correlated with enhanced survival compared to 1999-2003 (Figure 3E-H). Surgical treatment improved survival for stage III and stage IV NSCLC (Figure 4). Notably, stage I NSCLC showed significant increase in survival with radiotherapy treatment but not with surgery (P<0.0001). For patients who did not receive surgery or radiotherapy (n=131,022), survival improved across every stage, suggesting that overall quality of care improved, or patient selection was favorable (Figure 5).
Radiation and survival stratified by age
| Radiation | No Radiation | Radiation Difference | |||||
|---|---|---|---|---|---|---|---|
| MS | 95% CI | MS | 95% CI | *P value | MS Gain | ||
| In 151,453 patients who did not have surgery and age ≥ 65 years | |||||||
| 18 | 17, 19 | 10 | 10, 11 | <0.0001 | 8 | ||
| 13 | 13,15 | 7 | 6, 8 | <0.0001 | 6 | ||
| 10 | na | 3 | 3, 4 | <0.0001 | 7 | ||
| 4 | na | 2 | na | <0.0001 | 2 | ||
| 7 | na | 3 | na | <0.0001 | 4 | ||
| In 73,202 patients who did not have surgery and age < 65 years | |||||||
| 20 | 19, 22 | 19 | 17, 20 | <0.0001 | 1 | ||
| 18 | 15, 21 | 9 | 8, 11 | <0.0001 | 9 | ||
| 13 | 13, 14 | 7 | 7, 8 | <0.0001 | 6 | ||
| 6 | na | 4 | na | <0.0001 | 2 | ||
| 8 | na | 6 | 5, 6 | <0.0001 | 2 | ||
Radiation and survival stratified by gender
| Stage | Radiation | No Radiation | Radiation Difference | |||||
|---|---|---|---|---|---|---|---|---|
| MS | 95% CI | MS | 95% CI | *P value | MS Gain | |||
| In 124,301 patients who did not have surgery and were male | ||||||||
| I | 16 | 16, 17 | 10 | 10, 11 | <0.0001 | 6 | ||
| II | 14 | 13, 15 | 7 | 6, 8 | <0.0001 | 7 | ||
| III | 11 | 10, 11 | 4 | na | <0.0001 | 7 | ||
| IV | 5 | na | 3 | 2, 3 | <0.0001 | 2 | ||
| Total | 7 | na | 3 | na | <0.0001 | 4 | ||
| In 100,354 patients who did not have surgery and were female | ||||||||
| I | 21 | 20, 22 | 13 | 12, 14 | <0.0001 | 8 | ||
| II | 16 | 15, 18 | 8 | 7, 9 | <0.0001 | 8 | ||
| III | 12 | 12, 13 | 4 | 4, 5 | <0.0001 | 8 | ||
| IV | 5 | na | 3 | na | <0.0001 | 2 | ||
| Total | 8 | na | 4 | na | <0.0001 | 4 | ||
Radiation and survival stratified by histology
| Stage | Radiation | No Radiation | Radiation Difference | |||||
|---|---|---|---|---|---|---|---|---|
| MS | 95% CI | MS | 95% CI | *P value | MS Gain | |||
| In 43,467 patients who did not have surgery squamous cell carcinoma (SCC) | ||||||||
| I | 16 | 15, 17 | 9 | 9, 10 | <0.0001 | 7 | ||
| II | 13 | 12, 15 | 7 | 5, 9 | <0.0001 | 6 | ||
| III | 11 | na | 5 | 4, 5 | <0.0001 | 6 | ||
| IV | 5 | na | 3 | na | <0.0001 | 2 | ||
| Total | 9 | na | 4 | 4, 5 | <0.0001 | 5 | ||
| In 181,188 patients who did not have surgery and had non-SCC | ||||||||
| I | 12 | 19, 20 | 12 | 12, 13 | <0.0001 | <1 | ||
| II | 15 | 14, 17 | 8 | 7, 9 | <0.0001 | 7 | ||
| III | 12 | 11, 12 | 5 | na | <0.0001 | 7 | ||
| IV | 5 | na | 3 | na | <0.0001 | 2 | ||
| Total | 7 | na | 4 | na | <0.0001 | 3 | ||
Note: MS= median survival in months, CI = confidence interval, na= an interval that was too narrow to be computable.
*The P-values are for the overall difference between the time periods for each stage.
Discussion
In our analysis of 288,670 lung cancer patients treated between 1999 and 2008, radiotherapy treatment was correlated with improved OS. Notably, the number of NSCLC patients receiving radiation (32%) was nearly double that receiving surgery (16%). In an epidemiology study of cancer survivors between 2000 and 2030, Bryant et al. reports a projected increase in radiation-treated lung cancer survivors from 16-31% [24]. These numbers underscore the growing prevalence of radiotherapy in cancer therapeutics, even as the incidence rate of lung cancer declines over recent years [3, 24, 25]. The data from the current study also suggest that combined radiotherapy and surgical treatment is correlated with enhanced survival outcomes comparable to that of surgery for stage II/III NSCLC. This trend is consistent with previous studies which have demonstrated the potential for multimodal therapy to improve treatment and survival outcomes for NSCLC [26, 27].
The chronologic impact of technological advances in radiation was analyzed during two consecutive time periods: 1999-2003 and 2004-2008. The earlier time period (1999-2003) reflects the time before the full availability of advanced technologies in radiotherapy. The latter time period (2004-2008) reflects the time after the availability of advanced imaging and radiation-delivering technologies. In this study, radiotherapy treatment during 2004-2008 showed improved survival compared to 1999-2003. Interestingly, stage I NSCLC showed significant improvement in survival with radiotherapy treatment but not with surgery, possibly due to the introduction of new technologies such as stereotactic body radiation therapy (SBRT). While stereotactic radiation was first used cranially in the 1950s, SBRT became FDA approved in 2001 for extracranial treatment of tumors and quickly showed promise in the treatment of various tumor sites including the lung [28, 29]. SBRT uses multiple radiation beams to precisely deliver high doses to tumor targets in extracranial sites. Before the advent of SBRT, surgical resection was the standard treatment for stage I NSCLC, achieving 5-year survival rates of 60-70% [28]. The emergence of SBRT has improved lung cancer survival across various countries, with survival rates comparable to that of surgery [30-32]. The Netherlands, for example, has witnessed the growth of SBRT utilization in the treatment of lung cancer over the course of 10 years [33]. In a population-based study of elderly Dutch patients with stage I NSCLC, Haasbeek et al. [34] found increases in survival for patients seen between 2001 and 2009, the years encompassing the introduction and full availability of SBRT treatment.
In the 224,655 patients who did not undergo surgery, radiotherapy is correlated with greater OS across all stages.
Radiation improved overall survival.
| Stage | Radiation | No Radiation | Radiation Difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % pt | MS (95% CI) | N | %pt | MS ( 95% CI) | *P value | MS Gain (months) | |||
| I | 7473 | 14% | 18 (18, 19) | 9732 | 18% | 12 (11, 12) | <0.0001 | 6 | ||
| II | 1931 | 18% | 15 (14, 15) | 1484 | 14% | 7 (7, 9) | <0.0001 | 8 | ||
| III | 29795 | 40% | 11 (n/a, n/a) | 31935 | 43% | 4 (n/a, n/a) | <0.0001 | 7 | ||
| IV | 50325 | 43% | 5 (n/a, n/a) | 62865 | 54% | 3 (n/a, n/a) | <0.0001 | 2 | ||
| Unknown | 4109 | 13% | 12 (n/a, n/a) | 25006 | 78% | 5 (4, 5) | <0.0001 | 7 | ||
| Total | 93633 | 32% | 8 (n/a, n/a) | 131022 | 45% | 4 (n/a, n/a) | <0.0001 | 4 | ||
Note: MS=median survival in months, CI = confidence interval, pt = patient, na= an interval that was too narrow to be computable.
*The P-values are for the overall difference between the time periods for each stage.
Among NSCLC patients receiving radiotherapy without surgery, radiotherapy improved median survival across all stages.
Stage and overall survival stratified by time period.
| Stage | % of Overall Survival (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| # Pts | % pts | MS ( 95% CI ) | 1 Yr | 2 Yr | 3 Yr | 5 Yr | ||
| 1999-2008 Total | ||||||||
| I | 53764 | 19% | 49(48, 50) | 78.7 (78.3, 79.0) | 65.7 (65.3, 66.2) | 56.9 (56.4, 57.3) | 45.2 (44.7, 45.8) | |
| II | 10937 | 4% | 27 (26, 29) | 71.0 (70.1, 71.9) | 53.3 (52.3, 54.3) | 42.3 (41.2, 43.3) | 29.8 (28.7, 30.9) | |
| III | 74570 | 26% | 9 (9, 10) | 41.7 (41.3, 42.1) | 24.0 (23.7, 24.3) | 16.6 (16.3, 16.9) | 10.6 (10.4, 10.9) | |
| IV | 117228 | 41% | 4 (n/a, n/a) | 19.7 (19.5, 20.0) | 8.5 (8.3, 8.7) | 4.9 (4.7, 5.0) | 2.4 (2.3, 2.5) | |
| Unknown | 32171 | 11% | 7 (6, 7) | 34. 9 (34.4, 35.5) | 21.4 (20.9, 21.9) | 15.2 (14.7, 15.6) | 9.5 (9.1, 9.9) | |
| Total | 288670 | 100% | 8 (n/a, n/a) | 40.0 (39.9, 40.2) | 26.3 (26.2, 26.5) | 20.2 (20.1, 20.4) | 14.5 (14.3, 14.7) | |
| 1999-2003 Total | ||||||||
| I | 27469 | 20% | 44 (43, 46) | 76.1 (75.6, 76.6) | 63.3 (62.7, 63.8) | 54.4 (53.8, 55.0) | 43.2(42.6, 43.8) | |
| II | 4382 | 3% | 28 (27, 30) | 73.1 (71.7, 74.4) | 54.2 (52.7, 55.6) | 41.5 (40.1, 42.9) | 30.0 (28.7, 31.4) | |
| III | 40305 | 29% | 9 (n/a, n/a) | 39.3 (38.8, 39.8) | 22.1 (21.7, 22.5) | 15.1 (14.8, 15.5) | 9.6 (9.4, 9.9) | |
| IV | 53293 | 39% | 4 (n/a, n/a) | 17.8 (17.5, 18.1) | 7.2 (6.9, 7.4) | 4.1 (3.9, 4.2) | 2.0 (1.9, 2.1) | |
| Unknown | 12514 | 9% | 7 (6, 7) | 33.7 (32.9, 34.5) | 18.9 (18.2, 19.6) | 12.3 (11.7, 12.9) | 7.3 (6.8, 7.7) | |
| Total | 137963 | 100% | 8 (n/a, n/a) | 38.9 (38.6, 39.1) | 25.3 (25.0, 25.5) | 19.3 (19.1, 19.5) | 13.8 (13.6, 14.0) | |
| 2004-2008 Total | ||||||||
| I | 26295 | 17% | 56 (54, 58) | 81.6 (81.1, 82.1) | 68.6 (68.0, 69.3) | 60.1 (59.4, 60.9) | NA | |
| II | 6555 | 4% | 27 (26, 29) | 69.5 (68.3, 70.7) | 52.9 (51.5, 54.2) | 42.2 (40.7, 43.7) | NA | |
| III | 34265 | 23% | 10 (10, 11) | 44.8 (44.2, 45.3) | 26.7 (26.2, 27.3) | 18.8 (18.2, 19.3) | NA | |
| IV | 63935 | 42% | 4 (n/a, n/a) | 21.6 (21.2, 21.9 ) | 9.9 (9.6, 10.1) | 5.7 (5.5, 5.9) | NA | |
| Unknown | 19657 | 13% | 6 (6, 7) | 35.9 (35.2, 36.6) | 23.5 (22.9, 24.2) | 18.0 (17.3, 18.6) | NA | |
| Total | 150707 | 100% | 9 (8, 9) | 41.2 (41.0, 41.5) | 27.5 (27.3, 27.8) | 21.3 (21.1, 21.6) | NA | |
Note: MS= median survival in months, CI = confidence interval, na= an interval that was too narrow to be computable.
Survival between the time period of 1999-2003 and 2004-2008. A-D: Among all primary NSCLC patients (n=288,670), overall survival improved significantly for patients with stage I, stage, III, and stage IV NSCLC between the time periods 1999-2003 and 2004-2008 (P<0.0001). E-H: Among the 93,633 patients receiving radiotherapy, treatment given during the recent time period (2004-2008) is correlated with enhanced OS compared to the earlier time period (1999-2003).
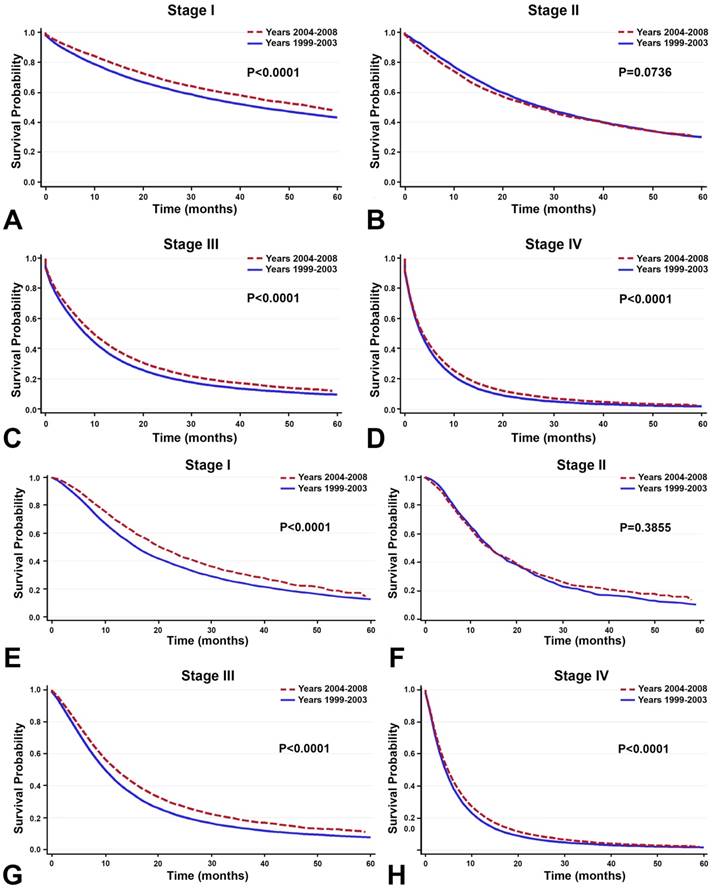
In the 10,838 patients receiving surgery, survival improved in the recent years (2004-2008) compared to the earlier years (1999-2003) only in stage III and stage IV NSCLC. Overall survival did not increase significantly for stage I and stage II NSCLC.
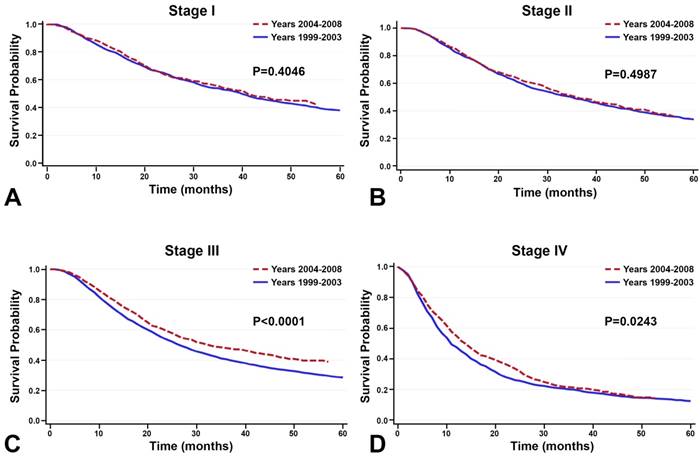
Among the 131,022 patients who did not receive surgery or radiotherapy, survival improved between the time periods 1999-2003 and 2004-2008 across every stage.
In the United States, the first phase I clinical trial of SBRT was conducted in 2003; Timmerman et al. [35] found that medically inoperable patients with early-stage NSCLC experienced significantly improved treatment outcomes with SBRT. In light of this encouraging data and in anticipation of widespread use of this innovative technology. RTOG236 the only SBRT multicenter trial was approved in the year of 2002, kicked off in 2003, and became widely adopted around 2004. The American Society of Therapeutic Radiology and Oncology (ASTRO) and the American College of Radiology (ACR) developed guidelines in 2004 for integrating SBRT into the standard treatment of lung cancer [36]. Since then, numerous ongoing studies have assessed the role of SBRT in various clinical settings and have found high rates of tumor control exceeding 90% [16, 37, 38]. Indeed, our data show improved survival during this time period after implementation of the 2004 ASTRO and ACR guidelines. Improved survival may also be impacted by novel imaging techniques which could contribute to stage migration, or the Will Rogers phenomenon. Further investigation is warranted to elucidate the nuanced roles of these novel technologies [39-42].
There are several limitations to our study. SEER data were observational and retrospective, and lack information on adjuvant chemotherapy and radiotherapy technique [43, 44]. Nonetheless, the standard of care for the treatment of NSCLC is constantly evolving [45]. It is possible that, over the course of this study, patients increasingly received bimodal or multimodal treatments which could not be fully assessed in this study. Importantly, recent advancements in targeted therapy and immunotherapy have ushered a new era of precision radiation oncology that harnesses radiobiological mechanisms and technology-driven improvements to improve therapeutic outcomes for patients with NSCLC [21, 46-48]. Combined immunoradiotherapy has shown promise in improving survival outcomes by capitalizing on the synergistic anti-tumor responses to the two treatment modalities, and several trials are underway to explore this topic [17, 18, 46-49]. Understanding the features of genomic instability that influence anti-tumor response and identifying the DNA repair biomarkers will help guide the use of immune-directed therapies combined with radiotherapy [9, 50-52]. In a phase I KEYNOTE-001 trial, radiotherapy was shown to improve progression-free survival and OS among NSCLC patients treated afterward with pembrolizumab [17]. These findings warrant further trials to more fully assess the long-term impact of radiotherapy in combination with immunotherapy[53].
Conclusions
This large population-based study of 288,670 patients with primary NSCLC shows that radiotherapy is correlated with improved survival outcomes and is increasingly utilized in the treatment of NSCLC. Our study represents one of the largest population-based studies performed to date of radiotherapy and survival in lung patients across all stages. Combined surgery and radiotherapy treatment correlate with improved survival, as compared to other treatment modalities. Our data also provide supporting evidence for the potential of recent advances in radiotherapeutic technologies to enhance survival outcomes in NSCLC. As treatment regimens evolve to utilize multimodal and targeted therapy alongside innovative technology, we enter a new era of personalized clinical oncology that promises to improve survival outcomes for patients with NSCLC through a tailored radiotherapeutic approach.
Abbreviations
NSCLC: non-small-cell lung cancer; SEER: Surveillance, Epidemiology, and End Results; (SEER); AJCC: American Joint Cancer Committee; OS: overall survival.
Acknowledgements
We are grateful to Woodworth family for their generous gift to make this research possible.
Funding
This work was supported in part by NIH grants R01CA142840 (PI: Kong). This project was funded in part by the National Cancer Institute, National Institutes of Health, Dept. of Health and Human Services, under Contract No. HHSN261201000028C and by NCI grant 5P30CAO22453-32.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30
2. Tyldesley S, Boyd C, Schulze K, Walker H, Mackillop WJ. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49:973-85
3. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-54
4. Van Houtte P, Rocmans P, Smets P, Goffin JC, Lustman-Marechal J, Vanderhoeft P. et al. Postoperative radiation therapy in lung caner: a controlled trial after resection of curative design. Int J Radiat Oncol Biol Phys. 1980;6:983-6
5. Phlips P, Rocmans P, Vanderhoeft P, Van Houtte P. Postoperative radiotherapy after pneumonectomy: impact of modern treatment facilities. Int J Radiat Oncol Biol Phys. 1993;27:525-9
6. Machtay M, Lee JH, Stevenson JP, Shrager JB, Algazy KM, Treat J. et al. Two commonly used neoadjuvant chemoradiotherapy regimens for locally advanced stage III non-small cell lung carcinoma: long-term results and associations with pathologic response. J Thorac Cardiovasc Surg. 2004;127:108-13
7. Okawara G, Ung YC, Markman BR, Mackay JA, Evans WK. Postoperative radiotherapy in stage II or IIIA completely resected non-small cell lung cancer: a systematic review and practice guideline. Lung Cancer. 2004;44:1-11
8. Jassem J. Combined chemotherapy and radiation in locally advanced non-small-cell lung cancer. Lancet Oncol. 2001;2:335-42
9. Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J. et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234-49
10. Koshy M, Malik R, Spiotto M, Mahmood U, Rusthoven CG, Sher DJ. Association between intensity modulated radiotherapy and survival in patients with stage III non-small cell lung cancer treated with chemoradiotherapy. Lung Cancer. 2017;108:222-7
11. Jegadeesh N, Liu Y, Gillespie T, Fernandez F, Ramalingam S, Mikell J. et al. Evaluating intensity-modulated radiation therapy in locally advanced non-small-cell lung cancer: Results from the national cancer data base. Clin Lung Cancer. 2016;17:398-405
12. Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE. et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56-62
13. Puri V, Crabtree TD, Bell JM, Broderick SR, Morgensztern D, Colditz GA. et al. Treatment outcomes in stage I lung cancer: A comparison of surgery and stereotactic body radiation therapy. J Thorac Oncol. 2015;10:1776-84
14. Videtic GMM, Donington J, Giuliani M, Heinzerling J, Karas TZ, Kelsey CR. et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;5:295-301
15. Kong FM, Ten Haken RK, Schipper M, Frey KA, Hayman J, Gross M. et al. Effect of midtreatment PET/CT-adapted radiation therapy with concurrent chemotherapy in patients with locally advanced non-small-cell lung cancer: a phase 2 clinical trial. JAMA Oncol. 2017;10:1358-65
16. Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P. et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630-7
17. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC. et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895-903
18. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919-29
19. Khalifa J, Amini A, Popat S, Gaspar LE, Faivre-Finn C, International Association for the Study of Lung Cancer Advanced Radiation Technology C. Brain metastases from NSCLC: Radiation therapy in the era of targeted therapies. J Thorac Oncol. 2016;11:1627-43
20. Counago F, Rodriguez A, Calvo P, Luna J, Monroy JL, Taboada B. et al. Targeted therapy combined with radiotherapy in non-small-cell lung cancer: a review of the Oncologic Group for the Study of Lung Cancer (Spanish Radiation Oncology Society). Clin Transl Oncol. 2017;19:31-43
21. Hiley CT, Le Quesne J, Santis G, Sharpe R, de Castro DG, Middleton G. et al. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet. 2016;388:1002-11
22. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350-5
23. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG. et al. American Joint Committee on Cancer Staging Manual, 6th ed. New York: Springer. 2004
24. Bryant AK, Banegas MP, Martinez ME, Mell LK, Murphy JD. Trends in radiation therapy among cancer survivors in the United States, 2000-2030. Cancer Epidemiol Biomarkers Prev. 2017;26:963-70
25. Rosenzweig KE, Gomez JE. Concurrent Chemotherapy and Radiation Therapy for Inoperable Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2017;35:6-10
26. Herskovic A, Chitti B, Christos P, Wernicke AG, Parashar B. Addition of surgery after radiation significantly improves survival in stage IIIB non-small cell lung cancer: A population-based analysis. World J Surg. 2017;41:758-62
27. Bott MJ, Patel AP, Crabtree TD, Morgensztern D, Robinson CG, Colditz GA. et al. Role for surgical resection in the multidisciplinary treatment of stage iiib non-small cell lung cancer. Ann Thorac Surg. 2015;99:1921-8
28. Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117:4566-72
29. Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol. 2014;32:2847-54
30. Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys. 2012;82:1149-56
31. Sun B, Brooks ED, Komaki RU, Liao Z, Jeter MD, McAleer MF. et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer. 2017;123:3031-9
32. Barton MK. Encouraging long-term outcomes reported in patients with stage I non-small cell lung cancer treated with stereotactic ablative radiotherapy. CA Cancer J Clin. 2017;67:349-50
33. Peguret N, Dahele M, Lagerwaard F, Senan S, Slotman BJ. A brief report of 10-year trends in the use of stereotactic lung radiotherapy at a dutch academic medical center. J Thorac Oncol. 2014;9:114-7
34. Haasbeek CJ, Palma D, Visser O, Lagerwaard FJ, Slotman B, Senan S. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol. 2012;23:2743-7
35. Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S. et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946-55
36. Potters L, Steinberg M, Rose C, Timmerman R, Ryu S, Hevezi JM. et al. American Society for Therapeutic Radiology and Oncology and American College of Radiology practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2004;60:1026-32
37. Bi N, Shedden K, Zheng X, Kong FM. Comparison of the effectiveness of radiofrequency ablation with stereotactic body radiation therapy in inoperable stage I non-small cell lung cancer: A systemic review and pooled analysis. Int J Radiat Oncol Biol Phys. 2016;95:1378-90
38. Nanda RH, Liu Y, Gillespie TW, Mikell JL, Ramalingam SS, Fernandez FG. et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: A National Cancer Data Base analysis. Cancer. 2015;121:4222-30
39. Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604-8
40. Geiger GA, Kim MB, Xanthopoulos EP, Pryma DA, Grover S, Plastaras JP. et al. Stage migration in planning PET/CT scans in patients due to receive radiotherapy for non-small-cell lung cancer. Clin Lung Cancer. 2014;15:79-85
41. Chee KG, Nguyen DV, Brown M, Gandara DR, Wun T, Lara PN Jr. Positron emission tomography and improved survival in patients with lung cancer: the Will Rogers phenomenon revisited. Arch Intern Med. 2008;168:1541-9
42. Dinan MA, Curtis LH, Carpenter WR, Biddle AK, Abernethy AP, Patz EF Jr. et al. Stage migration, selection bias, and survival associated with the adoption of positron emission tomography among medicare beneficiaries with non-small-cell lung cancer, 1998-2003. J Clin Oncol. 2012;30:2725-30
43. Lally BE, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;24:2998-3006
44. Dautzenberg B, Arriagada R, Chammard AB, Jarema A, Mezzetti M, Mattson K. et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Cancer. 1999;86:265-73
45. Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY. et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16:807-21
46. Soo RA, Stone EC, Cummings KM, Jett JR, Field JK, Groen HJ. et al. Scientific advances in thoracic oncology 2016. J Thorac Oncol. 2017;8:1183-209
47. Cassidy RJ, Zhang X, Patel PR, Shelton JW, Escott CE, Sica GL. et al. Next-generation sequencing and clinical outcomes of patients with lung adenocarcinoma treated with stereotactic body radiotherapy. Cancer. 2017;19:3681-90
48. Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS.Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol 2018; 15:477-94.
49. Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51
50. Mouw KW, Goldberg MS, Konstantinopoulos PA, D'Andrea AD. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov. 2017;7:675-93
51. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498-509
52. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365-79
53. Cheng M, Durm G, Hanna N, Einhorn LH, Kong FS. Can radiotherapy potentiate the effectiveness of immune checkpoint inhibitors in lung cancer? Future Oncology. 2017;13:2503-5
Author contact
![]() Corresponding author: Feng-Ming (Spring) Kong, M.D., Ph.D., Department of Radiation Oncology, Seidman Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106. Telephone: 216-983-4703, Fax: 216-201-6623, E-mail: fxk132edu
Corresponding author: Feng-Ming (Spring) Kong, M.D., Ph.D., Department of Radiation Oncology, Seidman Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106. Telephone: 216-983-4703, Fax: 216-201-6623, E-mail: fxk132edu

 Global reach, higher impact
Global reach, higher impact