3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2026; 17(3):542-554. doi:10.7150/jca.125922 This issue Cite
Review
MZB1 at the ER-immunity interface: from antibody folding to disease vulnerability in autoimmunity, inflammation, and cancer
1. Department of Clinical Laboratory, the Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming 650118, Yunnan, China.
2. Yunnan Key Laboratory of Laboratory Medicine, Kunming 650032, Yunnan, China.
3. Department of Breast Surgery, the Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming 650118, Yunnan, China.
4. Department of Ultrasound Department, the Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming 650118, Yunnan, China.
# Equal contributors.
Received 2025-9-27; Accepted 2026-1-19; Published 2026-2-11
Abstract
MZB1 (marginal zone B and B1 cell-specific protein 1) is an endoplasmic reticulum-resident molecular chaperone that is predominantly expressed in marginal zone B cells, B1 cells, plasma cells, and pDCs. Functioning as a co-chaperone of GRP94 and BiP, MZB1 selectively facilitates the proper folding and secretion of immunoglobulin M (IgM) and J-chain-containing immunoglobulin A (IgA) dimers, and maintains the homeostasis of highly secretory cells by upregulating the expression of partner proteins such as BiP/GRP94. This review highlights the emerging role of MZB1 across various pathological conditions. In autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), MZB1 contributes to aberrant autoantibody and cytokine secretion. MZB1 also modulates inflammatory responses in conditions such as colitis and periodontitis by regulating the B cell-skewed inflammatory microenvironment. In oncology, MZB1 is overexpressed in breast cancer (BC), lymphoma, and multiple myeloma (MM), where it is associated with enhanced tumor cell proliferation and poor prognosis. Conversely, in hepatocellular carcinoma (HCC) and gastric cancer (GC), MZB1 is transcriptionally silenced due to promoter hypermethylation. In summary, the tissue-specific expression pattern of MZB1 endows it with potential as both a diagnostic biomarker and therapeutic target, holding significant implications for the exploration of future cancer prognosis and treatment strategies.
Keywords: MZB1, endoplasmic reticulum, immune homeostasis, molecular chaperone, tumor microenvironment, targeted therapy
1. Introduction
The endoplasmic reticulum (ER)-resident protein MZB1 is a molecular chaperone encoded by a locus on chromosome 5q31.2. Its crystal structure displays a characteristic saposin-like fold and contains highly conserved cysteine residues as well as a C-terminal KDEL-like tetrapeptide motif that anchors it to the ER lumen. Due to these features, MZB1 is also referred to as CNPY5 or pERp1 [1, 2]. As the most abundantly expressed ER chaperone in marginal zone B cells, B1 cells, plasma cells, and pDCs(plasmacytoid dendritic cells) [3], MZB1 contributes to immune homeostasis through three primary mechanisms: (a) Promoting antibody maturation: as a co-chaperone of BiP/GRP94 (the ER-specific Hsp70/Hsp90 paralogs), it specifically aids in the oxidative folding and secretion of IgM and IgA (J-chain-containing dimer) [3-5]; (b) Maintaining cellular signaling: It regulates B-cell integrin activation, calcium homeostasis, and the high-level secretion of interferon-alpha (IFN-α) by pDCs [6, 7]; (c) Alleviating ER stress: By activating the ATF6 pathway, MZB1 upregulates chaperone protein expression, thereby supporting the survival of hypersecretory cells[6].
Recent studies have revealed a paradoxical dual role of MZB1 in disease. On one hand, MZB1 exerts pro-pathogenic effects: it promotes excessive autoantibody and IFN-α production in SLE [6, 8]; in RA, guanosine modification of MZB1 enhances autoantibody secretion [9]; it is highly expressed in cancers such as breast cancer (BC), lymphomas, and multiple myeloma (MM), and is positively correlated with tumour progression [10-12]. On the other hand, MZB1 also demonstrates protective functions: it helps maintain intestinal homeostasis in colitis through the IgA-J chain-mediated barrier [5]; in hepatocellular carcinoma (HCC) and gastric cancer (GC), it is predominantly silenced via promoter hypermethylation, is associated with tumour suppression, and serves as an effective prognostic indicator [13, 14]. These findings highlight the role of MZB1 as a key node linking endoplasmic reticulum quality control-immune response-disease pathology. However, its tissue-specific regulatory mechanisms (e.g., methylation silencing, post-translational modifications) and targeting strategies still need to be deeply explored. This review aims to systematically integrate the multidimensional roles of MZB1 in immune homeostasis, tumor microenvironment and disease, and provide theoretical basis for the development of novel diagnostic markers and targeted therapies.
2. Structure of MZB1
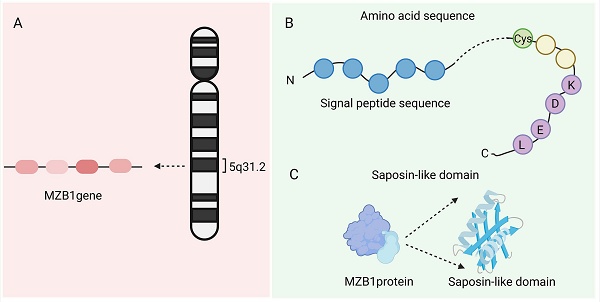
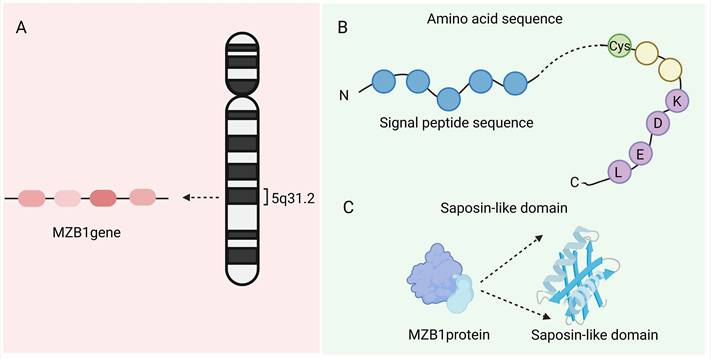
The MZB1 gene is located on chromosome 5q31.2 and contains four exons (NCBI Gene ID: 51237). It encodes an ER-resident protein with a molecular weight of approximately 18-19 kDa, comprising about 189 amino acids [1]. The MZB1 protein (PDB ID:7AAH) includes a signal peptide (which directs the protein into the ER lumen), a highly conserved cysteine residue (which forms disulfide bonds), and a C-terminal “KDEL(Lys-Asp-Glu-Leu)-like” tetrapeptide sequence that facilitates ER retention. MZB1, also known as CNPY5, belongs to the ER-resident CNPY protein family and features a saposin-like domain, a structure commonly involved in lipid or protein binding [1]. Crystal structure analysis confirms that MZB1 adopts a typical saposin fold with unique structural motifs, which collectively enable its function as a molecular chaperone in the ER. Owing to this role, it is also referred to as pERp1 (resident ER protein) [2]. Under normal physiological conditions, MZB1 is primarily expressed in B-cell lineages, particularly in marginal zone B cells, B1 cells, and plasma cells. It is one of the most abundantly expressed ER proteins in these cell types, highlighting its specialized role in supporting their secretory functions [3] (Figure 1).
Structure of MZB1. A. The location of the MZB1 gene. B. The signal peptide, highly conserved cysteine residues, and C-terminal 'KDEL-like' tetrapeptide sequence of MZB1 protein. C. Saposin-like domain of MZB1 protein.
Role of MZB1 in the immune system. A. The role of MZB1 in B cell development and function. B. The role of MZB1 in plasma cell differentiation and antibody secretion. C. The effect of MZB1 on the secretory function of plasmacytoid dendritic cells. D. The effect of MZB1 on the endoplasmic reticulum stress response.
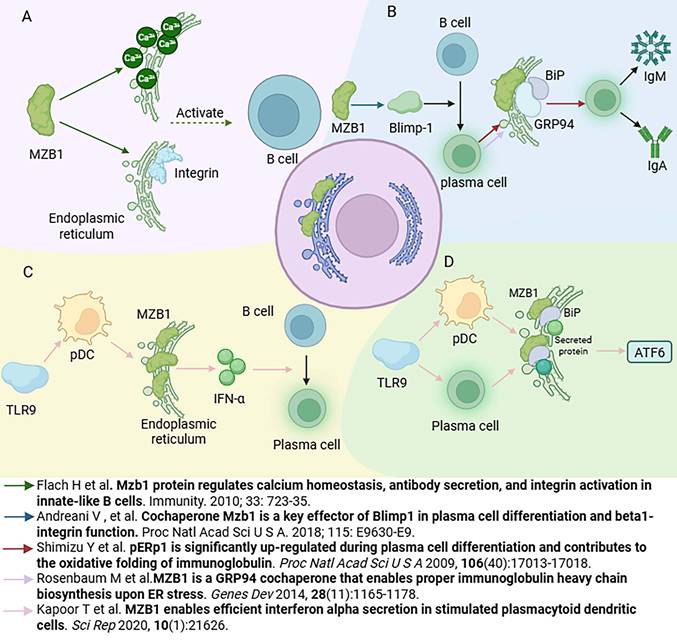
3. Role of MZB1 in the immune system
MZB1 plays a critical and multifaceted role in the immune system, functioning as a folding cofactor and quality control mediator for antibodies and integrins within the endoplasmic reticulum. Through its specialized structural domains and chaperone activity, MZB1 supports the proper assembly of immune molecules, maintains calcium homeostasis, and alleviates ER stress to preserve cellular balance [4, 6, 7, 15] (Figure 2).
3.1. B cell development and function
MZB1 is highly expressed in innate-like B cell subsets, particularly marginal zone B cells and B1 cells, and plays a crucial role in their development and function [3]. Marginal zone B cells and B1 cells are cell populations that mount a rapid antibody response to pathogens. Marc Rosenbaum et al. showed that MZB1 deficiency impairs the marginal zone B cell-mediated humoral immune response, in particular on early IgM antibody production to B cell-independent antigens [4]. MZB1 regulates Ca2+ homeostasis and endoplasmic reticulum Ca2+ reserves. In addition, MZB1 may influence integrin signalling by modulating the redox enzyme ERp57. Integrin primarily is involved in the adhesion process of marginal zone B cells to integrin ligands VCAM-1 or ICAM-1 upon chemokine induction.[16]. In MZB1-deficient mice, B1 cells exhibit reduced B cell receptor (BCR)-induced calcium mobilization and impaired β1 integrin-dependent adhesion capacity [7]. Thus, MZB1 contributes to the regulation of calcium signaling and adhesion molecule function in B cells, thereby facilitating B cell activation, adhesion, and migration.
3.2. Plasma cell differentiation and antibody secretion
The transcription factor Blimp-1 is a master regulator of plasma cell differentiation, and MZB1 acts as a key downstream effector in the Blimp-1 signaling pathway during the transition from B cells to plasma cells [15]. MZB1 is essential for the efficient production of antibodies by plasma cells [9]. Its role in IgM secretion has been confirmed in primary B cells: MZB1 downregulation impairs IgM secretion, while its overexpression enhances IgM production in marginal zone B (MZB) cells and follicular B (FoB) cells upon lipopolysaccharide (LPS) stimulation [7]. The proper folding and secretion of antibodies in the endoplasmic reticulum rely on the ER-resident chaperones BiP and GRP94, which ensure protein quality control and homeostasis [17]. MZB1 is a chaperone protein of GRP94/BiP.Yuichiro Shimizu's group demonstrated that MZB1 interacts with the BiP complex to facilitate oxidative folding of immunoglobulin domains [3]. Moreover, MZB1 forms a complex with GRP94 and acts as its co-chaperone, enhancing the association between GRP94 and the immunoglobulin μ heavy chain, thereby improving the efficiency of IgM antibody assembly and secretion [4, 18]. Similarly, MZB1 promotes the efficient folding and secretion of IgA antibodies, but has minimal impact on IgG production [5, 19]. In addition, MZB1 deficiency does not significantly affect the early stages of plasma cell differentiation, as expression of transcriptional regulators such as Blimp-1 and XBP1 remains unchanged. However, it compromises the secretory efficiency of plasma cells in the later differentiation stages [4]. Thus, MZB1 primarily functions during the effector phase of plasma cells by enhancing the folding and secretion of IgM and IgA antibodies, thereby expanding their secretory capacity.
3.3. Role of pDCs
In addition to B cells, MZB1 mRNA is also expressed in pDCs [20]. Although pDCs exhibit a lymphoid morphology at rest, they acquire dendritic features upon activation, hence the name “plasmacytoid dendritic cells” [21]. Tanya Kapoor et al. reported that MZB1 expression levels in murine pDCs are comparable to those in marginal zone B cells and higher than in follicular B cells [6]. Functionally, MZB1-deficient pDC were significantly impaired in their ability to secrete IFN-α in response to TLR9 stimulation, but had little effect on the small amount of IFN-α produced by low levels of stimulation (e.g., weak TLR7 stimulation), suggesting that MZB1 functions primarily under conditions of high-intensity secretion [6]. When large amounts of IFN-α are synthesized, MZB1 facilitates its proper folding and transport; in the absence of MZB1, IFN-α accumulates in the endoplasmic reticulum and fails to be efficiently secreted. In addition, pDC can promote the differentiation of B cells into plasma cells by secreting IFN-α [6]. Co-culture experiments showed that wild-type pDC significantly promoted B cell differentiation to CD138+ plasma cells and IgM production, whereas this function was significantly impaired in MZB1-deficient pDC. Supplementation with exogenous IFN-α or blockade of the IFN receptor restored or impaired the differentiation effect, respectively, suggesting that MZB1 deficiency leads to insufficient secretion of IFN-α by pDC to efficiently assist B cell differentiation [6]. In conclusion, MZB1 can stimulate pDC to secrete IFN-α. Moreover,given that pDCs are a major source of IFN-α in autoimmune diseases such as SLE [22, 23], MZB1 may serve as an important molecular link between innate and adaptive immunity.
3.4. Endoplasmic reticulum stress response
Plasma cells and pDC, which have the highest secretory load among hematopoietic cells, require robust ER protein folding, which involves induction of ER stress, increased expression of protein folding chaperones, and expansion of the ER [24, 25]. The accumulation of unfolded proteins in the ER triggers ER stress, which is alleviated through activation of the unfolded protein response (UPR), thereby restoring protein homeostasis. The UPR consists of three canonical signaling pathways: inositol-requiring enzyme 1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6) [26, 27]. MZB1 functions as a molecular "lubricant" within the UPR machinery, supporting highly secretory cells in managing their protein folding demands. However, the relationship between MZB1 and IRE1 / PERK is not clear. In particular, MZB1 activates the ATF6 pathway: under TLR9-mediated stimulation in plasma cells and pDCs, MZB1 enhances BiP-mediated binding of secretory proteins, promoting early activation of ATF6. This leads to upregulation of chaperone expression (e.g., BiP and GRP94) and facilitates ER expansion, thus helping the cell adapt to high secretory demands [6]. These findings suggest that MZB1, by indirectly modulating the UPR, enables secretory cells to resolve ER stress and maintain homeostasis under strong stimulation. In summary, MZB1 serves as a critical molecular link between the immunosecretory function and the ER stress response in highly secretory cells such as B cells and pDCs. On one hand, it ensures the efficient folding and secretion of large quantities of antibodies and cytokines; on the other, it activates protective UPR mechanisms to prevent apoptosis during peak secretory activity.
The effects of MZB1 on SLE and RA.
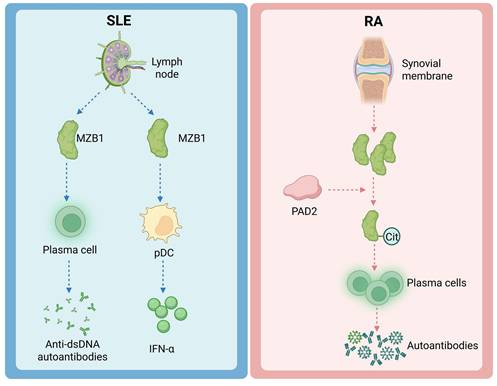
4. The relationship between MZB1 and immune-related diseases and its research progress
MZB1 is also important in a variety of autoimmune and immune-related diseases. Such diseases are often accompanied by abnormal humoral immune responses involving dysregulation of plasma cells and antibody production. SLE and RA are discussed below as representatives (Figure 3).
4.1. Systemic lupus erythematosus
SLE patients are characterized by B-cell hyperfunction and overproduction of cytokines IFN-α, TNF and IL-1 [28]. A study conducted proteomic analysis of lymph node biopsies from SLE patients identified MZB1 as one of the proteins differentially upregulated in lymphoid tissues of SLE patients [8]. Further immunohistochemical validation showed that MZB1 was abundantly located in the interfollicular zone and scattered in the germinal centers in lymph nodes of SLE patients, in association with CD138⁺ plasma cells and IRTA1⁺ marginal zone B cells [8]. Correspondingly, a similar phenomenon was observed in a murine model of spontaneous lupus, with elevated levels of MZB1 in splenic marginal zone B cells and plasma cells in aged rats [8]. Functional experiments suggest that MZB1 is closely related to the pathogenesis of lupus: treatment of lupus mice with endoplasmic reticulum stress inducers selectively induced apoptosis in plasma cells with high expression of MZB1, which resulted in a significant decrease in serum anti-dsDNA autoantibodies of the mice, which suggests that high titers of autoantibodies in the condition of lupus depend partly on plasma cells with high expression of MZB1 [8]. In addition, as mentioned previously, pDC are the main source of IFN-α in lupus patients [29], and high MZB1 expression can fuel pDC to secrete large amounts of IFN-α [6]. Collectively, these findings indicate that MZB1 is upregulated in two key pathogenic pathways in SLE—B cells and pDCs: on one hand, it supports the production of pathogenic autoantibodies by plasma cells, and on the other, it amplifies IFN-α-mediated immune responses via pDCs [6, 8]. Therefore, MZB1 has been proposed as a potential therapeutic target in SLE, with the aim of selectively depleting antibody-overproducing plasma cells [8]. Although this strategy remains in the early conceptual stage, it highlights the central role of MZB1 in SLE pathogenesis and its therapeutic relevance.
4.2. Rheumatoid arthritis
RA is a chronic inflammatory disease characterized by the presence of autoantibodies, such as rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies [30]. Plasma cells that produce large quantities of autoantibodies are commonly found in RA patients, and these cells can form ectopic lymphoid structures within synovial tissues. Notably, MZB1 is highly expressed in plasma cells within RA synovium [8]. In addition, an interesting mechanism comes from studies of arthritis-associated lung disease: RA patients often suffer from interstitial inflammation of the lungs, where high expression of citrullination enzyme 2 (PAD2) in the lungs is able to citrullinate the MZB1 protein [9]. Citrullination, a post-translational modification that converts arginine residues into citrulline, is strongly associated with the generation of anti-citrullinated protein/peptide antibodies (ACPAs) in RA pathogenesis [31]. Lee et al. identified MZB1 as one of the citrullinated proteins enriched in lung tissues of RA patients, and demonstrated that PAD2-mediated citrullination of MZB1 enhances the ability of plasma cells to secrete IgM and IgA antibodies [31]. This partly explains why RA patients produce excessive amounts of IgM-RF and IgA-like autoantibodies [32]. Beyond plasma cells, MZB1 has recently been reported to be highly upregulated in T cells and monocytes within leukocyte-rich RA synovial tissues [33]. MZB1 is also being explored as a potential biomarker to distinguish RA from spondyloarthritis (SpA) [34]. Additionally, recent studies suggest that MZB1 plays a role in B-cell maturation and antibody generation, and is associated with the development of anti-drug antibodies (ADAs), which may impact therapeutic efficacy in RA patients [35]. Thus, it is clear that MZB1 is not only increased in expression in RA, but also becomes part of the immune aberrant loop specific to RA. In the future, it is expected that MZB1 will be used as a node to develop novel therapies for RA, such as utilizing the role of MZB1 in regulating autoantibody production to alleviate the disease.
5. Relationship between MZB1 and inflammatory diseases and its research progress
As an important mediator involved in the correct folding of antibodies in the ER, MZB1 is often upregulated in humoral immunity. However, its role may vary in different inflammatory conditions, which might be due to the fact that it mainly depends on the different internal environment (Table 1).
5.1. Periodontitis
Periodontitis is a common chronic inflammatory disease that affects the supporting structures of the teeth, leading to progressive destruction of periodontal tissues and alveolar bone, which can ultimately result in tooth loss [36]. In the context of periodontitis, MZB1 may act as part of the local humoral immune response, regulating the synthesis of immune proteins and the differentiation of immune cells [37]. A microarray-based gene expression profiling study of aggressive periodontitis revealed that MZB1 is significantly upregulated in gingival tissues of affected patients [38]. Similarly, Esra Guzeldemir-Akcakanat et al. identified MZB1 as a signature molecule of chronic periodontitis through genome-wide transcriptomic and proteomic analyses [39]. These findings were further supported by transcriptome sequencing and histological studies, which consistently demonstrated elevated MZB1 expression in inflamed gingival tissues, providing new insights into the genetic drivers of periodontal inflammation [40, 41]. A recent study also reported changes in the composition of immune cells between healthy and diseased gingival tissues, including differences in B cells, T cells, dendritic cells, and neutrophils, with MZB1 expression highly correlated with B cell infiltration [42]. Furthermore, MZB1 expression was found to increase during T cell differentiation [43], suggesting broader immune regulatory roles. Mechanistically, MZB1 has been identified as a direct target of miR-185-5p, and it was shown to inhibit human periodontal ligament cell migration via the NF-κB signaling pathway. This may represent a key regulatory mechanism by which MZB1 modulates periodontal inflammation and tissue remodeling [44]. Although the current understanding of MZB1's molecular mechanisms in periodontitis remains limited, it presents a promising avenue for future research and may emerge as a novel target in periodontitis-related diagnostics or therapeutics.
The relationship between MZB1 and inflammatory diseases and its research progress
| Disease type | Changes in MZB1 expression | Key Functions/Mechanisms | Species | Research methodology |
|---|---|---|---|---|
| Periodontitis | Significantly upregulated in gingival tissues | Drives chronic inflammation [39] and affects immune protein synthesis [37] and B cell infiltration [42]. Inhibits periodontal ligament cell migration through the NF-κB pathway [44] | Human, mouse | RT-qPCR, Western blotting, Microarray, Transcriptomics, Histologic analysis. |
| Colorectitis | Overexpressed in ulcerative colitis | Promotes IgA secretion from plasma cells and maintains intestinal mucosal immunity MZB1 deficiency results in decreased IgA and exacerbates DSS-induced colitis [5] | Mouse | Flow Cytometry, Western blotting, Mouse model, RNA sequencing |
| Chronic endometritis | Upregulated in the endometrium | Involved in disease progression as an inflammation-associated gene, exact mechanism not defined [49]. | Human | Immunohistochemistry, RNA sequencing, RT-qPCR, |
| Type 2 chronic rhinosinusitis with nasal polyps | Significantly upregulated in B cells | Upregulated ER stress markers and IgE expression [50]. | Human | Western blotting, RNA sequencing, Immunohistochemistry |
| Acute pancreatitis | Elevated in pancreatic tissues and cells | Promotes pancreatic cell proliferation and inhibits apoptosis and mitochondrial dysfunction through the PI3K-AKT pathway [51]. | Mouse | RT-qPCR, Western blotting, Mouse model, In vitro experiments |
5.2. Colorectal inflammation
Chronic inflammation is known to be associated with the development of many types of cancer, and intestinal inflammation may induce oncogenic mutations and promote colorectal cancer [45]. IgA is the most abundant antibody in mucosal secretions, and plays an essential role in intestinal homeostasis regulation and mucosal immunity [46]. Dimeric IgA molecules are covalently linked via a joining J chain, which is essential for their translocation across epithelial barriers to mucosal surfaces [47]. Ermeng Xiong et al. found that MZB1-deficient mice have an increased susceptibility to dextran sulfate sodium (DSS)-induced colitis, and that IgA and IgM levels were elevated in the intestines of MZB1-overexpressing mice after DSS administration. In contrast, IgA levels did not increase in MZB1-deficient mice, while IgM levels increased only slightly. Exogenous addition of IgA restored the normal sensitivity of MZB1-deficient mice to DSS-induced colitis [5]. Mechanistically, MZB1 deficiency impairs IgA secretion by plasma cells and disrupts J chain—IgA association, thereby compromising the efficient transport of IgA to the intestinal mucosa. These findings provide reverse validation that MZB1 promotes IgA secretion and plays a protective role in suppressing the onset and progression of colitis [5]. In addition, a research team found that MZB1 was significantly overexpressed in ulcerative colitis by whole blood RNA sequencing. However, there is currently little relevant research available, and the specific role of MZB1 in the development of colitis requires further investigation [48].
5.3. Other inflammatory diseases
In addition to its roles in periodontitis and colitis, MZB1 is also expressed and functionally relevant in a range of other inflammatory conditions. For example, Kyoko Oshina et al. reported via RNA sequencing that MZB1 is among the upregulated genes in the endometrium of patients with chronic endometritis [49]. In type 2 chronic rhinosinusitis with nasal polyps (CRSwNP), the ER stress level of B cells was significantly elevated, and MZB1 showed co-localization with plasma cells and mature B cells and was significantly upregulated. Further experiments revealed that MZB1 stimulated upregulation of ER stress markers and IgE mRNA expression [50]. In addition, a recent study on MZB1 and acute pancreatitis (AP) used bioinformatic analysis and in vitro cell experiments to demonstrate that MZB1 expression levels are significantly elevated in AP cells and tissues. Furthermore, MZB1 overexpression can promote pancreatic cell proliferation and inhibit cell apoptosis, mitochondrial dysfunction, and inflammation by regulating the PI3K-AKT signaling pathway, thereby playing a key role in AP [51]. The possible reason for the up-regulation of MZB1 in inflammatory diseases is that MZB1 is expressed in B-cells and correlates with the secretion of immunoglobulins IgM and IgA, which also provides important clues for the study of MZB1 and inflammatory diseases.
6. MZB1 expression and role in tumors
MZB1 is abnormally expressed in a variety of tumors, which is correlated with the differentiation status of tumor cells, the immune microenvironment and disease prognosis. Tissue-specific secretory demands and UPR dependency may select for high expression of MZB1 (e.g., plasma-cell tumors, ER+ BC), whereas tumors with methylation drift may silence MZB1, losing ER-stress checkpoints. The following summarizes the research progress of MZB1 in several types of typical cancers (Figure 4 and Table 2).
Expression and function of MZB1 in tumours. A. The carcinogenic effects of MZB1. B. The anti-cancer effects of MZB1.
The expression of MZB1 in cancer and its prognosis
| Type of cancer | MZB1 expression | Correlation study | Species sample types and quantities, database | Prognosis | Reference documentation |
|---|---|---|---|---|---|
| Breast cancer (BC) | Up-regulation | RT-qPCR, Immunohistochemistry, Single-cell RNA-sequencing | 13 BC cell lines and two non-cancerous mammary cell lines, BC patients (n=114) and non-cancerous specimens, and the cancer genome atlas (TCGA) for breast cancer | Related to poor prognosis of breast cancer patients | [10,52,53] |
| Lymphoma | Up-regulation | RT-qPCR, Microarray data | 139 CLL patients | MZB1 is a prognostic marker for untreated and relapsed CLL, FL, and DLBCL | [11] |
| Multiple myeloma (MM) | Up-regulation | RT-qPCR, Western blotting, Proteomics analysis | 32 patients with multiple myeloma and 32 patients with non-hematologic malignancies | MZB1 can accelerate the cycle progression of MM plasma cells and lead to malignant development to some extent. | [12] |
| Hepatocellular carcinoma (HCC) | Low expression | RT-qPCR, Immunohistochemistry, Western blotting, Whole genome methylation screening, Methylation analysis | 162 cases of primary HCC, 15 HCC cell lines and 2 hepatoblastoma cell lines | MZB1 protein silencing is significantly and independently associated with poor prognosis. | [13] |
| Gastric cancer (GC) | Low expression | RT-qPCR, Immunohistochemistry, Transcriptome analysis, PCR array analysis, Methylation analysis | Primary gastric cancer and adjacent non-cancerous tissues of 200 patients, 11 cell types and 94 cases of patients with stage II/III gastric cancer who received radical surgery | The decreased expression of MZB1 is an independent predictor of recurrence in patients with stage II / III GC | [14] |
| Clear cell renal cell carcinoma(ccRCC) | Up-regulation | Western blotting, Immunohistochemistry, Single-cell sequencing data | 59 pairs of human ccRCC and adjacent normal tissues | MZB1 is associated with poor prognosis in ccRCC patients | [60] |
| Mucinous tubular cell carcinoma (MTSCC) | Up-regulation | Proteomics spectrum analysis, Immunohistochemistry, Immunofluorescence | 32 cases of MTSCC and 36 cases of type 1 papillary renal cell carcinoma (PRCC) | The expression of MZB1 in MTSCC is negatively correlated with the clinical stage of the tumor | [61] |
| Non-small cell lung cancer (NSCLC) | Up-regulation | Western blotting, RT-qPCR | 12 tumor samples from NSCLC patients and their corresponding adjacent tissues | MZB1 is a potential prognostic biomarker for NSCLC | [64] |
| Pancreatic cancer | Up-regulation | Proteomics analysis, Bioinformatics analysis | 72 patients with resectable PDAC, 3 pairs of high CD8 TIL and low CD8 TIL, and the TCGA data base | High expression of MZB1 correlates with disease-free and overall survival | [65,66] |
| Ovarian cancer | Low expression | Bioinformatics analysis | TCGA data base | High expression of MZB1 is positively correlated with improved clinical prognosis | [67] |
| Colorectal cancer | Low expression | Western blotting, RT-qPCR | 10 pairs of rectal adenocarcinoma and adjacent tissue samples | MZB1 inhibits proliferation, migration and invasion of rectal adenocarcinoma cells and promotes apoptosis of rectal adenocarcinoma cells | [68,69] |
6.1. Breast cancer
Recent studies have found that MZB1 is highly expressed in BC by bioinformatics analysis [52] and is associated with BC prognosis [53]. Similarly, a study using eXplainable Artificial Intelligence (XAI) found that MZB1 could be used as an early, non-invasive diagnostic and prognostic biomarker for BC [54]. Manabu Watanabe's team found that MZB1 expression was strongly correlated with hormone receptor status in BC, and they examined multiple mammary cell lines, which were detected mRNA expression of MZB1 only for estrogen receptor (ER⁺) breast cancer cells, whereas ER-negative cell lines and normal mammary epithelial cells barely expressed MZB1 [10]. Among ER⁺ BC patients, high MZB1 expression was associated with worse clinical outcomes: disease-free survival (DFS) was significantly shorter in MZB1-positive patients, and multivariate analyses identified MZB1 positivity as an independent prognostic factor for poor outcome [10]. The specific mechanism may involve heightened activity within the endoplasmic reticulum and protein synthesis pathways of ER⁺ breast cancer cells. Upregulation of MZB1 may confer enhanced protein folding and secretion capabilities to these cells, thereby promoting tumour cell survival and invasion [10]. However, this potential mechanism requires extensive validation through both in vivo and in vitro studies. In summary, MZB1 expression correlates with breast cancer progression, particularly within the hormone receptor-positive subtype.
6.2. Lymphoma
MZB1 is frequently overexpressed in B cell derived malignancies, including chronic lymphocytic leukemia (CLL), follicular lymphoma (FL), and diffuse large B-cell lymphoma (DLBCL), and its expression positively correlates with disease aggressiveness [11]. Herold et al. analyzed the gene expression profiles of peripheral blood tumor cells in patients with CLL, and initially found that high expression of MZB1 was associated with poor prognosis. This was subsequently validated in several independent cohorts, and in 139 CLL patients, both overall and progression-free survival were significantly shorter in the MZB1 mRNA high-expression group [11]. Similarly, publicly available datasets for FL and DLBCL revealed that elevated MZB1 expression was associated with worse clinical outcomes. Notably, in DLBCL, the prognostic significance of MZB1 remained consistent across different molecular subtypes, including both the activated B cell like (ABC) and germinal center B cell like (GCB) subtypes, indicating that MZB1 functions as an additional adverse prognostic factor regardless of molecular classification [11]. Given the critical role of MZB1 in B cell function, its overexpression may enhance the antibody-secreting capacity of tumor B cells, thereby promoting tumor cell survival [11]. Although MZB1 exhibits high expression levels across multiple study cohorts, this remains dependent on subtype and plasma cell characteristics.
6.3. Multiple myeloma
MM is a malignant proliferative disease of plasma cells, and MZB1 is highly expressed in MM and plays a tumor-promoting role [55]. Chanukuppa et al. conducted proteomic analyses and found that MZB1 protein levels were significantly elevated in bone marrow mononuclear cells from MM patients compared to healthy controls [12]. In the multiple myeloma cell line RPMI-8226, MZB1 knockdown via shRNA led to marked phenotypic changes: proliferation was slowed, cell cycle arrest was induced, and apoptosis was significantly increased. Soft agar colony formation assays further showed that MZB1-deficient myeloma cells had reduced anchorage-independent growth, indicating impaired tumorigenic potential [12]. Mechanistically, MZB1 knockdown resulted in impaired multiple proliferation-related signals: the phosphorylation levels of the key pro-survival pathways AKT and ERK were significantly reduced, and the expression of cyclins Cyclin D1, Cyclin A, and Cyclin B, which are required for the cell cycle, was down-regulated [12]. Additionally, the study suggested that MZB1 may interact with the immunoglobulin J chain to cooperatively promote the malignant phenotype of myeloma cells [12]. Emerging evidence also indicates that MZB1 is an aberrantly spliced gene in MM, and disruption of normal RNA splicing patterns is increasingly recognized as a driver of tumorigenesis [56]. Although current data remain limited, these findings collectively suggest that MZB1 functions as an oncogene in multiple myeloma by sustaining tumor cell proliferation and survival.
6.4. Hepatocellular carcinoma
In contrast to the cancer types discussed above, MZB1 is expressed at low levels in HCC. Matsumura et al. identified frequent MZB1 silencing in HCC by integrating DNA methylation microarray and gene expression profiling data, and found that hypermethylation of the MZB1 promoter region was a major mechanism contributing to its transcriptional suppression [13]. In a cohort of 162 patients with primary HCC, immunohistochemical analysis revealed that MZB1 protein expression was significantly reduced in tumor tissues, and Kaplan-Meier survival analysis showed that loss of MZB1 expression was associated with poorer prognosis [13]. Furthermore, in hepatocellular carcinoma cell lines with low MZB1 expression, extracellular transfection of MZB1 inhibited tumor cell proliferation and induced G1-phase cell cycle arrest [13]. Animal experiments have also shown that restoring MZB1 expression can slow tumor growth [13]. It is therefore inferred that MZB1 is associated with HCC, and its loss promotes the proliferation of hepatocellular carcinoma cells, thereby facilitating carcinogenesis. Given that MZB1 silencing is primarily driven by promoter CpG island hypermethylation, it may serve as a methylation-based biomarker or therapeutic target, particularly in the context of epigenetic therapies such as demethylation agents.
6.5. Gastric cancer
In GC, early microarray analysis found that MZB1 (formerly known as MGC29506) was downregulated in intestinal-type GC tissues, suggesting an association between MZB1 and GC development [57]. Lin Xia et al. found that the expression of MZB1 in GC tissues was lower than that in samples from adjacent non-tumor tissues by immunohistochemistry [58]. In addition, functional assays showed that GC cells induced by MZB1 gene transfection were arrested in the G0/G1 and S phases of the cell cycle and MZB1 inhibited the proliferation of GC cells [58]. Similarly, Mitsuro Kanda et al. showed by transcriptome analysis that the expression level of MZB1 was significantly reduced in primary GC tissues compared to the corresponding normal gastric mucosa, and that MZB1 knockdown increased the proliferation, invasion, and migration of the GC cells, further suggesting that MZB1 may act as a novel suppressor gene in GC[14].
6.6. Other solid tumors
In addition to the cancer types discussed above, several studies have provided preliminary evidence for the role of MZB1 in other solid tumors. In cutaneous squamous cell carcinoma (cSCC), MZB1 may co-express with the lncRNA-mRNA network and may be associated with tumour progression [59]. MZB1 expression is upregulated in clear cell renal cell carcinoma (ccRCC) and is associated with poor prognosis in ccRCC patients [60]. Conversely, Huiya Xu et al. determined through proteomic analysis that MZB1 exhibits a negative correlation with the clinical staging of mucoid tubular and spindle cell carcinoma (MTSCC) [61]. In addition, MZB1 may be an important hub gene in lung non-small cell lung cancer (NSCLC) tissues [62]. Moreover, there is a positive correlation between MZB1 and PD-L1 in lung squamous cell carcinoma [63]. Xiaohong Zhu et al. demonstrated that overexpression of MZB1 could counteract the inhibitory effects of miR-103-3p on the migration and metastasis of NSCLC cell lines [64]. Finally, several studies based on bioinformatics analyses and functional experiments have reported that high MZB1 expression is positively associated with prognosis in cancers such as pancreatic cancer [65, 66], ovarian cancer [67], and colorectal cancer [68, 69]. However, the specific molecular mechanisms behind these correlations have not been fully elucidated through experiments. Further validation can be achieved through knockdown and backfilling experiments using animal and cell models.
7. Discussion
In summary, the ER protein encoded by the MZB1 gene has important functions in the immune system at multiple levels. From a basic research perspective, MZB1 is a chaperone protein essential for antibody-secreting cells (plasma cells and some innate immune cells) to adapt to high secretory loads, regulating the assembly and folding of antibodies and cytokines as well as the ER stress response [6]. Through interactions with ER chaperones such as GRP94, MZB1 promotes the maturation of immunoglobulins IgM and IgA, as well as specific receptor proteins such as Toll-like receptors (TLRs) and integrins. In addition, it contributes to the maintenance of intracellular calcium homeostasis and integrin-mediated adhesion signaling [2, 4, 7]. In immune organs, MZB1 is highly expressed in marginal zone B cells, B1 cells, and plasma cells, contributing to rapid natural antibody response and high-affinity antibody production [70]. In pDC, MZB1 similarly safeguards the high secretion of IFN-α [6]. Together, these findings highlight MZB1 as a key “quality control” regulator of the humoral immune response, whose functional significance has only recently begun to receive widespread attention.
Clinical studies have shown that MZB1 is aberrantly expressed in a variety of disease states and becomes a “player” in disease development. MZB1's impact depends on whether the tissue context contains antibody-secreting programs (plasma cells/pDC secretory stress), epithelial tumor epigenetic state, and whether disease is driven by pathogenic humoral output (autoantibodies, IFN-I) and barrier-protective IgA. In autoimmune diseases such as SLE and RA, MZB1 is upregulated throughout the pathogenesis: in SLE, MZB1 promotes a dual excess of autoantibodies and inflammatory factors [6, 8], while in RA, post-translational modification of MZB1 exacerbates abnormal plasma cell activation [9]. These findings suggest that MZB1 may be an enabler of autoimmune responses, and inhibition of MZB1 expression is expected to alleviate the pathologic process of related diseases [8]. In inflammatory diseases, MZB1 plays multiple roles. For instance, MZB1 drives chronic inflammation in periodontitis, affecting the synthesis of immune proteins and the maturation of immune cells. It may play the role of a pro-inflammatory cytokine in hormone regulation, but this inference requires extensive experimental verification [37]. In colorectitis, high MZB1 expression promotes IgA secretion from plasma cells and enhances J chain-IgA association, which decreases susceptibility to colitis [5]. In cancer, MZB1 serves as a poor prognostic factor. Its elevated expression in certain solid tumours (such as breast cancer) correlates with tumour progression and adverse outcomes [10]. On the other hand, in certain tumours (such as HCC), it functions as a tumour suppressor gene, silenced by methylation, and its deletion is associated with tumour progression [13]. In B cell derived hematological malignancies, MZB1 is universally upregulated and associated with an aggressive disease phenotype, suggesting a dependency of malignant B cells on MZB1 [11]. Particularly in plasma cell tumors (myeloma), MZB1 has been shown to be a key protein in maintaining tumor proliferation and could be a potential therapeutic target for future MM [12]. These studies provide powerful clues for future studies exploring molecular biomarkers related to tumor prognosis and targeted therapy.
Future research on MZB1 will be divided into several directions:(1) Mechanistic level: to deeply analyze the molecular mechanism of MZB1 in regulating protein folding, for example, how its saposin domain interacts with immunoglobulin heavy chain, BiP/GRP94, and how modifications such as citrullination affect its function; (2) Disease models: construct mouse models with high expression or deficiency of MZB1, and observe phenotypic changes in autoimmune diseases, inflammatory diseases, and tumor models to clarify the causal role of MZB1 in pathological processes; (3) Clinical application: develop interventions targeting the MZB1 pathway. For example, small molecules or antibodies are used to block the action of MZB1 and its chaperone proteins in order to selectively inhibit disease-causing plasma cells in autoimmunity; and in tumors, treatment strategies such as activating the silenced MZB1 gene (e.g., with demethylating drugs) or targeting and eliminating MZB1-high tumor cells (e.g., with MZB1-specific T cell immunotherapy) are being explored. With the expansion of the functional spectrum of MZB1, this molecule has the potential to move from basic research to clinical translation, becoming a new focal point connecting immunology and oncology. In conclusion, research on the structure and function of the MZB1 gene provides important clues for understanding the coupling between endoplasmic reticulum homeostasis and immune function, and its roles in diseases warrant further exploration and utilization.
Abbreviations
MZB1: Marginal zone B and B1 cell-specific protein 1; ER: Endoplasmic reticulum; SLE: Systemic lupus erythematosus; RA: Rheumatoid arthritis; IFN-α: Interferon-alpha; TLR: Toll-like receptor; GRP94: Glucose-regulated protein 94; BiP: Binding immunoglobulin protein; pDC: Plasmacytoid dendritic cell; IgA: Immunoglobulin A; IgM: Immunoglobulin M; MM: Multiple myeloma; HCC: Hepatocellular carcinoma; GC: Gastric cancer; BC: Breast cancer; DLBCL: Diffuse large B-cell lymphoma; FL: Follicular lymphoma; CLL: Chronic lymphocytic leukemia; NSCLC: Non-small cell lung cancer; UPR: Unfolded protein response.
Acknowledgements
Funding
This work was supported by the National Natural Science Foundation of China (No. 81960542 and 82360614), the Joint Project of Yunnan Provincial Department of Science and Technology and Kunming Medical University (No. 202401AY070001-268), Yunnan Provincial Health Commission's Reserve Talent Development Program for Medical Disciplines, the Scientific Research Fund of Education Department of Yunnan Province (No. 2024J0330, 2025J0247).
Authorship contributions
Yu Zhang: Writing - review & editing, Writing - original draft, Software, Resources, Investigation, Conceptualization. Wei Yang: Writing - original draft, Software, Conceptualization. Xiaofang Yang: Writing - original draft, Software, Conceptualization. Yuqing Pan: Software, Data curation. Rong Guo: Writing - original draft, Data curation. Dong Chen: Investigation, Data curation. Xi Zhang: Writing - review & editing, Conceptualization, Investigation, Writing - review & editing, Funding acquisition. Shicong Tang: Visualization, Supervision, Project administration, Funding acquisition.
Competing interests
The authors have declared that no competing interest exists.
References
1. Schildknegt D, Lodder N, Pandey A, Chatsisvili A, Egmond M, Pena F. et al. Characterization of CNPY5 and its family members. Protein Sci. 2019;28:1276-89
2. Sowa ST, Moilanen A, Biterova E, Saaranen MJ, Lehtio L, Ruddock LW. High-resolution Crystal Structure of Human pERp1, A Saposin-like Protein Involved in IgA, IgM and Integrin Maturation in the Endoplasmic Reticulum. J Mol Biol. 2021;433:166826
3. Shimizu Y, Meunier L, Hendershot LM. pERp1 is significantly up-regulated during plasma cell differentiation and contributes to the oxidative folding of immunoglobulin. Proc Natl Acad Sci U S A. 2009;106:17013-8
4. Rosenbaum M, Andreani V, Kapoor T, Herp S, Flach H, Duchniewicz M. et al. MZB1 is a GRP94 cochaperone that enables proper immunoglobulin heavy chain biosynthesis upon ER stress. Genes Dev. 2014;28:1165-78
5. Xiong E, Li Y, Min Q, Cui C, Liu J, Hong R. et al. MZB1 promotes the secretion of J-chain-containing dimeric IgA and is critical for the suppression of gut inflammation. Proc Natl Acad Sci U S A. 2019;116:13480-9
6. Kapoor T, Corrado M, Pearce EL, Pearce EJ, Grosschedl R. MZB1 enables efficient interferon alpha secretion in stimulated plasmacytoid dendritic cells. Sci Rep. 2020;10:21626
7. Flach H, Rosenbaum M, Duchniewicz M, Kim S, Zhang SL, Cahalan MD. et al. Mzb1 protein regulates calcium homeostasis, antibody secretion, and integrin activation in innate-like B cells. Immunity. 2010;33:723-35
8. Miyagawa-Hayashino A, Yoshifuji H, Kitagori K, Ito S, Oku T, Hirayama Y. et al. Increase of MZB1 in B cells in systemic lupus erythematosus: proteomic analysis of biopsied lymph nodes. Arthritis Res Ther. 2018;20:13
9. Geary B, Sun B, Tilvawala RR, Barasa L, Tsoyi K, Rosas IO. et al. Peptidylarginine deiminase 2 citrullinates MZB1 and promotes the secretion of IgM and IgA. Front Immunol. 2023;14:1290585
10. Watanabe M, Shibata M, Inaishi T, Ichikawa T, Soeda I, Miyajima N. et al. MZB1 expression indicates poor prognosis in estrogen receptor-positive breast cancer. Oncol Lett. 2020;20:198
11. Herold T, Mulaw MA, Jurinovic V, Seiler T, Metzeler KH, Dufour A. et al. High expression of MZB1 predicts adverse prognosis in chronic lymphocytic leukemia, follicular lymphoma and diffuse large B-cell lymphoma and is associated with a unique gene expression signature. Leuk Lymphoma. 2013;54:1652-7
12. Chanukuppa V, Paul D, Taunk K, Chatterjee T, Sharma S, Shirolkar A. et al. Proteomics and functional study reveal marginal zone B and B1 cell specific protein as a candidate marker of multiple myeloma. Int J Oncol. 2020;57:325-37
13. Matsumura S, Imoto I, Kozaki K, Matsui T, Muramatsu T, Furuta M. et al. Integrative array-based approach identifies MZB1 as a frequently methylated putative tumor suppressor in hepatocellular carcinoma. Clin Cancer Res. 2012;18:3541-51
14. Kanda M, Tanaka C, Kobayashi D, Tanaka H, Shimizu D, Shibata M. et al. Epigenetic suppression of the immunoregulator MZB1 is associated with the malignant phenotype of gastric cancer. Int J Cancer. 2016;139:2290-8
15. Andreani V, Ramamoorthy S, Pandey A, Lupar E, Nutt SL, Lammermann T. et al. Cochaperone Mzb1 is a key effector of Blimp1 in plasma cell differentiation and beta1-integrin function. Proc Natl Acad Sci U S A. 2018;115:E9630-E9
16. Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619-47
17. Obaseki I, Ndolo CC, Adedeji AA, Popoola HO, Kravats AN. The structural and functional dynamics of BiP and Grp94: opportunities for therapeutic discovery. Trends Pharmacol Sci. 2025;46:453-67
18. van Anken E, Pena F, Hafkemeijer N, Christis C, Romijn EP, Grauschopf U. et al. Efficient IgM assembly and secretion require the plasma cell induced endoplasmic reticulum protein pERp1. Proc Natl Acad Sci U S A. 2009;106:17019-24
19. Suzuki K, Vogelzang A, Fagarasan S. MZB1 folding and unfolding the role of IgA. Proc Natl Acad Sci U S A. 2019;116:13163-5
20. Heng TS, Painter MW, Immunological Genome Project C. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091-4
21. McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79:17-27
22. Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594-606
23. Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135-43
24. Wiest DL, Burkhardt JK, Hester S, Hortsch M, Meyer DI, Argon Y. Membrane biogenesis during B cell differentiation: most endoplasmic reticulum proteins are expressed coordinately. J Cell Biol. 1990;110:1501-11
25. van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I. et al. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity. 2003;18:243-53
26. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081-6
27. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519-29
28. Anolik JH. B cell biology: implications for treatment of systemic lupus erythematosus. Lupus. 2013;22:342-9
29. Eloranta ML, Ronnblom L. Cause and consequences of the activated type I interferon system in SLE. J Mol Med (Berl). 2016;94:1103-10
30. Molendijk M, Hazes JM, Lubberts E. From patients with arthralgia, pre-RA and recently diagnosed RA: what is the current status of understanding RA pathogenesis? RMD Open. 2018;4:e000256
31. Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. Citrullination and autoimmunity. Autoimmun Rev. 2015;14:490-7
32. Togashi T, Ishihara R, Watanabe R, Shiomi M, Yano Y, Fujisawa Y. et al. Rheumatoid Factor: Diagnostic and Prognostic Performance and Therapeutic Implications in Rheumatoid Arthritis. J Clin Med. 2025;14:1529
33. Ramirez-Perez S, Oregon-Romero E, Reyes-Perez IV, Bhattaram P. Targeting MyD88 Downregulates Inflammatory Mediators and Pathogenic Processes in PBMC From DMARDs-Naive Rheumatoid Arthritis Patients. Front Pharmacol. 2021;12:800220
34. Wang J, Xue Y, Zhou L. Comparison of immune cells and diagnostic markers between spondyloarthritis and rheumatoid arthritis by bioinformatics analysis. J Transl Med. 2022;20:196
35. Yap CF, Nair N, Morgan AW, Isaacs JD, Wilson AG, Hyrich K. et al. Identifying Predictive Biomarkers of Response in Patients With Rheumatoid Arthritis Treated With Adalimumab Using Machine Learning Analysis of Whole-Blood Transcriptomics Data. Arthritis Rheumatol. 2025;77:1663-72
36. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809-20
37. Guzeldemir-Akcakanat E, Sunnetci-Akkoyunlu D, Balta-Uysal VM, Ozer T, Isik EB, Cine N. Differentially expressed miRNAs associated with generalized aggressive periodontitis. Clin Oral Investig. 2023;28:7
38. Guzeldemir-Akcakanat E, Sunnetci-Akkoyunlu D, Orucguney B, Cine N, Kan B, Yilmaz EB. et al. Gene-Expression Profiles in Generalized Aggressive Periodontitis: A Gene Network-Based Microarray Analysis. J Periodontol. 2016;87:58-65
39. Guzeldemir-Akcakanat E, Alkan B, Sunnetci-Akkoyunlu D, Gurel B, Balta VM, Kan B. et al. Molecular signatures of chronic periodontitis in gingiva: A genomic and proteomic analysis. J Periodontol. 2019;90:663-73
40. Lundmark A, Gerasimcik N, Bage T, Jemt A, Mollbrink A, Salmen F. et al. Gene expression profiling of periodontitis-affected gingival tissue by spatial transcriptomics. Sci Rep. 2018;8:9370
41. Sunnetci-Akkoyunlu D, Guzeldemir-Akcakanat E, Alkan B, Gurel B, Balta-Uysal VM, Akgun E. et al. Altered expression of MZB1 in periodontitis: A possible link to disease pathogenesis. J Periodontol. 2023;94:1285-94
42. Gao X, Jiang C, Yao S, Ma L, Wang X, Cao Z. Identification of hub genes related to immune cell infiltration in periodontitis using integrated bioinformatic analysis. J Periodontal Res. 2022;57:392-401
43. Baheti W, Dong D, Li C, Chen X. Identification of core genes related to exosomes and screening of potential targets in periodontitis using transcriptome profiling at the single-cell level. BMC Oral Health. 2025;25:28
44. Li D, Zhu Y, Zhang L, Shi L, Deng L, Ding Z. et al. MZB1 targeted by miR-185-5p inhibits the migration of human periodontal ligament cells through NF-kappaB signaling and promotes alveolar bone loss. J Periodontal Res. 2022;57:811-23
45. Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. 2017;32:43-53
46. Corthesy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185
47. Brandtzaeg P, Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984;311:71-3
48. Wacker EM, Uellendahl-Werth F, Bej S, Wolkenhauer O, Vesterhus M, Lieb W. et al. Whole blood RNA sequencing identifies transcriptional differences between primary sclerosing cholangitis and ulcerative colitis. JHEP Rep. 2024;6:100988
49. Oshina K, Kuroda K, Nakabayashi K, Tomikawa J, Kitade M, Sugiyama R. et al. Gene expression signatures associated with chronic endometritis revealed by RNA sequencing. Front Med (Lausanne). 2023;10:1185284
50. Huang Y, Xu Z, Yan Y, Li C, Yan J, Wei Y. et al. Excessive Endoplasmic Reticulum Stress in B Cells Associates With the Local Immunoglobulin Production in Severe Type 2 Chronic Rhinosinusitis With Nasal Polyps. Clin Exp Allergy. 2025;55:1194-207
51. Xu M, Feng Y, Xiang X, Liu L, Tang G. MZB1 regulates cellular proliferation, mitochondrial dysfunction, and inflammation and targets the PI3K-Akt signaling pathway in acute pancreatitis. Cell Signal. 2024;118:111143
52. Yi C, Yang J, Zhang T, Qin L, Chen D. Identification of Breast Cancer Subtypes Based on Endoplasmic Reticulum Stress-Related Genes and Analysis of Prognosis and Immune Microenvironment in Breast Cancer Patients. Technol Cancer Res Treat. 2024;23:15330338241241484
53. Wu J, Zhao G, Cai Y. Regulatory T cell-associated gene signature correlates with prognostic risk and immune infiltration in patients with breast cancer. Transl Cancer Res. 2024;13:6766-81
54. Kumar S, Das A. Peripheral blood mononuclear cell derived biomarker detection using eXplainable Artificial Intelligence (XAI) provides better diagnosis of breast cancer. Comput Biol Chem. 2023;104:107867
55. Ho M, Dasari S, Visram A, Drake M, Charlesworth C, Johnson K. et al. An atlas of the bone marrow bone proteome in patients with dysproteinemias. Res Sq. 2023;13:63
56. Bauer MA, Ashby C, Wardell C, Boyle EM, Ortiz M, Flynt E. et al. Differential RNA splicing as a potentially important driver mechanism in multiple myeloma. Haematologica. 2021;106:736-45
57. Katoh M, Katoh M. MGC29506 gene, frequently down-regulated in intestinal-type gastric cancer, encodes secreted-type protein with conserved cysteine residues. Int J Oncol. 2003;23:235-41
58. Xia L, Shen C, Fu Y, Tian L, Chen M. MGC29506 induces cell cycle arrest and is downregulated in gastric cancer. Cell Immunol. 2013;281:31-6
59. Hu Y, Li R, Chen H, Chen L, Zhou X, Liu L. et al. Comprehensive analysis of lncRNA-mRNAs co-expression network identifies potential lncRNA biomarkers in cutaneous squamous cell carcinoma. BMC Genomics. 2022;23:274
60. Zheng X, Tong T, Duan L, Ma Y, Lan Y, Shao Y. et al. VSIG4 induces the immunosuppressive microenvironment by promoting the infiltration of M2 macrophage and Tregs in clear cell renal cell carcinoma. Int Immunopharmacol. 2024;142:113105
61. Xu H, Li W, Zhu C, Cheng N, Li X, Hao F. et al. Proteomic profiling identifies novel diagnostic biomarkers and molecular subtypes for mucinous tubular and spindle cell carcinoma of the kidney. J Pathol. 2022;257:53-67
62. Niemira M, Collin F, Szalkowska A, Bielska A, Chwialkowska K, Reszec J. et al. Molecular Signature of Subtypes of Non-Small-Cell Lung Cancer by Large-Scale Transcriptional Profiling: Identification of Key Modules and Genes by Weighted Gene Co-Expression Network Analysis (WGCNA). Cancers (Basel). 2019;12:37
63. Zhong R, Zhang Y, Chen D, Cao S, Han B, Zhong H. Single-cell RNA sequencing reveals cellular and molecular immune profile in a Pembrolizumab-responsive PD-L1-negative lung cancer patient. Cancer Immunol Immunother. 2021;70:2261-74
64. Zhu X, Liu D, Wang Y, Dong M. Salidroside suppresses nonsmall cell lung cancer cells proliferation and migration via microRNA-103-3p/Mzb1. Anticancer Drugs. 2020;31:663-71
65. Tang S, Huang X, Jiang H, Qin S. Identification of a Five-Gene Prognostic Signature Related to B Cells Infiltration in Pancreatic Adenocarcinoma. Int J Gen Med. 2021;14:5051-68
66. Miyake K, Mori R, Homma Y, Matsuyama R, Okayama A, Murakami T. et al. MZB1 in borderline resectable pancreatic cancer resected after neoadjuvant chemoradiotherapy. J Surg Res. 2017;220:391-401
67. Zhu M, Zhou G, Chang F, Liu J. MZB1 regulates the immune microenvironment and inhibits ovarian cancer cell migration. Open Med (Wars). 2025;20:20251174
68. Wu W, Yang Z, Long F, Luo L, Deng Q, Wu J. et al. COL1A1 and MZB1 as the hub genes influenced the proliferation, invasion, migration and apoptosis of rectum adenocarcinoma cells by weighted correlation network analysis. Bioorg Chem. 2020;95:103457
69. Tang Y, Feng X, Lu Q, Cui C, Yu M, Wen Z. et al. MZB1-mediated IgA secretion suppresses the development and progression of colorectal cancer triggered by gut inflammation. Mucosal Immunol. 2024;17:450-60
70. Zhang Y, Li H, Pan Y, Cao J, Zhai L, Zhang X. Pan-cancer analysis of MZB1 expression and its association with immune infiltration and clinical prognosis. Nan Fang Yi Ke Da Xue Xue Bao. 2025;45:2006-18
Author contact
![]() Corresponding authors: Xi Zhang, Department of Clinical Laboratory, the Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming 650118, Yunnan, China. Shicong Tang, Department of Breast Surgery, the Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming 650118, Yunnan, China. E-mail addresses: zhangxiedu.cn (X. Zhang), tang_shicongcom (S. Tang).
Corresponding authors: Xi Zhang, Department of Clinical Laboratory, the Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming 650118, Yunnan, China. Shicong Tang, Department of Breast Surgery, the Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming 650118, Yunnan, China. E-mail addresses: zhangxiedu.cn (X. Zhang), tang_shicongcom (S. Tang).

 Global reach, higher impact
Global reach, higher impact