Impact Factor
ISSN: 1837-9664
J Cancer 2026; 17(3):507-514. doi:10.7150/jca.126692 This issue Cite
Research Paper
Clinical Outcomes and Individualized Seed Implantation Planning for Iodine-125 Seeds Brachytherapy in Lymph Node Metastases
Department of Minimally Invasive Intervention, State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou 510060, P. R. China.
Received 2025-10-4; Accepted 2026-1-20; Published 2026-1-30
Abstract
Background: Lymph node metastasis (LNM) critically influences cancer prognosis and treatment. This study explored the efficacy and prognostic factors of CT-guided radioactive iodine-125 (¹²⁵I) seed brachytherapy (RISB) for LNM and optimized the therapeutic dosage.
Methods: We conducted a single-center retrospective cohort study analyzing 81 cases with histologically confirmed LNM (≤ 5 cm) from diverse primary cancers treated with CT-guided RISB. Postoperative dosimetric parameters (D90, D100, V90, V100, V150, V200) were assessed. Treatment response was evaluated at 6 months using RECIST 1.1, calculating the objective response rate (ORR) and local control rate (LCR). Patients were categorized into objective response and non-objective response groups based on treatment efficacy, and factors influencing treatment efficacy were identified through logistic regression analysis. Based on the ROC curve, the Youden index method was used to determine the dose optimization cutoff value.
Results: The overall ORR was 71.6%, and LCR was 96.3%. The complication rate was 3.7%. Tumor size was an independent influencing factor for efficacy. Higher postoperative dosimetric parameters were associated with efficacy but were not independent influencing factors. ROC analysis identified the optimal D90 threshold as 102.7 Gy. The ORR in patients who achieved D90 > 102.7 Gy (n = 65, ORR = 81.54%) was significantly higher than in patients with D90 ≤ 102.7 Gy (n = 16, ORR = 31.25%) (p < 0.01). Complication rates did not differ between dose groups.
Conclusion: Patients with LNM undergoing RISB can achieve a significantly higher ORR by ensuring a postoperative D90 > 102.7 Gy, without increasing the risk of complications. This dose threshold serves as a practical reference for clinical dose planning. Tumor size independently influences better response, guiding patient selection.
Keywords: brachytherapy, clinical outcomes, implantation planning, lymph node metastasis
Introduction
LNM is one of the critical prognostic factors in cancer, influencing therapeutic decisions and patient outcomes across multiple malignancies. The incidence of LNM exhibits substantial variability among different cancer types. In non-small cell lung cancer (NSCLC), LNM prevalence ranges from 25.0% to 38.2% [1, 2], while in thyroid cancer, central LNM occurs in 35.3% of cases, with micrometastases (≤ 0.28 cm) detected in 57.8% of node-positive patients [3]. Breast cancer demonstrates an 8% sentinel lymph node (SLN) positivity rate, with 33.3% of these cases presenting non-SLN metastases [4]. LNM is a critical determinant of survival and prognostic outcomes across multiple cancer types, serving as a key indicator of disease progression and therapeutic response. The presence of metastatic tumor cells in regional lymph nodes is strongly correlated with increased recurrence risk, diminished disease-free survival (DFS), and reduced overall survival (OS) [5, 6]. In cervical cancer, nodal involvement decreases 5-year survival from 74.8% (T1) to 39.3% (T3) [7]. Similarly, renal cell carcinoma with ≥10 positive nodes exhibit a 38% 2-year survival rate compared to 64% for single-node involvement, underscoring the prognostic significance of nodal burden [8]. Consequently, positive LNM status typically necessitates aggressive therapeutic interventions.
The treatment of LNM remains a critical challenge in oncology, with current therapeutic approaches increasingly incorporating multimodal strategies that combine surgical, systemic, and localized interventions. However, the limitations of different treatments, their toxic side effects, and the heterogeneity of their efficacy remain critical bottlenecks demanding breakthroughs [9]. For instance, external beam radiotherapy (EBRT) is constrained by anatomical limitations and the tolerance thresholds of normal tissues. Minimally invasive interventional therapy, with its unique advantages of precision, efficiency, minimal invasiveness, repeatability, and the ability to overcome hypoxic resistance, has become an essential complementary approach to local treatments. RISB has emerged as a promising minimally invasive brachytherapy modality for managing LNM, particularly in cases resistant to conventional therapies such as surgery, EBRT, and systemic treatments. This technique delivers high-dose radiation directly to the tumor while sparing adjacent critical structures, owing to the rapid dose fall-off of low-energy γ-rays (27-35 keV) emitted by ¹²⁵I seeds [10, 11]. Clinical evidence has demonstrated substantial LCR and symptomatic improvement in metastatic lymph nodes across various malignancies, including thyroid, esophageal, and head and neck cancers. For example, in radioactive iodine-refractory differentiated thyroid cancer (RAIR-DTC), ¹²⁵I implantation achieved an LCR of 94.9% at 5 months post-treatment, with concurrent reductions in serum thyroglobulin levels and pain scores (P < 0.001) [12, 13]. Similarly, in esophageal cancer patients with recurrent LNM following prior radiotherapy, RISB resulted in a 69.9% tumor response rate and a 2-year local progression-free survival rate of 23% [14, 15]. Despite these promising outcomes, several challenges remain, including the suitability of RISB for all LNM cases due to tumor heterogeneity and the necessity for individualized dose optimization [14, 16]. For instance, poorly differentiated tumors, such as small cell carcinoma, may require higher initial doses or alternative isotopes like protactinium-103 [11, 16]. This retrospective study analyzed the efficacy and dose differences of RISB in the treatment of LNM from various cancers with the aim of investigating factors affecting their efficacy and dose optimization. Our study will inform the selection of patients who will undergo RISB for the treatment of LNM and the determination of the appropriate prescribed dose.
Methods
Patients
This retrospective cohort study enrolled patients with LNM who underwent RISB at the Sun Yat-sen University Cancer Center from September 2015 to September 2023. The inclusion criteria comprised: (1) age ≥ 18 years; (2) histologically confirmed LNM; (3) treatment with RISB, without external radiotherapy or other local treatments such as ablation before surgery; (4) lesion diameter ≤ 5 cm; (5) efficacy could be accurately evaluated; (6) minimum postoperative follow-up duration of 6 months. Exclusion criteria included: (1) suboptimal seed positioning confirmed by postoperative imaging; and (2) incomplete clinical documentation in medical records.
RISB procedure
Preoperative assessment involves precise tumor localization through contrast-enhanced computed tomography (CT), with acquired images subsequently integrated into the treatment planning system (TPS). The clinical target volume (CTV) is meticulously delineated, followed by TPS-based calculations of seed quantity and activity required to deliver the prescribed radiation dose. Concurrently, seed spatial distribution is optimized, and organs at risk (OARs) adjacent to the tumor are contoured, thereby finalizing the preoperative treatment plan. Intraoperatively, under CT guidance, seed needles are precisely positioned at predetermined tumor coordinates. Seed implantation is executed using a retraction needle technique with 5-10 mm spacing intervals, strictly adhering to the TPS-generated preoperative plan. Continuous patient monitoring is maintained throughout the procedure, with immediate symptomatic management of any complications. Postoperative evaluation commences with immediate CT imaging for dose verification using TPS analysis. Long-term follow-up incorporates serial imaging studies, with tumor response assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [17]. Concurrent monitoring of potential radiation-induced damage to adjacent normal tissues ensures an optimal balance between precise local radiotherapy delivery and patient safety.
Data
Comprehensive clinical data, postoperative TPS dose verification parameters, and therapeutic efficacy outcomes were systematically collected for all enrolled patients. The clinical dataset encompassed demographic characteristics (age, gender), tumor-related parameters (location, size), surgical details, and histopathological classification of the primary tumor. TPS dose verification metrics included dosimetric parameters such as the prescribed dose received by 90% and 100% of the target area (D90, D100), as well as the target volume covered by 90%, 100%, 150%, and 200% of the prescribed dose lines (V90, V100, V150, V200), complemented by the activity of implanted radioactive seeds. The therapeutic response was evaluated at the 6-month postoperative follow-up, with the ORR calculated as the proportion of complete response (CR) and partial response (PR) cases relative to the total patient cohort, expressed as (CR + PR)/total cases × 100%. Similarly, the LCR was determined by incorporating stable disease (SD) cases, calculated as (CR + PR + SD)/total cases × 100%.
Statistical analysis
Statistical analyses were performed using R language version 4.3.0 (The R Foundation for Statistical Computing, Vienna, Austria). Continuous variables with normal distribution were presented as mean ± standard deviation and analyzed using independent samples t-test. Non-normally distributed continuous variables were expressed as median (interquartile range) and compared using the Wilcoxon rank-sum test. Categorical variables were reported as frequencies (percentages) and analyzed using either the chi-square test or Fisher's exact test, as appropriate. The optimal cutoff value was determined through receiver operating characteristic (ROC) curve analysis using the Youden index method. Variables were screened using univariate logistic regression analysis (P < 0.05), and these significant variables were subsequently incorporated into multivariate logistic regression analysis to identify independent factors associated with treatment outcomes. All statistical tests were two-tailed, with a p-value < 0.05 considered statistically significant.
Results
Baseline characteristics
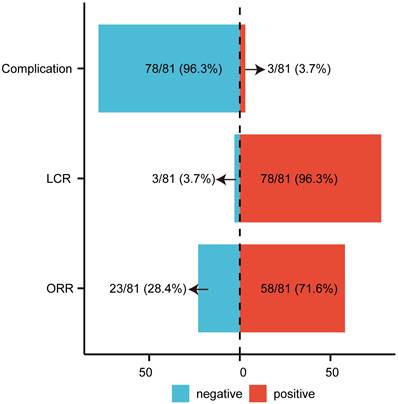
Following the application of inclusion and exclusion criteria, 81 cases of LNM were enrolled in the study (Table 1), with a mean age of 51.49 years. Objective response (CR + PR) was observed in 58 cases (71.60%), while 23 cases (28.40%) exhibited non-objective response (SD + progressive disease [PD]) (Figure 1). Baseline characteristics including age, gender, cancer location, and complication rates were comparable between the objective response group and the non-objective response group, but no significant differences were observed (all P > 0.05; Table 1). However, significant differences were identified in tumor size, D90, D100, V90, V100, V150, and V200 (P < 0.05). The objective response group demonstrated a smaller median tumor diameter (18.06 mm) compared to the non-objective response group (27.10 mm) (Table 1). Postoperative dosimetric parameters (D90, D100, V90, V100, V150, and V200) were significantly higher in the objective response group than in the non-objective response group (Table 1). The overall postoperative LCR was 96.3% (Figure 1). Complications occurred in 3 cases (3.7%) (Figure 1), including one case of minor pneumothorax (Supplementary Figure 1) and two cases of minor bleeding. No instances of seed migration, needle tract metastasis, or severe pain requiring pharmacological intervention were reported. The minor pneumothorax resolved spontaneously, and the bleeding was controlled with hemostatic agents. The treatment demonstrated significant efficacy and safety. Pathological analysis identified 18 primary tumor types in LNM cases, with hepatocellular carcinoma being the most prevalent (32.1%) (Supplementary Table 1).
The Efficacy and Complications of Radioactive Iodine-125 Seed Brachytherapy in the Treatment of LNM. ORR objective response rate; LCR local control rate; LNM lymph node metastasis.
Patient characteristics
| Variables | Total (n = 81) | SD*+PD (n = 23) | CR+PR (n = 58) | Statistic | P |
|---|---|---|---|---|---|
| Age (years), Mean ± SD | 51.49 ± 11.72 | 54.52 ± 13.52 | 50.29 ± 10.83 | T = 1.47 | 0.14 |
| Sex, n (%) | χ² = 0.34 | 0.56 | |||
| Male | 56 (69.14) | 17 (73.91) | 39 (67.24) | ||
| Female | 25 (30.86) | 6 (26.09) | 19 (32.76) | ||
| Cancer location, n (%) | - | 0.56 | |||
| head and neck | 8 (9.88) | 4 (17.39) | 4 (6.90) | ||
| chest | 11 (13.58) | 4 (17.39) | 7 (12.07) | ||
| abdomen | 53 (65.43) | 13 (56.52) | 40 (68.97) | ||
| pelvic | 5 (6.17) | 1 (4.35) | 4 (6.90) | ||
| lower limbs | 4 (4.94) | 1 (4.35) | 3 (5.17) | ||
| Tumor size (mm), M (Q₁, Q₃) | 19.70 (16.00, 27.80) | 27.10 (19.45, 34.90) | 18.06 (14.22, 23.02) | Z = -3.47 | < 0.01 |
| D90, Mean ± SD | 12276.09 ± 2601.80 | 10807.31 ± 2507.85 | 12858.53 ± 2420.28 | T = -3.40 | < 0.01 |
| D100, Mean ± SD | 7442.40 ± 2220.36 | 6334.83 ± 2052.29 | 7881.61 ± 2145.32 | T = -2.96 | < 0.01 |
| V90, M (Q₁, Q₃) | 0.94 (0.89, 0.97) | 0.89 (0.83, 0.95) | 0.95 (0.93, 0.98) | Z = -2.71 | < 0.01 |
| V100, M (Q₁, Q₃) | 0.91 (0.85, 0.94) | 0.87 (0.79, 0.92) | 0.91 (0.88, 0.95) | Z = -2.72 | < 0.01 |
| V150, Mean ± SD | 0.68 ± 0.13 | 0.62 ± 0.15 | 0.70 ± 0.11 | T = -2.92 | < 0.01 |
| V200, Mean ± SD | 0.51 ± 0.14 | 0.45 ± 0.15 | 0.53 ± 0.13 | T = -2.41 | 0.02 |
| Complication, n (%) | - | 1.00 | |||
| without complication | 78 (96.30) | 22 (95.65) | 56 (96.55) | ||
| with complication | 3 (3.70) | 1 (4.35) | 2 (3.45) |
t: t-test, Z: Mann-Whitney test, χ²: Chi-square test, -: Fisher exact, SD: standard deviation, M: Median, Q₁: 1st Quartile, Q₃: 3rd Quartile, CR: complete response, PR: partial response, SD*: stable disease, PD: progressive disease, D90/D100: dose received in 90/100 percent of target areas, V90/V100/V150/V200: volume of target area covered by 90%, 100%, 150%, 200% prescription dose lines.
Factors affecting ORR
Univariate and multivariate logistic regression analyses (Table 2) demonstrated that tumor size emerged as an independent factor significantly associated with ORR. In contrast, postoperative dosimetric parameters, including D90, D100, V90, V100, V150, and V200, were not identified as independent factors but were observed to exert related influences on ORR. Furthermore, demographic and anatomical variables such as age, gender, and tumor location exhibited no statistically significant correlation with ORR.
Dose optimization
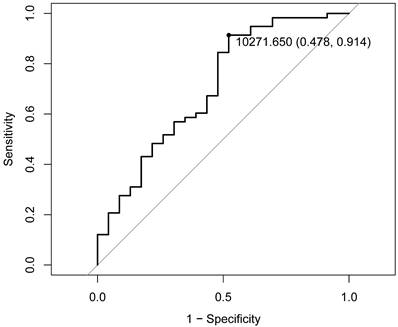
The ROC curve analysis, employing the Youden index method, demonstrated that a D90 value of 102.7 Gy represents the optimal threshold for distinguishing the objective response population (Figure 2). Based on this threshold, the study cohort was stratified into high-dose (D90 > 102.7 Gy, n = 65, 80.25%) and low-dose (D90 ≤ 102.7 Gy, n = 16, 19.75%) groups (Table 3). Statistical analysis revealed a significant difference in ORR between the two groups (P<0.01), with the high-dose group having an ORR of 81.54% and the low-dose group having an ORR of only 31.25% (Table 3), which was significantly lower than that of the high-dose group. There were no statistically significant differences in age, gender, tumor location, or complication incidence between the high-dose and low-dose groups (P > 0.05). Notably, while complications were exclusively observed in the high-dose group, statistical evaluation confirmed that a D90 exceeding 102.7 Gy does not significantly increase the risk of complications (P > 0.05). These findings suggest that administering doses above 102.7 Gy in the context of RISB for LNM is both therapeutically effective and clinically safe.
ROC curve. ROC analysis identified the optimal D90 threshold as 102.7 Gy. D90 prescribed dose received by 90% of the target area.
Univariate and multivariate logistic regression analyses of ORR
| Variables | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| β | P | OR (95%CI) | β | P | OR (95%CI) | |
| Age | -0.03 | 0.15 | 0.97 (0.93 ~ 1.01) | |||
| Sex | ||||||
| Male | 1.00 (Reference) | |||||
| Female | 0.32 | 0.56 | 1.38 (0.47 ~ 4.07) | |||
| Cancer location | ||||||
| abdomen | 1.00 (Reference) | |||||
| head and neck | -1.12 | 0.15 | 0.33 (0.07 ~ 1.49) | |||
| chest | -0.56 | 0.42 | 0.57 (0.14 ~ 2.26) | |||
| pelvic | 0.26 | 0.82 | 1.30 (0.13 ~ 12.70) | |||
| lower limbs | -0.03 | 0.98 | 0.97 (0.09 ~ 10.20) | |||
| Tumor size | -0.13 | < 0.01 | 0.88 (0.82 ~ 0.94) | -0.16 | <0.01 | 0.85 (0.77 ~ 0.93) |
| D90 | 0.01 | < 0.01 | 1.01 (1.01 ~ 1.01) | 0.00 | 0.09 | 1.00 (1.00 ~ 1.00) |
| D100 | 0.01 | < 0.01 | 1.01 (1.01 ~ 1.01) | -0.00 | 0.14 | 1.00 (1.00 ~ 1.00) |
| V90 | 13.28 | < 0.01 | 5.86E+05 (99.55 ~ 3.45E+09) | -24.35 | 0.48 | 0.00 (0.00 ~ 7.87E+18) |
| V100 | 11.10 | < 0.01 | 6.60E+04 (44.00 ~ 9.89E+07) | 35.46 | 0.35 | 2.51E+15 (0.00 ~ 9.23E+47) |
| V150 | 5.70 | < 0.01 | 298.97 (4.14 ~ 2.16E+04) | -13.27 | 0.47 | 0.00 (0.00 ~ 8.71E+09) |
| V200 | 4.38 | 0.02 | 79.69 (1.84 ~ 3.45E+03) | -2.95 | 0.78 | 0.05 (0.00 ~ 4.99E+07) |
ORR: objective response rate, OR: Odds Ratio, CI: Confidence Interval, D90/D100: dose received in 90/100 percent of target areas, V90/V100/V150/V200: volume of target area covered by 90%, 100%, 150%, 200% prescription dose lines.
Characteristics of patients in the high-dose group (D90 ≥ 102.7 Gy) and low-dose group (D90 < 102.7 Gy).
| Variables | Total (n = 81) | High-dose (n = 65) | Low-dose (n = 16) | Statistic | P |
|---|---|---|---|---|---|
| Age (years), Mean ± SD | 51.49 ± 11.72 | 50.57 ± 10.47 | 55.25 ± 15.70 | T = -1.44 | 0.15 |
| Sex, n (%) | χ² = 0.00 | 1.00 | |||
| Male | 56 (69.14) | 45 (69.23) | 11 (68.75) | ||
| Female | 25 (30.86) | 20 (30.77) | 5 (31.25) | ||
| RECIST 1.1, n (%) | χ² = 13.59 | < 0.01 | |||
| PD+SD* | 23 (28.40) | 12 (18.46) | 11 (68.75) | ||
| CR+PR | 58 (71.60) | 53 (81.54) | 5 (31.25) | ||
| Complication, n (%) | - | 1.00 | |||
| without complication | 78 (96.30) | 62 (95.38) | 16 (100.00) | ||
| with complication | 3 (3.70) | 3 (4.62) | 0 (0.00) | ||
| Cancer location, n (%) | - | 0.98 | |||
| head and neck | 8 (9.88) | 6 (9.23) | 2 (12.50) | ||
| chest | 11 (13.58) | 9 (13.85) | 2 (12.50) | ||
| abdomen | 53 (65.43) | 43 (66.15) | 10 (62.50) | ||
| pelvic | 5 (6.17) | 4 (6.15) | 1 (6.25) | ||
| lower limbs | 4 (4.94) | 3 (4.62) | 1 (6.25) | ||
| D90, Mean ± SD | 12276.09 ± 2601.80 | 13138.27 ± 2065.63 | 8773.48 ± 1261.32 | T = 8.07 | < 0.01 |
t: t-test, χ²: Chi-square test, -: Fisher exact, SD: standard deviation, CR: complete response, PR: partial response, SD*: stable disease, PD: progressive disease, D90: dose received in 90 percent of target areas.
Discussion
LNM represents a critical event in the progression of numerous malignant neoplasms, manifesting at various stages of tumor development. Beyond its role as an indicator of local tumor dissemination, LNM has been implicated in facilitating distant metastasis through diverse molecular mechanisms [18], thereby significantly influencing patient outcomes [19, 20]. RISB has emerged as an effective and safe therapeutic modality for LNM, particularly in cases where surgical intervention is contraindicated, systemic therapies yield suboptimal results or disease recurrence occurs following EBRT. In the present study, RISB demonstrated notable efficacy, with an ORR of 71.6% (44 cases of PR), a LCR of 96.3% (20 cases of SD) at 6 months, and a remarkably low complication rate of 3.7%.
A retrospective analysis of 24 patients with metastatic tumors in the right subclavian tracheal lymph nodes demonstrated an ORR of 87.5% following RISB, including 4 cases of CR and 17 cases of PR, with a significant reduction in the mean diameter of metastatic lymph nodes from 40.21 mm to 12.25 mm (p < 0.001) [21]. In a separate study involving 11 patients with iliac LNM, the ORR was 72.73% (8 cases of PR) at the 2-month follow-up, with 2 cases of SD and a LCR of 90.91% [22]. Among 15 patients with oral and maxillofacial squamous cell carcinoma who underwent postoperative RISB, the LCR reached 95.3% at 6 months [23]. The ORR and LCR observed in our study were generally consistent with those reported in prior investigations [22, 23], although the ORR was lower than that reported by He et al. [21, 24]. These discrepancies may be attributed to differences in the types of primary tumors evaluated across studies. Furthermore, our study encompassed a larger sample size and a broader spectrum of primary cancers. Previous research has identified key determinants of treatment efficacy, including radiation dose parameters (e.g., D90), tumor characteristics (e.g., lesion size, primary tumor stage), and short-term treatment response [12, 14]. This study corroborates that tumor size is an independent predictor of ORR, while radiation dose parameters, such as D90 and D100, are influential but not independent factors, aligning with earlier findings. We hypothesize that this may be due to an intrinsic relationship between tumor size and achievable dose parameters. As a comprehensive variable encompassing anatomical complexity and biological characteristics, tumor size's potent predictive effect may partially mask or mediate the apparent influence of dose parameters. Chen et al. [25] demonstrated in their study on recurrent retroperitoneal LNM that tumor volumes ≤ 49.8 cm³ were associated with significantly prolonged local control. Collectively, smaller tumor volumes correlate with improved treatment outcomes, likely due to central necrosis in larger tumors resulting from inadequate blood supply, which leads to heterogeneous dose distribution following particle implantation [26, 27].
Previous investigations have not comprehensively addressed the dosage optimization required to enhance therapeutic efficacy, and research in this domain remains limited. Consequently, this study aims to identify the optimal dosage to improve treatment outcomes. Our findings indicate that a postoperative D90 exceeding 102.7 Gy significantly enhances therapeutic efficacy, with the ORR increasing from 31.25% in the low-dose group to 81.54% in the high-dose group. The expert consensus [28] on the prescription dose from the American Brachytherapy Society states that the postoperative D90 should at least reach the prescription dose. Based on these findings, we propose that the prescribed dose for LNM treated with RISB should be at least 102.7 Gy. In recurrent head and neck cancers, a D90 ≥ 130 Gy correlated with superior local control (72.7% for cervical lymph node recurrences vs. 39.9% for primary site recurrences) [29]. For thyroid cancer LNM, the recommended prescription dose ranges from 120 to 150 Gy, with no significant difference in D90 between postoperative and preoperative plans [30]. Notably, the recommended prescription dose in our study is lower than those reported in prior studies, which may be attributed to the inclusion of diverse tumor types (18 distinct malignancies) in our cohort, as opposed to the recurrent or refractory cases predominantly examined in earlier research.
This study represents a single-center retrospective investigation, with a subsequent multicenter prospective study planned to validate the proposed prescription dosage. Furthermore, although the research encompasses various primary tumor types, the limited sample size for certain tumor categories necessitates the future expansion of the study cohort to enhance statistical power and generalizability.
Conclusion
This study confirms that CT-guided RISB is an effective and safe local treatment option for LNM. More importantly, through dosimetric analysis, we propose a clear dose optimization target (postoperative D90 > 102.7 Gy) for the LNM population, providing crucial scientific evidence for developing more standardized RISB treatment protocols in the future.
Abbreviations
CT: computed tomography; CTV: clinical target volume; CR: complete response; DFS: disease-free survival; D90, D100: prescribed dose received by 90% and 100% of the target area; EBRT: external beam radiotherapy; LNM: lymph node metastasis; LCR: local control rate; NSCLC: non-small cell lung cancer; ORR: objective response rate; OS: overall survival; OARs: organs at risk; PR: partial response; PD: progressive disease; RISB: radioactive iodine-125 (¹²⁵I) seed brachytherapy; RAIR-DTC: radioactive iodine-refractory differentiated thyroid cancer; ROC: receiver operating characteristic; SLN: sentinel lymph node; SD: stable disease; TPS: treatment planning system; V90, V100, V150, V200: target volume covered by 90%, 100%, 150%, and 200% of the prescribed dose lines.
Supplementary Material
Supplementary figure and table.
Acknowledgements
We thank the patients enrolled in this study.
Funding
This work was supported by research grants from the National Natural Science Foundation of P.R. China (No. 82472082, No. 82302322).
Ethics approval and consent to participate
This study was performed in accordance with the standards of the Helsinki Declaration. With the approval of the Ethics Committee of Sun Yat-sen University Cancer Center (Ethical Review Number: SL-B2025-653-01), the patient's informed consent was waived.
Consent for publication
All authors read and approved the final manuscript.
Availability of data and materials
Due to patient privacy protection, the raw data supporting the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Dongcun Huang (Data curation: Lead; Investigation: Lead; Methodology: Lead;
Software: Lead; Visualization: Lead; Writing - original draft: Lead); Zhihui Zhong (Data curation: Equal; Formal analysis: Equal; Investigation: Supporting; Writing - original draft: Supporting); Meigui Xiao (Data curation: Equal; Resources: Equal); Fujun Zhang (Conceptualization: Supporting; Resources: Equal; Supervision: Equal; Writing - review & editing: Supporting); Letao Lin (Conceptualization: Lead; Funding acquisition: Lead; Supervision: Lead; Writing - review & editing: Lead).
Competing Interests
The authors have declared that no competing interests exist.
References
1. Wei B, Jin X, Lu G, Zhao T, Xue H, Zhang Y. A novel nomogram to predict lymph node metastasis in cT1 non-small-cell lung cancer based on PET/CT and peripheral blood cell parameters. BMC Pulmonary Medicine. 2023 23
2. Moulla Y, Gradistanac T, Wittekind C, Eichfeld U, Gockel I, Dietrich A. Predictive risk factors for lymph node metastasis in patients with resected non-small cell lung cancer: a case control study. Journal of Cardiothoracic Surgery. 2019 14
3. Zhang S, Zhang R, Wang C, Gong W, Zheng C, Fang Q. et al. Unnecessity of Routine Dissection of Right Central Lymph Nodes in cN0 Papillary Thyroid Carcinoma Located at the Left Thyroid Lobe. Frontiers in Oncology. 2021 11
4. Syed A, Eleti S, Kumar V, Ahmad A, Thomas H. Validation of Memorial Sloan Kettering Cancer Center nomogram to detect non-sentinel lymph node metastases in a United Kingdom cohort. Giornale di Chirurgia - Journal of Surgery. 2018 39
5. Deng XF, Jiang L, Liu QX, Zhou D, Hou B, Cui K. et al. Lymph node micrometastases are associated with disease recurrence and poor survival for early-stage non-small cell lung cancer patients: a meta-analysis. Journal of Cardiothoracic Surgery. 2016 11
6. Ahmad A, Schrembs P, Martin B, Anthuber M, Schenkirsch G, Märkl B. The prognostic significance of lymph node size in node-positive colon cancer. Plos One. 2018 13
7. Wang M, Ma M, Yang L, Liang C. Development and validation of a nomogram for predicting pelvic lymph node metastasis and prognosis in patients with cervical cancer. Frontiers in Oncology. 2022 12
8. Zareba P, Russo P. The prognostic significance of nodal disease burden in patients with lymph node metastases from renal cell carcinoma. Urologic Oncology: Seminars and Original Investigations. 2019;37:302.e1-e6
9. Zhou K, Li Z-Z, Cai Z-M, Zhong N-N, Cao L-M, Huo F-Y. et al. Nanotheranostics in cancer lymph node metastasis: The long road ahead. Pharmacological Research. 2023 198
10. Li M, Li X, Qiu B, Chen Y, Jiang P, Sun H. et al. Experts consensus on 3D-printing template-assisted CT-guided radioactive iodine-125 seed implantation for recurrent soft tissue carcinoma in China. Clinical and Experimental Medicine. 2025 25
11. Ji Z, Jiang Y, Guo F, Peng R, Sun H, Fan J. et al. Safety and efficacy of CT-guided radioactive iodine-125 seed implantation assisted by a 3D printing template for the treatment of thoracic malignancies. Journal of Cancer Research and Clinical Oncology. 2019;146:229-36
12. Wan Q, Tan L, Tang X, Wang W, Su Y, Wu Z. et al. The clinical value of iodine-125 seed implantation in the treatment of iodine-refractory differentiated thyroid carcinoma. Frontiers in Endocrinology. 2024 15
13. Su Y, Wang J, Huang L, Xie L, Yu X, Zha J. Clinical efficacy of iodine-125 ((125)I) seed implantation in patients with iodine-refractory differentiated thyroid cancer. American Journal of Cancer Research. 2023 13(10)
14. Wu L, Zhao X, Tian S, Zhang K, He C, Feng Y. et al. Efficacy and toxicity of Iodine-125 seed implantation for lymph node recurrence secondary to esophageal cancer after radiotherapy: a multicenter retrospective study. Radiation Oncology. 2023 18
15. Li Y, Jiang Y, Wang J. Safety and efficacy of CT-guided radioactive iodine-125 seed implantation as a salvage treatment for recurrent head and neck cancer after two or more courses of radiotherapy. Radiation Oncology. 2023 18
16. Li W, Dan G, Jiang J, Zheng Y, Zheng X, Deng D. Repeated iodine-125 seed implantations combined with external beam radiotherapy for the treatment of locally recurrent or metastatic stage III/IV non-small cell lung cancer: a retrospective study. Radiation Oncology. 2016 11
17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer. 2009;45:228-47
18. Peng J-M, Su Y-L. Lymph node metastasis and tumor-educated immune tolerance: Potential therapeutic targets against distant metastasis. Biochemical Pharmacology. 2023 215
19. Meng F, Yuan J, Zhang X, Liu J, Li H. Influence of parotid lymph node metastasis on distant metastasis in parotid gland cancer. Frontiers in Oncology. 2023 13
20. Liu H, Zhang H, Zhang C, Liao Z, Li T, Yang T. et al. Pan-Soft Tissue Sarcoma Analysis of the Incidence, Survival, and Metastasis: A Population-Based Study Focusing on Distant Metastasis and Lymph Node Metastasis. Frontiers in Oncology. 2022 12
21. Yuan H, Song H-Y, Hu H-T, Cheng H-T, Li H-L. CT-guided iodine-125 brachytherapy is an effective palliative treatment for the right lower paratracheal lymph nodes metastasis previously treatment failure. Brachytherapy. 2024;23:617-22
22. Wang J, Zhang H, Yu H, Liang Y, Wang Z, Di X. et al. Preliminary investigation of computed tomography-guided iodine-125 seed implantation treatment efficacy in patients with iliac lymph nodes metastases. Journal of Cancer Research and Therapeutics. 2019 15
23. Jie W-p, Bai J-y, Li B-b. Clinicopathologic Analysis of Oral Squamous Cell Carcinoma After 125I Interstitial Brachytherapy. Technology in Cancer Research & Treatment. 2018 17
24. He C, Liu Y, Li Y, Yang L, Li Y-T, Li S-L. et al. Efficacy and safety of computed tomography-guided 125I brachytherapy for lymph node metastatic from hepatocellular carcinoma. Journal of Cancer Research and Therapeutics. 2018;14:754-9
25. Chen Y, Jiang Y, Ji Z, Jiang P, Xu F, Zhang Y. et al. Dosimetry, efficacy, and safety of three-dimensional printing noncoplanar template-assisted and CT-guided 125I seed implantation for recurrent retroperitoneal lymphatic metastasis after external beam radiotherapy. Brachytherapy. 2020;19:380-8
26. Li M, Liu P, Wang H, Wang B, Zhou J, Jin Y. et al. Computed tomography-guided iodine-125 radioactive seed implantation in small-cell lung cancer: A retrospective study. Journal of Contemporary Brachytherapy. 2022;14:536-41
27. Li L, Tian S, Han X, Tian J, Zhang C. Computed tomography-guided radioactive iodine-125 seed implantation for liver malignancies in challenging locations. Journal of Cancer Research and Therapeutics. 2024;20:1165-72
28. Stewart A, Parashar B, Patel M, O'Farrell D, Biagioli M, Devlin P. et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15:1-11
29. Jiang P, Wang J, Ran W, Jiang Y, Tian S, Sun H. Five-year outcome of ultrasound-guided interstitial permanent 125I seeds implantation for local head and neck recurrent tumors: a single center retrospective study. Journal of Contemporary Brachytherapy. 2019;11:28-34
30. Zhang W, Hao S, Wang Z, Ding T, Zhang G. 125I seed implantation for lymph node metastasis from radioactive iodine-refractory differentiated thyroid carcinoma: a study on short-term efficacy and dosimetry. Frontiers in Oncology. 2024 14
Author contact
![]() Corresponding authors: Letao Lin, linltorg.cn; Fujun Zhang, zhangfjorg.cn.
Corresponding authors: Letao Lin, linltorg.cn; Fujun Zhang, zhangfjorg.cn.

 Global reach, higher impact
Global reach, higher impact