Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(15):4270-4283. doi:10.7150/jca.119757 This issue Cite
Review
Molecular Mechanisms and Novel Therapeutics Targeting Ferroptosis in Gastric Cancer: A Literature Review
1. Graduate Institute of Health Industry Technology, Center for Drug Research and Development, College of Human Ecology, Chang Gung University of Science and Technology, Taoyuan 333, Taiwan.
2. Department of Neurology, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan.
3. Department of Chemical Engineering, R&D Center of Biochemical Engineering Technology, Ming Chi University of Technology, New Taipei City 301, Taiwan.
4. Department of General Surgery, New Taipei Municipal TuCheng Hospital, New Taipei 236, Taiwan.
5. College of Medicine, Chang Gung University, Taoyuan 333, Taiwan.
6. Department of General Surgery, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan.
7. Department of Nursing, Division of Basic Medical Sciences, Chang-Gung University of Science and Technology, Taoyuan 333, Taiwan.
† Authors contributed equally to this work.
Received 2025-6-17; Accepted 2025-10-7; Published 2025-10-20
Abstract
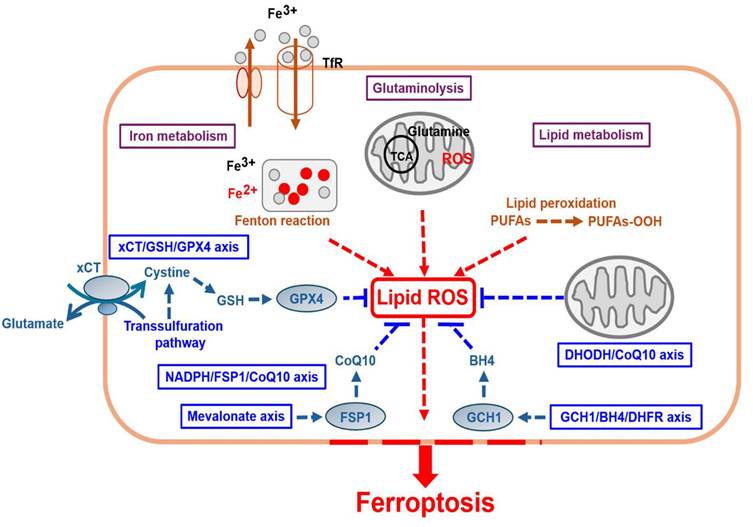
Since the discovery of ferroptosis, which plays an important role in gastric cancer (GC), its activation has been crucial for developing tumor therapeutic strategies. Recently, ferroptosis activation has become a research hotspot for GC treatment approaches. Energy and metabolism dysfunctions involving lipids, amino acids, iron, sugars, and nucleotides caused by GC cells in a typical hypoxic microenvironment are important disease characteristics. However, the immune escape mechanism of GC cells limits the occurrence of programed cell death, a controllable form of which is ferroptosis. First, excessive reactive oxygen species production induces changes in intracellular iron ion levels, resulting in an imbalance in the antioxidant defense system. Finally, excessive accumulation of intracellular lipid peroxidation byproducts destroys cell membrane consistency and causes cell death. The promotion of ferroptosis in GC cells has been widely employed as a method for inhibiting tumor growth and chemotherapy resistance, which is helpful for developing anti-GC targeted treatments. Because GC cells are sensitive to ferroptosis-inducing agents, some traditional antitumor drugs (e.g., cisplatin) and Chinese herbal or natural medicines (e.g., artemisinin) exert anticancer effects by inducing this process. In this article, we summarize the basic molecular mechanisms underlying ferroptosis and the involved tumor markers, along with associated chemotherapy drugs and natural medicines. To activate ferroptosis in GC, new targeted drug therapies can be used within the clinical treatment field to kill GC cells and enhance tumor sensitivity to chemotherapy.
Keywords: ferroptosis, gastric cancer, iron, biomarker, reactive oxygen species, antioxidant, redox, lipid peroxidation
Introduction
In 2003, Dr. B.R. Stockwell first discovered ferroptosis while screening for small-molecule drugs (e.g., erastin), which selectively kill tumor cells [1]. In 2012, Dr. Dixon officially named it “ferroptosis” because the process involves iron dependency. Ferroptosis is classified as regulated cell death (RCD) by various intracellular small-molecule inducers and inhibitors [2].
Ferroptosis involves the activation of several energy and metabolic processes and substances, such as iron, amino acids, and polyunsaturated fatty acids, leading to intracellular redox imbalance and dysregulated generation and degradation of lipid reactive oxygen species (ROS) [3] (Fig. 1 and Table 1). Recent studies, including cancer research, have shown that ferroptosis can participate in gastric cancer (GC) progression, where pro- or anti-ferroptotic pathways are involved [4]. This study discusses the mechanisms underlying ferroptosis and summarizes recent research findings to provide a reference for ferroptosis-based anti-GC strategies and contribute to the future development of new therapeutic approaches in clinical practice.
In this review, PART I delineates the molecular mechanisms underlying ferroptosis, with particular emphasis on the reprogramming of iron, lipid, and amino acid metabolism in GC cells. PART II identifies ferroptosis-related proteins as potential biomarkers, highlighting their clinical relevance in disease progression and poor prognosis. PART III explores the therapeutic potential of inducing ferroptosis through conventional chemotherapeutic agents, while PART IV extends this perspective to Chinese herbal medicines and natural compounds, underscoring their promise in overcoming chemoresistance. Finally, PART V addresses the remaining challenges of ferroptosis-based anti-GC therapy. Collectively, these sections underscore both the opportunities and the challenges that must be resolved to ensure the successful translation of ferroptosis-targeted strategies from experimental research into clinical application.
PART I: The molecular mechanisms underlying ferroptosis activation in tumor cells (lipid peroxidation, intracellular iron accumulation, and loss of antioxidant defense)
1) Iron metabolism dysregulation (intracellular iron accumulation)
Iron metabolism is important for some cellular activities, including oxygen transport, respiration, and DNA synthesis [5]. Iron is a key cofactor of cellular growth and metabolism. Ferric iron (Fe³⁺), which is carried by transferrin in circulation, is endocytosed into cells after binding to transferrin receptor 1 (TfR1) on an unaffected cell membrane [6]. Within cells, ferric iron undergoes reduction to ferrous iron (Fe²⁺) and released into cytoplasm, and excessive iron is stored in ferritin (FE) [7]. The latter is a universal intra- and extra-cellular protein that stores iron and is stored in ferroportin (FPN/iron transporter) [8-10]. Free iron ions are released from ferritin degradation via NCOA4-dependent ferritinophagy [11]. Iron primarily circulates through continuous redox cycling between Fe²⁺ and Fe³⁺ through the nonenzymatic Fenton reaction [12], leading to excess redox-active iron.
Ferroptosis-related proteins and molecular mechanisms.
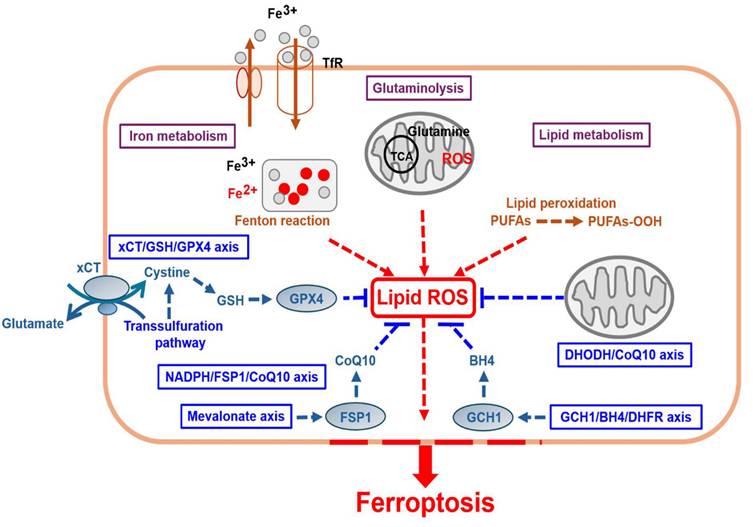
However, several tumor cells have dysregulated iron metabolism because of their rapid growth, with a higher overall intracellular iron concentration [13]. Tumor cells increase iron uptake and storage, while reducing iron efflux. Moreover, iron can produce excessive ROS through the Fenton reaction, activating lipid peroxidation and making tumor cells more susceptible to ferroptosis. Therefore, various iron metabolism pathways and organelles (mitochondria or lysosomes) can modulate ferroptosis [14]. Consequently, iron chelators [15] can inhibit ferroptosis, whereas iron-loaded nanoparticles [16] can induce ferroptosis in tumor cells.
2) Lipid metabolism (lipid ROS accumulation, redox imbalance, and lipid peroxidation of polyunsaturated fatty acids (PUFAs) leading to membrane damage)
In unaffected cells, glucose enters the cell to undergo glycolysis to produce pyruvate. Subsequently, pyruvate enters mitochondria and undergoes catalysis into tricarboxylic acid (TCA). The intermediates produced in this process contribute to lipid synthesis and the production of PUFAs. Iron-containing enzymes, such as arachidonate lipoxygenase (ALOX), mediate the lipid peroxidation pathway and promote the oxidation of free PUFAs. In cell membranes, PUFAs are transformed by acyl-coenzyme A synthetase long-chain family member 4 (ACSL4) into PUFA-CoA, which is then further catalyzed by lysophosphatidylcholine acyltransferase 3 (LPCAT3) into PL-PUFAs [17]. However, in cancer cells, redox imbalance leads to excessive ROS production, further driving the peroxidation of specific membrane lipids. PL-PUFAs are highly susceptible to peroxidation by LOX, in which they are converted to phospholipid hydroperoxide (PLOOH). Excess lipid ROS, catalyzed by ALOX5, reacts with free radicals to form lipid peroxides, finally activating ferroptosis. Therefore, any factors that affect the expression and activity of ALOX5 can control the occurrence of ferroptosis [18]. Furthermore, controlling the synthesis or degradation of PUFAs influences the cellular sensitivity to it. Moreover, the activity of iron-containing enzymes depends on iron availability, and the latter can also promote nonenzymatic lipid auto-oxidation through the Fenton reaction. If cells fail to effectively destroy peroxidized lipids, membrane damage occurs, leading to cell death [19]. Iron chelators (iron scavengers) serve as effective inhibitors of lipid peroxidation and contribute to the occurrence of anti-ferroptosis [20].
3) Glutaminolysis
Glutaminolysis is a cellular metabolic process in which glutamine is converted into α-ketoglutarate (α-KG), involved in the TCA cycle to provide energy and metabolic intermediates. Recent studies have shown that glutaminolysis plays an essential role in regulating ferroptosis, primarily by influencing intracellular ROS, glutathione (GSH), and lipid peroxidation, thereby modulating cell survival and death. Glutaminolysis plays a dual role in regulating ferroptosis because it can promote lipid peroxidation progressing to ferroptosis and suppress the latter through antioxidant mechanisms. Regulating glutaminolysis can therefore be considered a new strategy for promoting ferroptosis, while providing new research directions for cancer treatment based on different tumor types and metabolic characteristics [21].
4) The classic xCT/GSH/GPX4 axis involves glutathione/glutathione peroxidase 4 metabolism (loss of antioxidant defense and redox imbalance)
In hypoxic microenvironments, tumor cells can generate energy using cystine in addition to glycolysis. Cystine/glutamate (Gln) is transported into cells via the Xc-(solute carrier family 3 member 2/SLC3A2) [22]/xCT (solute carrier family 7 member 11/SLC7A11) [2] antiporter, and it is transformed into cysteine by the action of glutaminase (GLS), producing NADPH. Subsequently, GPX4 can promote the formation from GSH to glutathione disulfide (GSSG) and NADPH to NADP+ while directly converting endogenous phospholipid hydroperoxide (PLOOH) into harmless PL-OH [23]. Therefore, both xCT (SLC7A11) and GPX4 exert crucial duplicated effects on antioxidant defense and ferroptosis inhibition by regulating intracellular GSH accumulation [24].
The second key component, the thioredoxin reductase 1 (TXNRD1)/thioredoxin (TXN)-dependent antioxidant system, mediates the reduction of cystine to cysteine through trans-sulfuration [25]. In certain malignancies, the TXN-dependent cellular system is commonly activated, and inhibiting GSH and TXN pathways is a well-established method for effectively triggering cell death. In some cancer cells types, the trans-sulfuration pathway is continuously activated to increase cysteine levels [26]. The enzymes involved in this pathway, including glycine N-methyltransferase (GNMT), S-adenosylhomocysteine hydrolase (SAHH), cystathionine β-synthase (CBS), and cystathionine gamma-lyase (CTH), sensitize cancer cells to ferroptosis and slow down tumor progression [27]. Furthermore, GSH helps regulate the redox state within cancer cells, reducing their sensitivity to ferroptosis. Furthermore, some reports have indicated that amino acid transport (e.g., glutamine/Glu/SLC1A5/ASCT2/SLC38A1) [28, 29] and α-KG promote the TCA cycle, which increases ROS production and facilitates ferroptosis [30].
5) GPX4-independent regulatory pathwaysUnlike GPX4, which acts as a central suppressor of ferroptosis, the following four pathways regulate ferroptosis independently of GPX4
a) Ferroptosis suppressor protein (FSP1)/coenzyme Q10 (CoQ10/Ubiquinone)/nicotinamide adenine dinucleotide phosphate (NAD(P)H) axis
FSP1 is a CoQ oxidoreductase that protects cells from ferroptosis [31]. Mechanistically, FSP1 possesses NADH-CoQ reductase activity, enabling the reduction of CoQ10 and NADPH to form CoQ10H2 (ubiquinol) and NADP+. This process directly reduces lipid radical formation or promotes the antioxidant regeneration of α-tocopherol (α-TOH/vitamin E), thereby inhibiting lipid peroxidation and ferroptosis. FSP1 exerts a concomitant inhibitory effect with GPX4 on ferroptosis [32, 33].
b) Mevalonate (MVA) axis
The MVA pathway is an integral part of ferroptosis regulation via CoQ10 synthesis, cholesterol metabolism, and protein translation. CoQ10 and FSP1 can hinder ferroptosis via antioxidant mechanisms, whereas cholesterol metabolism imbalance can promote ferroptosis by affecting GPX4 stability. Therefore, the regulation of the MVA pathway can be a new therapeutic strategy for cancer or other diseases [34].
c) Dihydroorotate dehydrogenase (DHODH) axis
DHODH plays a significant role in ferroptosis regulation by representing a mitochondrial antioxidant defense mechanism. DHODH is engaged in the de novo pyrimidine synthesis pathway in the inner mitochondrial membrane, where it facilitates the oxidation of dihydroorotate to orotate and reduction of ubiquinone (CoQ10) to ubiquinol (CoQ10H2). Recent studies have suggested that DHODH can suppress ferroptosis in the mitochondria by inhibiting lipid peroxidation by maintaining CoQ10 in this form. This function is similar to that of FSP1, which operates in cytosol. DHODH inhibition has been shown to promote ferroptosis by promoting ferroptosis-mediated cell death, particularly in cells with low GPX4 activity, making it a potential therapeutic target [35].
d) GTP cyclohydrolase (GCH1)/tetrahydrobiopterin (THB/BH4)/DHFR/NADPH axis
The GCH1/DHFR/BH4 activation pathway plays a crucial protective role in ferroptosis regulation by inhibiting intracellular antioxidant mechanisms and lipid peroxidation, thereby preventing cell death. In unaffected cells, GCH1/BH4 activates phospholipid mono-unsaturation. This pathway influences the composition of phospholipid fatty acids, stimulating the synthesis and incorporation of MUFAs. The latter are more resistant to lipid peroxidation than PUFAs by reducing cellular susceptibility to ferroptosis. Subsequently, BH4 inhibits lipid peroxidation as an antioxidant. BH4 is a potent antioxidant molecule that directly scavenges lipid peroxyl radicals, reducing lipid peroxidation accumulation and thereby inhibiting ferroptosis. Studies have reported that high GCH1 expression promotes BH4 synthesis, thus making cells more resistant to ferroptosis [36]. DHFR maintains the BH4 cycle and enhances antioxidant capacity. DHFR is involved in the recycling of BH4, thereby ensuring stable intracellular BH4 levels and maintaining its antioxidant function. DHFR inhibition decreases BH4 levels, making cells more prone to lipid peroxidation and ferroptosis. GCH1 overexpression and ferroptosis resistance then occur in cancer cells [37]. Studies have found that GCH1 upregulation in certain cancer cells increases BH4 levels, thus enhancing resistance to ferroptosis. This suggests that the GCH1/BH4 pathway can be a cancer treatment target. Inhibiting GCH1 or BH4 can increase ferroptosis sensitivity in tumor cells, thereby enhancing therapeutic efficacy. The GCH1/DHFR/BH4 activation pathway regulates ferroptosis through antioxidant mechanisms and phospholipid composition modifications [38]. Activation of this pathway prevents lipid peroxidation and ferroptosis, whereas its inhibition can enhance ferroptosis sensitivity, providing new strategies for cancer treatment.
PART II: Ferroptosis-related biomarkers in GC
Early diagnostics of GC is complicated, causing high recurrence and mortality rates, along with low therapeutic response rates. Therefore, determining biomarkers that assist in GC diagnostics and targeted treatment is particularly important. Currently, biomarkers associated with ferroptosis (Table 2) are associated with the detection of GC.
Ferroptosis-related proteins and molecular mechanisms.
| Biological category | Genes name | Proteins name | Effect on ferroptotic types | Mode of action | Reference | |
|---|---|---|---|---|---|---|
| Iron metabolism | DMT1 | Divalent metal transporter1 | Induces | Mediates the release of Fe2+ into a labile iron pool | [7] | |
| FPN | Ferroportin | Induces | Increasing iron-dependent lipid ROS accumulation | [10] | ||
| FTH1 | Ferritin heavy chain1 | Induces | Iron accumulation Stores intracellular iron | [9] | ||
| STEAP3 | Six-transmembrane epithelial antigen of prostate3 | Induces | Converts Fe3+ to Fe2+ | [12] | ||
| TfR1 | Transferrin receptor1 | Induces | Iron accumulation Imports iron into cells | [6] | ||
| Lipid metabolism | ACSL4 | Acyl-CoA synthetase long-chain family member 4 | Induces | Lipid ROS accumulation convertsAA/AdA intoAACoA/AdA CoA | [17] | |
| LOX | Lipoxygenases | Induces | Lipid peroxidation accumulation Generate lipid ROS by oxidizing AA-PE and AdA-PE | [18] | ||
| Glutaminolysis (glutamate uptake) | ASCT2 (SLC1A5) | Glutamine transporter | Inhibits | Glutamate/cystine transportation | [28] | |
| SLC38A1 | Glutamine transporter | Inhibits | Glutamate/cystine transportation | [28, 29] | ||
| Amino acid metabolism (cystine uptake) GPX4-dependent antioxidant axis | GCL | Glutamate-cysteine ligase | Inhibits | Cysteine and glutamate to form the dipeptide gamma-glutamyl cysteine (γ-GC) | [39] | |
| GPX4 | Glutathione peroxidase 4 | Inhibits | Prevents lipids hydroperoxide formation | [23] | ||
| GSR | Glutathione reductase | Inhibits | Catalyzes the reduction of GSSG to GSH using electrons provided by NADPH/H+ | [40] | ||
| SLC3A2 | Solute carrier family 3 membrane 2 | Inhibits | Maintains SLC7A11stability | [22] | ||
| SLC7A11 | Solute carrier family 7 membrane 11 | Inhibits | Promotes cystine uptake | [2] | ||
| TXNRD1 | Thioredoxin reductase 1 | Inhibits | Reduces thioredoxin and activates Trans-sulfuration pathway | [25] | ||
| GPX4-independent antioxidant axis | ||||||
| Ⅰ. | FSP1/CoQ10/NAD(P)H axis | CoQ10 | Coenzyme Q10 | Inhibits | Mitochondrial oxidative phosphorylation | [32] |
| FSP1 | Ferroptosis suppressor protein-1 | Inhibits | Parallel to the GSH-GPX4 pathway | [33] | ||
| Ⅱ. | Mevalonate pathway | HMG-CoA reductase | 3-hydroxy-3-methylglutaryl-CoA reductase | Inhibits | GPX4 maturation | [41] |
| Ⅲ. | DHODH axis | DHODH | Dihydroorotate dehydrogenase | Inhibits | Coordinates with GPX4 to block ferroptosis in the mitochondrial inner membrane by reducing ubiquinone to ubiquinol | [35] |
| Ⅳ. | GCH1/BH4/DHFR/NAD(P)H axis | THB/BH4 | Tetrahydrobiopterin | Inhibits | BH4 synthesis is a critical pathway involved in GPX4 inhibition | [36] |
| DHFR | Dihydrofolate reductase | Inhibits | Reduces dihydrofolic acid to tetrahydrofolic acid | [38] | ||
| GCH1 | GTP Cyclohydrolase 1 | Inhibits | Increases antioxidant BH4 and CoQ10 abundance | [37] | ||
1. High expression of TfR1
Because of the increased iron demand of GC cells, the ability of GC cells to acquire iron depends on the number of TfR1s. Although tumors often upregulate TfR1 to acquire iron, they also upregulate ferritin to sequester iron and reduce free iron levels. Furthermore, their activation can promote the storage of free iron, thereby inducing ferroptosis [42]. Dysregulation or dysfunction of the iron transport system is considered an important factor for triggering ferroptosis.
2. Low expression of lipoxygenases 15 (LOX15)
Recently, Zhang et al. [43] found that exosomes containing miR-522 released by cancer-associated fibroblasts in the GC tumor microenvironment inhibit ferroptosis in GC cells by acting on LOX15, thereby promoting tumor growth and reducing GC sensitivity to chemotherapy with cisplatin and paclitaxel.
3. Low expression of cysteine dioxygenase 1 (CDO1)
CDO1 promotes the oxidation of cysteine. Hao et al. [44] found that CDO1 affects ferroptosis by limiting cysteine synthesis for that of GSH. The low CDO1 expression in GC cells may lead to the upregulation of GPX4 levels in GC cells, thereby inhibiting ferroptosis.
4. High expression of perilipin 2 (PLIN2)
PLIN2 is a protein associated with intracellular lipid metabolism and can act as an oncogene. PLIN2 participates in the regulation of the ferroptosis pathway in GC cells through genes, such as PRDM11 and IPO7. Its overexpression leads to the formation of a relatively hypoxic intracellular environment, which in turn inhibits ferroptosis and facilitates GC progression [45].
5. High expression of stearoyl-CoA desaturase 1 (SCD1)
SCD1 is a key enzyme in cellular fatty acid conversion and is a known oncogene in GC. SCD1 is highly expressed in GC tissues and can promote the proliferation and migration capabilities of GC cells. Studies have reported that in GC, SCD1 elevates the expression levels of ferroptosis inhibitors, such as GPX4 and SLC7A11, thereby increasing the resistance of GC cells to ferroptosis. SCD1 suppression significantly activates ferroptosis and hinders the growth of GC cells, revealing the mechanism of action of the oncogene SCD1 from the perspective of ferroptosis [46].
6. High expression of glutathione peroxidase 4 (GPX4)
GPX4 is a crucial inhibitor of ferroptosis and is commonly used as a biomarker for antioxidant systems in various disease models. The data analysis by Wang et al. [47] on the expression of GPX4 in GC suggests that the transcriptional level (mRNA) of GPX4 is significantly higher in GC tissues and indicates poor prognosis.
7. High expression of solute carrier family 7 member 11 (SLC7A11/Xc)
SLC7A11 is a key gene that inhibits ferroptosis. It plays an important role in preserving redox homeostasis. Although SLC7A11 primarily exerts a tumor-promoting effect, its overexpression also exposes lethal vulnerability in cancer cells, making them more sensitive to glucose or glutamine starvation [48].
8. Low expression of FSP1
Iron-dependent oxidative damage to cell membrane is a characteristic of ferroptosis, and this can be counteracted by FSP1 [49].
9. High expression of activated transcription factor 2 (ATF2)
Recent studies have shown that ATF2 is noticeably upregulated in GC tissues, which indicates poorer clinical prognosis. In particular, ATF2 suppresses sorafenib-provoked ferroptosis, thereby reducing a drug's anticancer efficacy. ATF2 negatively regulates ferroptosis by upregulating heat shock protein 110 (HSPH1/HSPH105/HSPH110), which stabilizes SLC7A11 and prevents ferroptotic cell death [50].
10. High expression of Aurora kinase A (AURKA)
AURKA was found to be upregulated in GC and is a poor prognostic factor. Loss of ARID1A expression is associated with poor survival rates in GC. Downregulation of miR-4715-3p leads to AURKA overexpression, maintaining GPX4 levels and suppressing ferroptosis; restoring miR-4715-3p or inhibiting AURKA downregulates GPX4 and induces GC cell death [51].
11. High expression of B-cell receptor-associated protein 31 (BAP31)
BAP31 is involved in tumor progression, whereas the roles and action of BAP31 in GC remain widely unclear. We detected BAP31 upregulation in GC, which indicates poor survival rates. BAP31 knockdown suppressed cell growth and promoted G1/S arrest. Moreover, BAP31 attenuation elevated the lipid peroxidation level of a membrane and promoted cellular ferroptosis. Mechanistically, BAP31 modulated cell proliferation and ferroptosis by directly binding to VDAC1 with further oligomerization and polyubiquitination. HNF4A was linked to BAP31 as a promoter and promoted transcription. Furthermore, the knockdown of BAP31 tended to make GC cells sensitive to 5-fluoruracil (5-FU) and the ferroptosis inducer, erastin, in vivo and in vitro [52]. Our review suggests that BAP31 can be a predictive factor for GC; furthermore, we recommend the elaboration of its treatment strategy.
12. Low expression of E-cadherin (CDH1)
In diffuse-type GC (DGC) cells, loss-of-function mutations in E-cadherin lead to higher sensitivity to nonapoptotic, iron-dependent types of cell death, such as ferroptosis. Homotypic interactions between its molecules and cells suppress ferroptosis by activating the Hippo pathway. Furthermore, single-nucleotide mutations detected in DGC abrogate the homophilic binding ability of the molecular marker, thereby reducing its capability to inhibit ferroptosis in cell culture and xenograft models. Importantly, although its loss in malignant cells is a key factor for epithelial-mesenchymal transition (EMT) and subsequent metastases, we found that circulating DGC cells lacking E-cadherin expression decreased metastatic potential due to higher susceptibility to ferroptosis. In summary, this study suggests that E-cadherin is a biomarker that enables prognosis determination for the primary tumor and circulating DGC cell sensitivity to ferroptosis, serving as a guide for future therapeutic schemes aimed at promoting ferroptosis [53].
13. High expression of CDGSH iron sulfur domain 1 (CISD1)
CISD1/mitoNEET and CISD2 hinder ferroptosis by decreasing mitochondrial iron uptake. CISD1 is a member of the CDGSH domain-containing family and can participate in the modulation of mitochondrial oxidative capacity. Overexpression of CISD1 can reduce mitochondrial iron accumulation and increase resistance to ferroptosis [54].
14. High expression of doublecortin-like kinase 3 (DCLK3)
DCLK3 is associated with poor prognoses in GC and modulates ferroptosis and mitochondria functioning in vitro and in vivo. DCLK3 was found to be upregulated in GC, and high DCLK3 expression is significantly correlated with low survival rates in GC. Here, DCLK3 knockdown lowered GC cell proliferation, provoked ferroptotic cell death, and increased oxidative stress levels. Logistic regression analysis revealed that TCF4 was an independent prognostic factor for GC. In particular, DCLK3 facilitated higher TCF4 expression and that of TCF4 downstream target genes (c-Myc and cyclin D1). Furthermore, DCLK3 overexpression promoted GC cell proliferation while decreasing ferroptotic cell death and oxidative stress. The underlying mechanism may involve the upregulation of TCF4, c-Myc, and cyclin D1. Our study suggests that DCLK3 regulates the levels of iron and reactive oxygen and is engaged in the TCF4 pathway, thereby facilitating GC cell growth. This indicates that DCLK3 can serve as a prognostic marker and therapeutic target in GC [55].
15. Low expression of glutaminase 2 (GLS2)
GLS2 activates ferroptosis by converting glutamate, which is a known suppressor of GC cell growth, which thus indicates poor prognosis in GC. Studies have reported the significant role of GLS2 as a tumor suppressor in malignancies, such as GC [56].
16. High expression of microsomal glutathione S-Transferase 1 (MGST1)
MGST1 expression indicates poor prognosis, activating the Wnt/β-catenin pathway by modulating AKT and hindering ferroptosis in GC. MGST1 expression was higher in GC and exhibited a correlation with low overall survival rates [57].
17. High expression of nuclear factor-erythroid 2 (NRF2)
High expression of NRf2, a transcription factor, may have a dual impact on the prognosis of patients with gastric adenocarcinoma. Ferroptosis may participate in tumor formation and affect the resistance of particular cancers, including GC, to medications [58].
18. Low expression of polycyanin B (PB)
A new GPX4 inhibitor called PB has been demonstrated to inhibit the expression of GPX4 in GC cells, leading to their death. Polyphyllin B hinders tumor growth by regulating iron metabolism and promoting ferroptosis [59].
19. High expression of SLC1A5, ANGPTL4, and CGAS (ferroptosis-related genes/FRG signature)
A novel FRG signature that could make a prognosis and show tumor immune microenvironment was constructed. In our study, a novel ferroptosis-related signature was created to predict outcomes in patients with GC. The ferroptosis-related signature reflected ferroptosis in GC, and with univariate Cox regression analysis, survival genes were evaluated to elaborate a prognostic model involving three genes (i.e., SLC1A5, ANGPTL4, and CGAS), which could predict survival rates in GC [60].
20. High expression of signal transducer activator of transcription 3 (STAT3)
STAT3 plays a critical role in oncogenic signaling and is activated in many types of human cancer. As an oncogene, STAT3 is a major signal transduction pathway involved in multiple cellular processes (e.g., proliferation and survival) [61]. Additionally, STAT3 is a key negative regulator of ferroptosis in GC. Inhibition of STAT3 downregulates antioxidant and iron homeostasis genes (such as SLC7A11, GPX4, and FTH1), induces lipid peroxidation and Fe²⁺ accumulation, and reverses 5-FU chemoresistance [62].
21. High expression of zinc finger protein 36 homolog (ZFP36)
Genetic and pharmacological inhibition of STAT3 triggers ferroptosis by the transcriptional regulation of GPX4, SLC7A11, and FTH1 in GC [63].
In summary, if there is overexpression of amino acid antioxidants, or the low expression of lipid peroxidation-promoting enzymes, the effect is considered to be the same: the inhibition of ferroptosis. These findings reflect specific molecular targets and theoretical references for those of redox homeostasis to prevent and treat ferroptosis-related diseases.
Overview of the roles of ferroptosis-related biomarkers in GC.
| Biological pathways | Biomarker genes | Biomarker proteins | Regulator of pro-/antiferroptotic proteins | Mode of action | Expression in GC tissue | Type of biomarker | Reference |
|---|---|---|---|---|---|---|---|
| Iron metabolism | TfR1 | Transferrin receptor | Activator | Iron accumulation | High expression | Prognosis | [42] |
| Lipid metabolism | LOX15 | Lipoxygenases15 | Antiferroptotic proteins | ●miR-522 target ●Depletes lipid ROS ●Chemoresistance | Low expression | Prognosis Chemoresistance | [43] |
| CDO1 | Cysteine dioxygenase1 | Antiferroptotic proteins | Enhance antioxidant defense | Low expression | Prognosis | [44] | |
| PLIN2 | Perilipin 2 | Antiferroptotic proteins | Lipid ROS accumulation | High expression | Prognosis | [45] | |
| SCD1 | Stearoyl-CoA desaturase1 | Antiferroptotic proteins | Enhance antioxidant defense | High expression | Prognosis Development | [46] | |
| xCT/GSH/GPX4-dependent antioxidant axis | GPX4 | Glutathioneperoxidase4 | Antiferroptotic proteins | Enhance antioxidant defense | High expression | Prognosis | [47] |
| SLC7A11 (Xc) | Solute carrier family7 membrane11 | Antiferroptotic proteins | Enhance antioxidant defense | High expression | Prognosis | [48] | |
| GPX4-independent antioxidant axis | FSP1 | Ferroptosis suppressor protein-1 | Antiferroptotic proteins | Enhance antioxidant defense | High expression | Prognosis | [49] |
| Other metabolism | ATF2 | Activating transcription factor 2 | Pro-ferroptotic proteins | miR-496 target | High expression | Prognosis | [50] |
| AURKA | Aurora Kinase A | Pro-ferroptotic proteins | miR-4715-3p target | High expression | Prognosis | [51] | |
| BAP31 | B-cell receptor-associated protein 31 | Antiferroptotic proteins | Interaction with Voltage-Dependent Anion Channel 1 (VDAC1), regulates ferroptosis. | High expression | Prognosis | [52] | |
| CDH1 | E-cadherin | Pro-ferroptotic proteins | Activation of Hippo signaling pathway | Low expression | Prognosis | [53] | |
| CISD1 | CDGSH iron sulfur domain 1 | Antiferroptotic proteins | Regulation of mitochondrial iron metabolism | High expression | Prognosis | [54] | |
| DCLK3 | Doublecortin-Like Kinase 3 | Antiferroptotic proteins | Oxidative stress (mitochondria) | High expression | Prognosis | [55] | |
| GLS2 | Glutaminase 2 | Antiferroptotic proteins | miR-103a-3p target | Low expression | Development | [56] | |
| MGST1 | Microsomal Glutathione S-Transferase 1 | Antiferroptotic proteins | Enhancing the Wnt/β-Catenin Pathway | High expression | Prognosis | [57] | |
| NRF2 | Nuclear factor-erythroid 2 | Antiferroptotic proteins | ●Reduce ROS accumulation ●Depletes lipid peroxidation | High expression | Prognosis | [58] | |
| PB | Polycyanin B | Pro-ferroptotic proteins | Regulate the expression of LC3B, TFR1, NOCA4, and FTH1 | Low expression | Prognosis | [59] | |
| SLC1A5, ANGPTL4, CGAS | Ferroptosis-related genes/FRG signature | Pro-ferroptotic proteins | Erastin-induced | High expression | Prognosis | [60] | |
| STAT3 | Signal transducer and activator of transcription 3 | Pro-ferroptotic proteins | miR-125b-5p target | High expression | Prognosis | [61] | |
| ZFP36 | Zinc finger protein 36 homolog | Pro-ferroptotic proteins | Erastin-induced | High expression | Prognosis | [63] |
PART III: Ferroptosis-related chemotherapeutic drugs for GC
Recently, the relationship between chemotherapy action and ferroptosis has been considered an important topic in cancer research. Certain chemotherapeutic drugs can enhance anticancer effects by inducing lipid peroxidation, affecting GSH metabolism, or inhibiting antioxidant systems to promote ferroptosis. Currently, those commonly used in GC treatment are mainly represented by platinum-based fluoropyrimidines and taxanes. However, multidrug resistance in patients with GC remains a major issue in chemotherapy. Cancer cells may undergo both apoptosis and ferroptosis simultaneously. As ferroptosis represents a novel mode of RCD that is completely not associated with apoptosis, it provides a new approach for overcoming chemotherapy drug resistance. Targeting ferroptosis to counteract chemotherapy resistance and integrating it within other therapeutic strategies represents a promising method for improving cancer treatment outcomes. Table 3 presents the chemotherapy drugs associated with ferroptosis in GC.
1. Platinum-based chemotherapy (cisplatin and oxaliplatin)
Studies have demonstrated that ATF3 inhibits the Nrf2/Keap1/xCT signaling pathway. Therefore, targeting this pathway with the aim to induce ferroptosis leads to increased intracellular ROS levels, depletion of intracellular GSH, or downregulation of GPX4, thus promoting lipid peroxidation accumulation. This helps overcome cisplatin resistance because GC cells are sensitized to cisplatin through ferroptosis [64, 65]. Another chemotherapy drug, oxaliplatin, may act on ferroptosis through the miR-181a-5p/SIRT1 pathway [66, 67].
2. Paclitaxel-based (sorafenib)
Sorafenib is a tyrosine kinase inhibitor that promotes ferroptosis through the SLC7A11-related pathway mediated by the downstream target gene HSPH1 of ATF2. This further supports the potential of chemotherapy combined with ferroptosis-targeted therapeutic strategies. Furthermore, silencing sirtuin 6 (SIRT6) inactivates the Keap1/Nrf2 signaling pathway, which helps overcome sorafenib resistance [50].
3. 5-FU-based (capecitabine and 5-FU)
ADP-ribosylation factor 6 (ARF6) regulates erastin-induced ferroptosis and lipid peroxidation. Inhibiting ARF6 can reduce capecitabine resistance in GC cells. Another chemotherapy drug, 5-FU, promotes ROS-mediated ferroptosis. Drug resistance in cancer cells is a major cause of treatment failure. Numerous studies have indicated that drug resistance mechanisms in GC are often associated with dysregulated iron metabolism, antioxidant pathways, and prolonged exposure to ROS at excessive levels, along with the accumulation of lipid peroxides [68]. Subsequently, ferroptosis resistance is considered a key molecular mechanism contributing to tumor drug resistance [69].
PART IV: Ferroptosis-related Chinese herbal or natural medicines for GC
Recent studies have found that traditional Chinese medicines (TCMs) and their natural byproducts are involved in ferroptosis pathways in tumor cells and can regulate ferroptosis in GC cells, ultimately achieving therapeutic effects. If TCM-derived compounds-induced ferroptosis is prevented by ferrostatin-1, liproxstatin-1, or deferoxamine (an iron chelator) but not by pan-caspase inhibitors (such as Z-VAD-FMK), and is associated with lipid peroxidation, GPX4 inhibition, iron dependence, and distinct mitochondrial morphological alterations, it provides direct evidence of ferroptosis and represents the standard for distinguishing ferroptosis from apoptosis, pyroptosis and necroptosis [2, 70]. Herbal medicine has unique advantages in cancer treatment, including multiple targets, fewer side effects, strong efficacy, lower economic burden, structural stability, high safety, low cost, and easy accessibility. In addition to prolonging patients' survival, it also improves their quality of life, and its clinical efficacy has been increasingly recognized by both medical professionals and patients. The pathways associated with ferroptosis in GC cells and the mechanisms through which herbal medicine exerts its effects, are worth further exploration to provide theoretical support for clinical GC treatment.
Chemotherapeutic drug-related ferroptosis for GC.
| Chemotherapy drugs used for GC treatment | Chemotherapy drug names | Pharmacological effects | Mode of action | Reference |
|---|---|---|---|---|
| Platinum-based | Cisplatin | Binds to DNA, affecting the synthesis of DNA, RNA, and protein | Silencing SIRT6 affects the Keap1/Nrf2 signaling pathway | [64, 65] |
| Platinum-based | Oxaliplatin | Thymidylate synthase | ●miR-181a-5p may influence ferroptosis by regulating SIRT1 ●SIRT1-induced ferroptosis may be associated with GPX4 downregulation | [66, 67] |
| Paclitaxel-based | Sorafenib | Multi-tyrosine kinase inhibitors | ●HSPH1 regulated by ATF2 plays a role in controlling ferroptosis. ●ATF3 inhibits the Nrf2/Keap1/xCT signaling pathway | [50] |
| 5-FU-based | Capecitabine | DNA synthesis inhibitor | ARF6 silencing enhances erastin-induced ferroptosis and lipid peroxidation | [68] |
| 5-FU-based | 5-FU | DNA synthesis inhibitor | Promotes ROS-mediated ferroptosis | [69] |
Considering recent research on the relationship between TCM and ferroptosis in GC cells, this review focuses on how herbal medicine regulates this process in GC treatment. It aims at providing new insights into therapeutic strategies for GC. Currently, drug therapy remains the primary approach in cancer treatment. However, acquired drug resistance continues to be a major challenge in clinical practice. Combination therapy can enhance drug efficacy, reduce side effects, and slow or prevent the development of resistance. Studies have shown that certain Chinese herbal medicines and their active components possess both anticancer and chemosensitizing effects, significantly increasing the sensitivity of tumor cells to chemotherapy and molecular-targeted drugs, thus improving therapeutic outcomes and reducing adverse reactions. Table 4 summarizes the mechanisms and applications of Chinese herbal medicines and their natural byproducts involved in targeting ferroptosis in cancer treatment, offering new insights into cancer therapy and overcoming drug resistance.
1. Amentoflavone (AF), a natural bioflavonoid compound, suppresses GC cell proliferation and promotes ferroptotic cell death. In particular, AF upregulates miR-496, which directly targets and downregulates transcription factor 2 (ATF2). ATF2 inhibits ferroptosis; thus, its suppression by miR-496 facilitates ferroptotic processes in GC cells. miR-496 inhibition reverses AF's effects, highlighting the critical role of the miR-496/ATF2 axis in mediating ferroptosis in GC (AGS and HGC-27) [71].
2. Metformin, a widely used antidiabetic medication, induces ferroptosis in GC cells through the disruption of the STAT1-PRMT1 axis in HGC-27 and AGS cells, inhibition of the Nrf2 pathway, activation of p53, and enhancement of chemosensitivity [72, 73].
3. Baicalein, a flavonoid compound derived from the TCM herb Scutellaria baicalensis, promotes ferroptosis in GC cells through multiple mechanisms, including the disruption of iron homeostasis, inhibition of antioxidant defense, activation of the p53 pathway, and enhancement of chemotherapy sensitivity. Baicalein regulated p53-mediated SLC7A11 downregulation, leading to lipid peroxidation accumulation. It also induced ferroptosis by reducing MDA levels and PTGS2 expression while increasing SGC-7901 cell survival [74].
4. Artemisinin, a sesquiterpene lactone derived from Artemisia annua, is renowned for its antimalarial properties. Recent studies have unveiled its potential in inducing ferroptosis in various cancer cells, including those in GC, by regulating iron metabolism, inducing oxidative stress, and having selective cytotoxicity [75].
5. The root of Chinese kiwifruit Actinidia chinensis Planch (ACP) is a well-known TCM that has been approved for use as an adjuvant treatment for various diseases, including leukemia and lung, gastric, colon, and liver cancers, apart from promoting apoptosis in cancer cells. ACP extract is rich in ursolic acid (UA) and oleanolic acid (OA). Gao et al. [76] found that the expression of GPX4 and SLC7A11 was significantly inhibited in HGC-27 cells treated with ACP, ultimately accelerating the occurrence of ferroptosis. These outcomes suggest that targeting ferroptosis can be a novel approach for treating GC. Interestingly, GC cells of different histological types may exhibit varying degrees of sensitivity to ferroptosis-targeting drugs, with mesenchymal GC stem cells expressing it more than intestinal-type GC cells [77]. In contrast, this may be attributable to the former containing more lipid-associated with ferroptosis; it is related to the increased activity of NRF2-dependent antioxidant pathways and SLC40A1 in intestinal-type GC cells.
6. Yiqi Jiedu Huayu decoction (YJHD) provokes ferroptosis in cisplatin-resistant GC cells by inhibiting the AKT/GSK3β/NRF2 signaling pathway, leading to decreased GPX4 expression and higher oxidative stress. This mechanism contributes to overcoming chemoresistance and highlights the potential of YJHD as an adjunctive therapy in GC treatment [78, 79].
7. Ophiopogonin B (OP-B), a natural compound extracted from Ophiopogon japonicus, promotes ferroptosis in GC cells through specific molecular mechanisms. Ophiopogonin B induces ferroptosis in GC cells by suppressing the GPX4/xCT antioxidant system, resulting in elevated lipid peroxidation and iron accumulation. This mechanism highlights OP-B's potential as a therapeutic agent targeting ferroptosis in GC [80].
8. Jiyuan oridonin A (JDA) is a natural compound obtained from the TCM herb Jiyuan Rabdosia rubescens. Its derivative, compound a2 (a2), provokes ferroptosis in GC cells through several mechanisms, including downregulation of GPX4, iron accumulation via autophagy, with efficacy superior to that of 5-FU and favorable pharmacokinetics [81].
9. Tanshinone IIA (Tan IIA), an extract from the TCM Salvia miltiorrhiza (Danshen), is widely used in clinical settings to treat cardiovascular diseases. Furthermore, it has shown antitumor activity in various human cancer cell lines, including liver, gastric, colon, and lung cancers. Recently, Guan et al. [82] discovered that ferroptosis is a mechanism through which Tan IIA exerts its antitumor effects in GC. In BGC-823 and NCI-H87 cells, Tan IIA promotes lipid peroxidation and downregulates the expression of SLC7A11, thereby inducing ferroptosis and inhibiting GC cell proliferation. The ferroptosis inhibitor Fer-1 attenuates its anticancer effects. Furthermore, Tan IIA can upregulate wild-type p53 expression, suppress SLC7A11 expression in GC cells, and reduce intracellular cysteine and GSH levels, ultimately leading to ferroptosis [82]. Furthermore, Tan IIA promotes GCSC stemness markers (e.g., Octamer binding transcription factor OCT3/4 (OCT3/4) and Aldehyde dehydrogenase 1 family member A1 (ALDH1A1)), which reduces chemoresistance and enhances chemotherapy sensitivity [83].
In summary, several TCMs and their natural byproducts contribute to ferroptosis in GC cells. These herbal medicines have shown good efficacy in treating GC corresponding to their indications. They primarily induce ferroptosis by downregulating GPX4 and SLC7A11 (xCT) and upregulating p53, promoting lipid peroxidation and iron ion accumulation. This provides new research directions and potential applications for GC treatment. Therefore, these herbal medicines may inhibit the proliferation of GC cells by modulating ferroptosis.
Part V: Further challenges of anti-GC therapy using ferroptosis
Recent studies on ferroptosis have enhanced our comprehension of its molecular mechanisms. In particular, the selective induction of ferroptosis in GC cells has become a prominent area, providing a new approach for cancer treatment. In this review, we elaborated on the mechanism underlying ferroptosis, including iron, lipids, amino acids, material metabolism, and nucleotides, whereas biomarker research on ferroptosis is of vital importance in the progression, diagnostics, treatment, and prognosis of GC.
Ferroptosis-related Chinese herbal medicine/natural medicine/traditional Chinese medicine (TCM) for GC.
| Class | Chinese herbal medicine/Natural medicine/ traditional Chinese medicine (TCM) | Active compound | Direct targets | Mode of action | Research model in vitro or in vivo (cell/animal) | Reference |
|---|---|---|---|---|---|---|
| Bioflavonoid | Selaginella tamariscina | Amentoflavone (AF) | miR-496/ATF2 axis | Increases ROS and lipid peroxidation, along with decreased GPX4 activity | AGS HGC-27 | [71] |
| Biguanides | Galega officinalis | Metformin | STAT1 | Disrupts the STAT1-PRMT1 axis | HGC-27 AGS | [72, 73] |
| Flavonoids | Huang Qin (Scutellaria baicalensis) | Baicalein | SLC7A11 p53 Fer-1 | ●p53-mediated SLC7A11 downregulation, leading to lipid peroxidation accumulation ●Baicalin-induced ferroptosis by reducing MDA levels and PTGS2 expression, improving cell survival | SGC-7901 | [74] |
| Sesquiterpene lactone | Artemisia annua (Artemisinin) | Artesunate (ART) Dihydroartemisinin (DHA) | p53 | Downregulates VEGF, upregulates calpain-2 expression | SGC-7901 BGC-823 MGC-803 AGS MKN74 | [84] [75] |
| TCM | Actinidia Chinensis Planch (ACP) | Ursolic acid (UA) Oleanolic acid (OA) | System Xc- GPX4 axis | Inhibits GPX4 and xCT expression, leading to ROS accumulation | HGC-27 | [76] [77] |
| TCM | Yiqi Jiedu Huayu decoction (YJHD) | ND | ACSL4 p53 | Downregulates GPX4 and SLC7A11 | HGC-27 Xenograft in zebrafish embryos | [78, 79] |
| Terpenes | Maidong (Ophiopogon japonicus) | Ophiopogonin B (OP-B) | GPX4 SLC7A11 | Inhibits GPX4/Xc-system, leading to lipid ROS accumulation and reduced lipid ROS scavenging | AGS NCI-N87 Xenograft in mouse model | [80] |
| Terpenes | Jiyuan Donglingcao (Rabdosia rubescens) | Compound a2/ Jiyuan oridonin A (JDA) | GPX4 | Decreased GPX4 induced the accumulation of Fe2+ | MGC-803 MKN-45 Xenograft in mouse model | [81] |
| Quinones | Danshen (Salvia miltiorrhiza Bunge) | Tanshinone ⅡA (Tan ⅡA) Dihydroisotanshinone I (DT) | p53 | Upregulates p53, decreases GSH and L-cysteine levels | BGC-823 NCI-N87 Xenograft in NOD SCID mice | [82] |
| SLC7A11 | Downregulates SLC7A11 and GPX4, GCSC stemness markers (OCT3/4, ALDH1A1) | GC stem cells (GCSC) | [83] |
The highly active metabolic capacity of GC cells makes them particularly susceptible to ferroptosis; therefore, treatment targeting the ferroptosis pathway and involving various chemotherapeutic drugs shows therapeutic potential to overcome drug resistance. However, many limitations should be acknowledged. Many GC cells can escape ferroptosis by activating or remodeling stress pathways, thus limiting the antitumor effects of ferroptosis inducers. With the continuous development of research, the regulatory mechanism underlying ferroptosis has been increasingly investigated. Researchers are seeking factors that make cells sensitive or resistant to ferroptosis. First, the molecular mechanism and metabolic basis of GC cells' escape from ferroptosis have not yet been elucidated. Second, the clinical application of ferroptosis in treating GC still requires a large amount of data and extensive experiments. More importantly, the complex interactions between ferroptosis and the tumor microenvironment causing tumor heterogeneity in GC can also have an impact.
Many TCMs and natural products have shown synergistic effects with conventional chemotherapy or targeted drugs in various types of GC, which can reduce systemic toxicity, improve treatment efficacy, and have lower costs, thus opening up new avenues for treatment. Although the potential of TCM and natural products in cancer therapy through ferroptosis has been confirmed, further in-depth studies are required to determine their effectiveness and safety, which will be the core focus of future research.
Abbreviations
α-KG: α-ketoglutarate; α-TOH: α-tocopherol; γ-GC: gamma-glutamyl cysteine; 5-FU: 5-fluoruracil; ACP: Actinidia chinensis Planch; ACSL4: acyl-coenzyme A synthetase long-chain family member 4; AF: Amentoflavone; ALOX: arachidonate lipoxygenase; ALDH1A1: Aldehyde dehydrogenase 1 family member A1; AURKA: Aurora kinase A; Artemisinin: Artemisia annua; ARF6: ADP-ribosylation factor 6; ART: Artesunate; ATF2: activating transcription factor 2; BAP31: B-cell receptor-associated protein 31; CBS: cystathionine β-synthase; CDH1: E-cadherin; CDO1: cysteine dioxygenase 1; GC: gastric cancer; CISD1: CDGSH iron sulfur domain 1; CTH: cystathionine gamma-lyase; CoQ10: coenzyme Q10; DGC: diffuse-type gastric cancer; DHA: Dihydroartemisinin; DCLK3: doublecortin-like kinase 3; DHA: Dihydroartemisinin; DHFR: Dihydrofolate reductase; DHODH: Dihydroorotate dehydrogenase; DMT1: Divalent metal transporter1; DT: Dihydroisotanshinone I; EMT: epithelial-mesenchymal transition; Fe²⁺: ferrous iron; Fe³⁺: ferric iron; FE: ferritin; FPN: ferroportin; FSP1: Ferroptosis suppressor protein 1; FRG: ferroptosis-related genes signature; FTH1: Ferritin heavy chain1; GCH1: GTP cyclohydrolase1; GC: gastric cancer; GCL: Glutamate-cysteine ligase; Gln: glutamate; GLS: glutaminase; GNMT: glycine N-methyltransferase; GPX4: glutathione peroxidase 4; GSH: glutathione; GSR: Glutathione reductase; GSSG: glutathione disulfide; HMG-CoA reductase: 3-hydroxy-3-methylglutaryl-CoA reductase; HSPH1/HSPH105/HSPH110: heat shock protein 110; JDA: Jiyuan oridonin A; LPCAT3: lysophosphatidylcholine acyltransferase 3; LOX15: lipoxygenases 15; MGST1: microsomal glutathione S-Transferase 1; MVA: Mevalonate; NAD(P)H: nicotinamide adenine dinucleotide phosphate; NRF2: nuclear factor-erythroid 2; OA: oleanolic acid; OCT3/4: Octamer binding transcription factor 3/4; OP-B: Ophiopogonin B; PB: polycyanin B; PLOOH: phospholipid hydroperoxide; PLIN2: perilipin 2; PUFAs: polyunsaturated fatty acids; RCD: regulated cell death; ROS: reactive oxygen species; SCD1: stearoyl-CoA desaturase 1; SAHH: S-adenosylhomocysteine hydrolase; SIRT6: sirtuin 6; SLC3A2: solute carrier family 3 member 2; SLC7A11: solute carrier family 7 member 11; STAT3: signal transducer activator of transcription 3; STEAP3: Six-transmembrane epithelial antigen of prostate3; Tan IIA: Tanshinone IIA; TCA: tricarboxylic acid; TCM: traditional Chinese medicines; TfR1: transferrin receptor 1; THB: tetrahydrobiopterin; TXN: thioredoxin; TXNRD1: thioredoxin reductase 1; UA: ursolic acid; VDAC1: Voltage-Dependent Anion Channel 1; YJHD: Yiqi Jiedu Huayu decoction; ZFP36: zinc finger protein 36 homolog.
Acknowledgements
Funding
This review was supported by grants from Ministry of Science and Technology of Taiwan (NSTC 113-2635-B-255-001/NMRPF3P0121), Chang Gung Memorial Hospital (CMRPF1N0011, CORPF1P0041, ZMRPF3P0131) and Chang Gung University of Science and Technology (ZRRPF3P0091).
Author Contributions
Ming-Ming Tsai and Hsi-Lung Hsieh: Writing—Original Draft, Writing—Review & Editing. Ming-Ming Tsai and Ming-Chin Yu: Funding acquisition. Hui-Ching Tseng and Yi-Hsuan Wu: Writing—Review & Editing; All the authors reviewed the results and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285-96
2. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-72
3. Venkataramani V. Iron Homeostasis and Metabolism: Two Sides of a Coin. Adv Exp Med Biol. 2021;1301:25-40
4. Li B, Cheng K, Wang T, Peng X, Xu P, Liu G. et al. Research progress on GPX4 targeted compounds. Eur J Med Chem. 2024;274:116548
5. Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61:933-52
6. Gammella E, Buratti P, Cairo G, Recalcati S. The transferrin receptor: the cellular iron gate. Metallomics. 2017;9:1367-75
7. Galy B, Ferring-Appel D, Becker C, Gretz N, Grone HJ, Schumann K. et al. Iron regulatory proteins control a mucosal block to intestinal iron absorption. Cell Rep. 2013;3:844-57
8. Enko D. Physiology of Iron Metabolism. Clin Lab. 2025 71
9. Shpyleva SI, Tryndyak VP, Kovalchuk O, Starlard-Davenport A, Chekhun VF, Beland FA. et al. Role of ferritin alterations in human breast cancer cells. Breast Cancer Res Treat. 2011;126:63-71
10. Vlasveld LT, Janssen R, Bardou-Jacquet E, Venselaar H, Hamdi-Roze H, Drakesmith H. et al. Twenty Years of Ferroportin Disease: A Review or An Update of Published Clinical, Biochemical, Molecular, and Functional Features. Pharmaceuticals (Basel). 2019 12
11. Guggisberg CA, Kim J, Lee J, Chen X, Ryu MS. NCOA4 Regulates Iron Recycling and Responds to Hepcidin Activity and Lipopolysaccharide in Macrophages. Antioxidants (Basel). 2022 11
12. Song X, Xie Y, Kang R, Hou W, Sun X, Epperly MW. et al. FANCD2 protects against bone marrow injury from ferroptosis. Biochem Biophys Res Commun. 2016;480:443-9
13. Liu K, Huang L, Qi S, Liu S, Xie W, Du L. et al. Ferroptosis: The Entanglement between Traditional Drugs and Nanodrugs in Tumor Therapy. Adv Healthc Mater. 2023;12:e2203085
14. Gu R, Xia Y, Li P, Zou D, Lu K, Ren L. et al. Ferroptosis and its Role in Gastric Cancer. Front Cell Dev Biol. 2022;10:860344
15. Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv H. et al. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127-36
16. Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90-9
17. Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91-8
18. Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237-48
19. Kim SW, Kim Y, Kim SE, An JY. Ferroptosis-Related Genes in Neurodevelopment and Central Nervous System. Biology (Basel). 2021 10
20. Prabhune NM, Ameen B, Prabhu S. Therapeutic potential of synthetic and natural iron chelators against ferroptosis. Naunyn Schmiedebergs Arch Pharmacol. 2025;398:3527-55
21. Bai C, Hua J, Meng D, Xu Y, Zhong B, Liu M. et al. Glutaminase-1 Mediated Glutaminolysis to Glutathione Synthesis Maintains Redox Homeostasis and Modulates Ferroptosis Sensitivity in Cancer Cells. Cell Prolif. 2025: e70036.
22. Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond). 2018;38:12
23. Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W. et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10:1617
24. Arner ESJ, Schmidt EE. Unresolved questions regarding cellular cysteine sources and their possible relationships to ferroptosis. Adv Cancer Res. 2024;162:1-44
25. Hsieh MS, Ling HH, Setiawan SA, Hardianti MS, Fong IH, Yeh CT. et al. Therapeutic targeting of thioredoxin reductase 1 causes ferroptosis while potentiating anti-PD-1 efficacy in head and neck cancer. Chem Biol Interact. 2024;395:111004
26. Miller CG, Holmgren A, Arner ESJ, Schmidt EE. NADPH-dependent and -independent disulfide reductase systems. Free Radic Biol Med. 2018;127:248-61
27. Chen NC, Yang F, Capecci LM, Gu Z, Schafer AI, Durante W. et al. Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J. 2010;24:2804-17
28. Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell. 2015;59:298-308
29. Xu J, Bai X, Dong K, Du Q, Ma P, Zhang Z. et al. GluOC Induced SLC7A11 and SLC38A1 to Activate Redox Processes and Resist Ferroptosis in TNBC. Cancers (Basel). 2025 17
30. He R, Wei Y, Peng Z, Yang J, Zhou Z, Li A. et al. alpha-Ketoglutarate alleviates osteoarthritis by inhibiting ferroptosis via the ETV4/SLC7A11/GPX4 signaling pathway. Cell Mol Biol Lett. 2024;29:88
31. Fujii J, Yamada KI. Defense systems to avoid ferroptosis caused by lipid peroxidation-mediated membrane damage. Free Radic Res. 2023;57:353-72
32. Santoro MM. The Antioxidant Role of Non-mitochondrial CoQ10: Mystery Solved!. Cell Metab. 2020;31:13-5
33. Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693-8
34. Sun Q, Liu D, Cui W, Cheng H, Huang L, Zhang R. et al. Cholesterol mediated ferroptosis suppression reveals essential roles of Coenzyme Q and squalene. Commun Biol. 2023;6:1108
35. Amos A, Amos A, Wu L, Xia H. The Warburg effect modulates DHODH role in ferroptosis: a review. Cell Commun Signal. 2023;21:100
36. Vasquez-Vivar J, Shi Z, Tan S. Tetrahydrobiopterin in Cell Function and Death Mechanisms. Antioxid Redox Signal. 2022;37:171-83
37. Liu M, Kong XY, Yao Y, Wang XA, Yang W, Wu H. et al. The critical role and molecular mechanisms of ferroptosis in antioxidant systems: a narrative review. Ann Transl Med. 2022;10:368
38. Zheng J, Conrad M. Ferroptosis: when metabolism meets cell death. Physiol Rev. 2025;105:651-706
39. Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489-92
40. Wu W, Geng Z, Bai H, Liu T, Zhang B. Ammonium Ferric Citrate induced Ferroptosis in Non-Small-Cell Lung Carcinoma through the inhibition of GPX4-GSS/GSR-GGT axis activity. Int J Med Sci. 2021;18:1899-909
41. Yin Z, Shen G, Fan M, Zheng P. Lipid metabolic reprogramming and associated ferroptosis in osteosarcoma: From molecular mechanisms to potential targets. J Bone Oncol. 2025;51:100660
42. Cheng X, Fan K, Wang L, Ying X, Sanders AJ, Guo T. et al. TfR1 binding with H-ferritin nanocarrier achieves prognostic diagnosis and enhances the therapeutic efficacy in clinical gastric cancer. Cell Death Dis. 2020;11:92
43. Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D. et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19:43
44. Jiang X, Yan Q, Xie L, Xu S, Jiang K, Huang J. et al. Construction and Validation of a Ferroptosis-Related Prognostic Model for Gastric Cancer. J Oncol. 2021;2021:6635526
45. Sun X, Yang S, Feng X, Zheng Y, Zhou J, Wang H. et al. The modification of ferroptosis and abnormal lipometabolism through overexpression and knockdown of potential prognostic biomarker perilipin2 in gastric carcinoma. Gastric Cancer. 2020;23:241-59
46. Sen U, Coleman C, Sen T. Stearoyl coenzyme A desaturase-1: multitasker in cancer, metabolism, and ferroptosis. Trends Cancer. 2023;9:480-9
47. Sugezawa K, Morimoto M, Yamamoto M, Matsumi Y, Nakayama Y, Hara K. et al. GPX4 Regulates Tumor Cell Proliferation via Suppressing Ferroptosis and Exhibits Prognostic Significance in Gastric Cancer. Anticancer Res. 2022;42:5719-29
48. Lin Y, Dong Y, Liu W, Fan X, Sun Y. Pan-Cancer Analyses Confirmed the Ferroptosis-Related Gene SLC7A11 as a Prognostic Biomarker for Cancer. Int J Gen Med. 2022;15:2501-13
49. Tamura K, Tomita Y, Kanazawa T, Shinohara H, Sakano M, Ishibashi S. et al. Lipid Peroxidation Regulators GPX4 and FSP1 as Prognostic Markers and Therapeutic Targets in Advanced Gastric Cancer. Int J Mol Sci. 2024 25
50. Xu X, Li Y, Wu Y, Wang M, Lu Y, Fang Z. et al. Increased ATF2 expression predicts poor prognosis and inhibits sorafenib-induced ferroptosis in gastric cancer. Redox Biol. 2023;59:102564
51. Gomaa A, Peng D, Chen Z, Soutto M, Abouelezz K, Corvalan A. et al. Epigenetic regulation of AURKA by miR-4715-3p in upper gastrointestinal cancers. Sci Rep. 2019;9:16970
52. Lin L, Que R, Wang J, Zhu Y, Liu X, Xu R. Prognostic value of the ferroptosis-related gene SLC2A3 in gastric cancer and related immune mechanisms. Front Genet. 2022;13:919313
53. Minikes AM, Song Y, Feng Y, Yoon C, Yoon SS, Jiang X. E-cadherin is a biomarker for ferroptosis sensitivity in diffuse gastric cancer. Oncogene. 2023;42:848-57
54. Zang J, Cui M, Xiao L, Zhang J, Jing R. Overexpression of ferroptosis-related genes FSP1 and CISD1 is related to prognosis and tumor immune infiltration in gastric cancer. Clin Transl Oncol. 2023;25:2532-44
55. Cheng J, Tang YC, Dong Y, Qin RL, Dong ZQ. Doublecortin-like kinase 3 (DCLK3) is associated with bad clinical outcome of patients with gastric cancer and regulates the ferroptosis and mitochondria function in vitro and in vivo. Ir J Med Sci. 2024;193:35-43
56. Li D, Cao D, Zhang Y, Yu X, Wu Y, Jia Z. et al. Integrative pan-cancer analysis and experiment validation identified GLS as a biomarker in tumor progression, prognosis, immune microenvironment, and immunotherapy. Sci Rep. 2025;15:525
57. Li Y, Xu X, Wang X, Zhang C, Hu A, Li Y. MGST1 Expression Is Associated with Poor Prognosis, Enhancing the Wnt/beta-Catenin Pathway via Regulating AKT and Inhibiting Ferroptosis in Gastric Cancer. ACS Omega. 2023;8:23683-94
58. Li X, Qian J, Xu J, Bai H, Yang J, Chen L. NRF2 inhibits RSL3 induced ferroptosis in gastric cancer through regulation of AKR1B1. Exp Cell Res. 2024;442:114210
59. Hu C, Zu D, Xu J, Xu H, Yuan L, Chen J. et al. Polyphyllin B Suppresses Gastric Tumor Growth by Modulating Iron Metabolism and Inducing Ferroptosis. Int J Biol Sci. 2023;19:1063-79
60. Wang F, Chen C, Chen WP, Li ZL, Cheng H. Development and Validation of a Novel Ferroptosis-Related Gene Signature for Predicting Prognosis and the Immune Microenvironment in Gastric Cancer. Biomed Res Int. 2021;2021:6014202
61. Liu YP, Qiu ZZ, Li XH, Li EY. Propofol induces ferroptosis and inhibits malignant phenotypes of gastric cancer cells by regulating miR-125b-5p/STAT3 axis. World J Gastrointest Oncol. 2021;13:2114-28
62. Expression of concern. Ophiopogonin B induces the autophagy and apoptosis of colon cancer cells by activating JNK/c-Jun signaling pathway [Biomedicine & Pharmacotherapy 108 (2018) 1208-1215]. Biomed Pharmacother. 2022;155:113820
63. Zhang S, Li Z, Hu G, Chen H. Integrative single-cell and multi-omics analyses reveal ferroptosis-associated gene expression and immune microenvironment heterogeneity in gastric cancer. Discov Oncol. 2025;16:57
64. Feng S, Li Y, Huang H, Huang H, Duan Y, Yuan Z. et al. Isoorientin reverses lung cancer drug resistance by promoting ferroptosis via the SIRT6/Nrf2/GPX4 signaling pathway. Eur J Pharmacol. 2023;954:175853
65. Fu D, Wang C, Yu L, Yu R. Induction of ferroptosis by ATF3 elevation alleviates cisplatin resistance in gastric cancer by restraining Nrf2/Keap1/xCT signaling. Cell Mol Biol Lett. 2021;26:26
66. Zhong S, Wang Z, Yang J, Jiang D, Wang K. Ferroptosis-related oxaliplatin resistance in multiple cancers: Potential roles and therapeutic Implications. Heliyon. 2024;10:e37613
67. Qu X, Liu B, Wang L, Liu L, Zhao W, Liu C. et al. Loss of cancer-associated fibroblast-derived exosomal DACT3-AS1 promotes malignant transformation and ferroptosis-mediated oxaliplatin resistance in gastric cancer. Drug Resist Updat. 2023;68:100936
68. Geng D, Wu H. Abrogation of ARF6 in promoting erastin-induced ferroptosis and mitigating capecitabine resistance in gastric cancer cells. J Gastrointest Oncol. 2022;13:958-67
69. Yuan J, Khan SU, Yan J, Lu J, Yang C, Tong Q. Baicalin enhances the efficacy of 5-Fluorouracil in gastric cancer by promoting ROS-mediated ferroptosis. Biomed Pharmacother. 2023;164:114986
70. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486-541
71. Tang F, Xu Y, Gao E, Zhang W, Zhang F, Xiang Y. et al. Amentoflavone attenuates cell proliferation and induces ferroptosis in human gastric cancer by miR-496/ATF2 axis. Chem Biol Drug Des. 2023;102:782-92
72. Sun S, Shen J, Jiang J, Wang F, Min J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct Target Ther. 2023;8:372
73. Wang K, Chen Y, Zhang M, Wang S, Yao S, Gong Z. et al. Metformin suppresses gastric cancer progression by disrupting the STAT1-PRMT1 axis. Biochem Pharmacol. 2024;226:116367
74. Shao L, Zhu L, Su R, Yang C, Gao X, Xu Y. et al. Baicalin enhances the chemotherapy sensitivity of oxaliplatin-resistant gastric cancer cells by activating p53-mediated ferroptosis. Sci Rep. 2024;14:10745
75. Zhang Y, Xu G, Zhang S, Wang D, Saravana Prabha P, Zuo Z. Antitumor Research on Artemisinin and Its Bioactive Derivatives. Nat Prod Bioprospect. 2018;8:303-19
76. Gao Z, Deng G, Li Y, Huang H, Sun X, Shi H. et al. Actinidia chinensis Planch prevents proliferation and migration of gastric cancer associated with apoptosis, ferroptosis activation and mesenchymal phenotype suppression. Biomed Pharmacother. 2020;126:110092
77. Lee JY, Nam M, Son HY, Hyun K, Jang SY, Kim JW. et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci U S A. 2020;117:32433-42
78. Xi S, Ding W, Weng D, Zeng Y, Gao K, Wu Q. et al. Chrysophanol induces apoptosis and ferroptosis of gastric cancer cells by targeted regulation of mTOR. Chem Biol Drug Des. 2024;103:e14417
79. Song S, Wen F, Gu S, Gu P, Huang W, Ruan S. et al. Network Pharmacology Study and Experimental Validation of Yiqi Huayu Decoction Inducing Ferroptosis in Gastric Cancer. Front Oncol. 2022;12:820059
80. Zhang L, Li C, Zhang Y, Zhang J, Yang X. Ophiopogonin B induces gastric cancer cell death by blocking the GPX4/xCT-dependent ferroptosis pathway. Oncol Lett. 2022;23:104
81. Liu Y, Song Z, Liu Y, Ma X, Wang W, Ke Y. et al. Identification of ferroptosis as a novel mechanism for antitumor activity of natural product derivative a2 in gastric cancer. Acta Pharm Sin B. 2021;11:1513-25
82. Guan Z, Chen J, Li X, Dong N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci Rep. 2020 40
83. Ni H, Ruan G, Sun C, Yang X, Miao Z, Li J. et al. Tanshinone IIA inhibits gastric cancer cell stemness through inducing ferroptosis. Environ Toxicol. 2022;37:192-200
84. Hu Y, Guo N, Yang T, Yan J, Wang W, Li X. The Potential Mechanisms by which Artemisinin and Its Derivatives Induce Ferroptosis in the Treatment of Cancer. Oxid Med Cell Longev. 2022;2022:1458143
Author contact
![]() Corresponding author: Email: mmtsaicgust.edu.tw (M.M.T.); Tel.: +886-3-2118999 EXT. 5640/Fax: 886-3-2118866.
Corresponding author: Email: mmtsaicgust.edu.tw (M.M.T.); Tel.: +886-3-2118999 EXT. 5640/Fax: 886-3-2118866.

 Global reach, higher impact
Global reach, higher impact