Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(18):5423-5431. doi:10.7150/jca.60390 This issue Cite
Research Paper
Serial surveillance by circulating tumor DNA profiling after chimeric antigen receptor T therapy for the guidance of r/r diffuse large B cell lymphoma precise treatment
1. Bone Marrow Transplantation Center, the First Affiliated Hospital, Zhejiang University School of Medicine
2. Institute of Hematology, Zhejiang University
3. Zhejiang Province Engineering Laboratory for Stem Cell and Immunity Therapy
4. Liangzhu Laboratory, Zhejiang University Medical Center, 1369 West Wenyi Road, Hangzhou 311121, China
5. Nanjing Geneseeq Technology Inc., Nanjing, Jiangsu, China
6. Chaim Sheba Medical Center, Tel Hashomer, Israel, Tel Hashomer, Israel
# Contributed equally.
Abstract
Background: Circulating tumor DNA (ctDNA) released from tumor cells carries the tumor-associated genetic and epigenetic characteristics of cancer patients. Next-generation sequencing (NGS) facilitates the application of ctDNA profiling for identification and monitoring of minimal residual disease (MRD) in cancer, and can serve as the guidance for precise treatment.
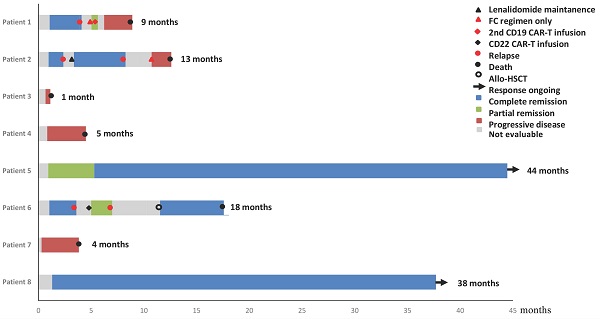
Methods: In this study, we profiled genomic alterations in the baseline, relapsed, and progressive tumor samples of eight diffuse large B cell lymphoma (DLBCL) patients (NCT03118180) after chimeric antigen receptor T (CAR-T) cell therapy.
Results: The median follow-up was 41 months. 4 (50%) patients achieved complete remission (CR), 1 (12.5%) patient achieved partial remission (PR), and the other 3 (37.5%) patients showed no response. 3 of 5 patients who achieved remission relapsed within 4 months after CAR-T therapy, while the rest 2 patients remained CR for more than 3 years. Based on the positron emission tomography-computed tomography (PET-CT) scan, the current gold standard for evaluating response to therapy in lymphoma, the sensitivity and specificity of our ctDNA profiling in detecting tumor-related ctDNA mutations were 94.7% and 83.3%, respectively. The median numbers of baseline plasma ctDNA mutations in patients who remained long-term CR and patients who relapsed or became refractory to CAR-T therapy were 3 and 14.3, respectively. GNA13, SOCS1, TNFAIP3 and XPO1 mutations appeared to be associated with poor prognosis after CAR-T cell therapy. Our results also suggested that lenalidomide might relieve relapsed lymphoma with mutations in NFKBIA 202C>T (p.Q68*) and NFKBIE 433A>T (p.K145*) by targeting NF-Kappa B signaling. In addition, the inhibitor selinexor may be another choice for refractory or relapse (r/r) DLBCL patients after CAR-T cell treatment.
Conclusion: Serial ctDNA monitoring is an emerging technology for the surveillance of disease status and prognosis prediction. In this work, we demonstrated the use of serial ctDNA monitoring in r/r DLBCL patients after CD19-targeted CAR-T cell therapy. Our longitudinal NGS profiling revealed the changes of ctDNA mutation in accordance with prognosis, and shed some light on exploring more targeted treatment options together with CAR-T cell therapy.
Keywords: ctDNA, DLBCL, CAR-T, precise treatment

 Global reach, higher impact
Global reach, higher impact