3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(7):2105-2112. doi:10.7150/jca.53242 This issue Cite
Research Paper
Peripheral blood biomarkers predict immune-related adverse events in non-small cell lung cancer patients treated with pembrolizumab: a multicenter retrospective study
1. Division of Pharmaceutical Care Sciences, Center for Social Pharmacy and Pharmaceutical Care Sciences, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
2. Division of Pharmaceutical Care Sciences, Keio University Graduate School of Pharmaceutical Sciences, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
3. Department of Pharmacy, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan.
4. Department of Biomedical Statistics and Bioinformatics, Kyoto University Graduate School of Medicine, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan.
5. Department of Thoracic Oncology, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan.
6. Division of Hospital Pharmacy Science, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
7. Department of Pharmacy, Keio University Hospital, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan.
8. Keio Cancer Center, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan.
9. Division of Pulmonary Medicine, Department of Medicine, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan.
Abstract
Background: Pembrolizumab is currently the standard treatment for patients with advanced non-small cell lung cancer (NSCLC). However, the association between immune-related adverse events (irAEs) and peripheral blood cell counts remains unclear. We aimed at identifying peripheral blood cell counts that may predict the development of pembrolizumab-induced irAEs.
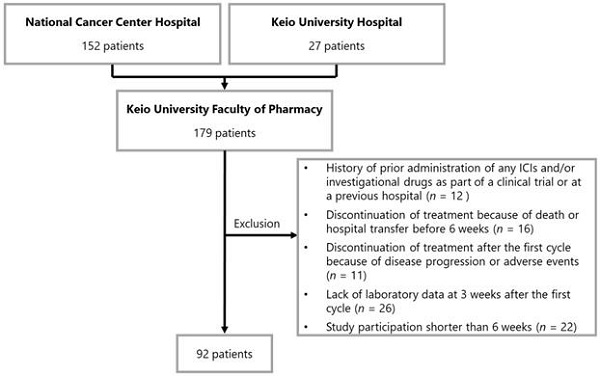
Methods: We retrospectively analyzed data on consecutive patients with advanced NSCLC who received pembrolizumab monotherapy as first-line or later-line therapy at the National Cancer Center Hospital and Keio University Hospital. We used data between December 2015 and November 2018. The primary endpoint was the relationship between peripheral blood cell count data and early-onset irAEs during the 6-weeks study period. Receiver operating characteristic (ROC) curve and multivariable logistic regression analyses were performed.
Results: In total, 92 patients were evaluated, of whom 45 (48.9%) had at least one irAE during the first 6-weeks after treatment initiation. The ROC curves revealed that the optimal cutoff of pretreatment absolute lymphocyte count (ALC), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) for onset of irAEs were 1459, 2.320, 1.538, and 165, respectively. Multivariable logistic regression analyses revealed that pretreatment ALC>1450 and LMR>1.6 were significantly associated with a reduced risk for onset of any irAEs, whereas pretreatment NLR>2.3 and PLR>165 were significantly associated with an increased risk.
Conclusions: The findings suggest that considering the routine availability of blood cell count data before the initiation of treatment with pembrolizumab, it may be useful in identifying early-onset irAEs during the 6-weeks study period in clinical practice.
Keywords: immune checkpoint inhibitor, blood cell count, absolute lymphocyte count, neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, platelet-to-lymphocyte ratio.

 Global reach, higher impact
Global reach, higher impact