3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(5):1555-1562. doi:10.7150/jca.53482 This issue Cite
Research Paper
Apatinib as non-first-line treatment in patients with Intrahepatic Cholangiocarcinoma
1. Department of Liver Surgery, Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC), Peking Union Medical College Hospital, Beijing, China.
2. Pancreas Center, The First Affiliated Hospital of Nanjing Medical University; Pancreas Institute, Nanjing Medical University, Nanjing 210000, China.
3. Department of General Surgery, Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC), Peking Union Medical College Hospital, Beijing, China.
#These authors contributed equally to this work.
Abstract
Purpose: There is limited standard treatment for patients with advanced cholangiocarcinoma after refractory of chemotherapy. Apatinib is a tyrosine kinase inhibitor targeting VEGFR-2, which exhibited broad-spectrum antitumor activities in previous studies. We aim to evaluate the efficacy and safety of apatinib as non-first-line treatment in patients with advanced cholangiocarcinoma.
Methods: This was a prospective open-label phase II trial (NCT03251443). Patients with pathology-confirmed cholangiocarcinoma after prior systemic therapy were enrolled. Participants were treated with apatinib 500 mg orally once daily. The primary end point was overall response rate (ORR).
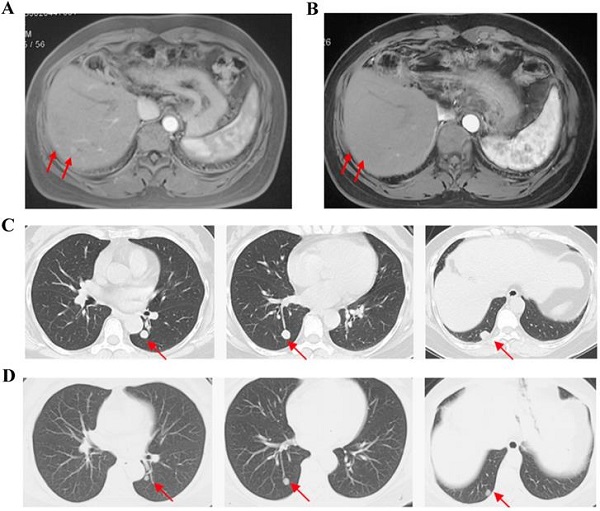
Results: Between August 8, 2017 and November 13, 2018, 30 patients participated in this study, and 26 patients received apatinib treatment except 4 patients withdrew consent before the first dosage. For full analysis set, the ORR was 11.5% and the disease control rate was 50.0%. 3 patients (11.5%) achieved partial response and no patients achieved complete response. The median progression free time was 2.0 (95% CI: 0.7-3.3) months and median overall survival was 9. 0 (95% CI: 4.6-13.4) months. The most common adverse events of any grade were fatigue (80.8%), hypertension (73.1%) and decreased appetite (38.5%). Grade 3 adverse events occurred in 23.1% patients and no grade 4 adverse events occurred. The most common grade 3 adverse events were hypertension (23.1%) and elevated transaminase (11.5%).
Conclusion: Apatinib as non-first-line monotherapy has potential therapeutic efficacy in patients with advanced cholangiocarcinoma.
Keywords: cholangiocarcinoma, anti-angiogenesis therapy, Apatinib, efficacy, safety

 Global reach, higher impact
Global reach, higher impact