3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(5):1318-1333. doi:10.7150/jca.49174 This issue Cite
Research Paper
Immune checkpoint inhibitor (ICI) combination therapy compared to monotherapy in advanced solid cancer: A systematic review
1. Cancer Center, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong An Road, Xi Cheng District, Beijing, 100050, China.
2. Department of Traditional Chinese Medicine, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong An Road, Xi Cheng District, Beijing, 100050, China.
Abstract
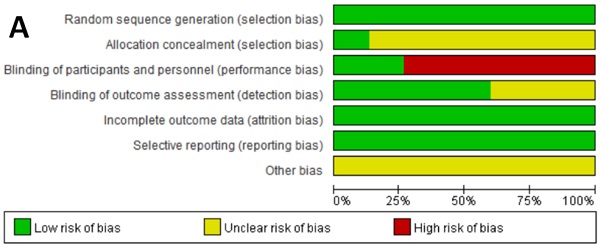
Aim: To evaluate the efficacy and safety of immune checkpoint inhibitor (ICI) two-drug combination therapy in patients with advanced malignancy.
Methods: We searched PubMed, PMC, EMBASE, EBSCO, Cochrane Central Register of Controlled Trials (CENTRAL), American Society of Clinical Oncology (ASCO and the European Society of Medical Oncology (ESMO) to identify primary research reporting the survival outcomes and safety of ICI combination therapy in patients with advanced malignancy. We performed a meta-analysis that evaluated the risk ratio (RR) and its 95% confidence interval (CI) for objective response rates (ORR) and disease control rates (DCR), hazard ratio (HR) and 95% CI for progression-free survival (PFS) and overall survival time (OS), and RR and 95% CI for adverse events (AEs).
Results: The final 10 studies (15 cohorts) and 2410 patients were included in the meta-analysis. The ICI combination therapy resulted in improved ORR (RR 1.82, 95% CI 1.31-2.54, p = 0.0004), DCR (RR 1.41, 95% CI 1.29-1.55, p < 0.0001), PFS (HR 0.83, 95% CI 0.74-0.94, p = 0.003) and OS (HR 0.90, 95% CI 0.82-0.98, p = 0.02) in patients with advanced malignant tumors. The incidence of some high grade (≥3) AEs increased, such as fatigue, nausea, diarrhea, colitis, rash, pruritus, elevated transaminase and lipase.
Conclusion: Our study showed that ICI combination therapy can improve ORR, DCR, PFS and OS in patients with advanced malignancy. Compared with ICI monotherapy, ICI combination therapy was more likely to induce severe AEs.
Keywords: ICI, combination, efficacy, malignancy, meta-analysis, safety

 Global reach, higher impact
Global reach, higher impact