3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(5):1318-1333. doi:10.7150/jca.49174 This issue Cite
Research Paper
Immune checkpoint inhibitor (ICI) combination therapy compared to monotherapy in advanced solid cancer: A systematic review
1. Cancer Center, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong An Road, Xi Cheng District, Beijing, 100050, China.
2. Department of Traditional Chinese Medicine, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong An Road, Xi Cheng District, Beijing, 100050, China.
Received 2020-6-7; Accepted 2020-12-6; Published 2021-1-1
Abstract
Aim: To evaluate the efficacy and safety of immune checkpoint inhibitor (ICI) two-drug combination therapy in patients with advanced malignancy.
Methods: We searched PubMed, PMC, EMBASE, EBSCO, Cochrane Central Register of Controlled Trials (CENTRAL), American Society of Clinical Oncology (ASCO and the European Society of Medical Oncology (ESMO) to identify primary research reporting the survival outcomes and safety of ICI combination therapy in patients with advanced malignancy. We performed a meta-analysis that evaluated the risk ratio (RR) and its 95% confidence interval (CI) for objective response rates (ORR) and disease control rates (DCR), hazard ratio (HR) and 95% CI for progression-free survival (PFS) and overall survival time (OS), and RR and 95% CI for adverse events (AEs).
Results: The final 10 studies (15 cohorts) and 2410 patients were included in the meta-analysis. The ICI combination therapy resulted in improved ORR (RR 1.82, 95% CI 1.31-2.54, p = 0.0004), DCR (RR 1.41, 95% CI 1.29-1.55, p < 0.0001), PFS (HR 0.83, 95% CI 0.74-0.94, p = 0.003) and OS (HR 0.90, 95% CI 0.82-0.98, p = 0.02) in patients with advanced malignant tumors. The incidence of some high grade (≥3) AEs increased, such as fatigue, nausea, diarrhea, colitis, rash, pruritus, elevated transaminase and lipase.
Conclusion: Our study showed that ICI combination therapy can improve ORR, DCR, PFS and OS in patients with advanced malignancy. Compared with ICI monotherapy, ICI combination therapy was more likely to induce severe AEs.
Keywords: ICI, combination, efficacy, malignancy, meta-analysis, safety
Introduction
In recent years, with the in-depth study of tumor immunology, immune checkpoint inhibitors, CAR-T cells, tumor vaccines and other immunotherapy strategies have continuously emerged, which has led to rapid development of tumor immunotherapy. In theory, when tumor cells occur accidentally in the body, they will be recognized and cleared by the immune system, thereby maintaining the normal physiological activities of the body. However, in actual situations, the antigen expressed by tumor cells is mutated or not expressed, so that some tumor cells can escape from this equilibrium state, and further develop and metastasize, that is, immune escape occurs [1,2]. In 2002, Schreiber and others first proposed the theory of tumor immune editing, which believed that tumor cells can go through the three stages of immune elimination, stalemate and escape, and finally escape the monitoring of the immune system [3].
Currently, immune checkpoint inhibitors (ICIs) have made significant progress in the treatment of various cancers. The immune checkpoint pathway is regulated by the interaction of ligands and receptors, so proteins related to this can become potential targets for ICIs. The current research on ICIs mainly focuses on CTLA-4 (cytotoxic T lymphocyte associated antigen-4) antibody, PD-1 (programmed cell death protein 1) antibody and PD-L1 (programmed cell death-ligand 1) antibody. It has been gradually approved for the treatment of patients with melanoma, non-small cell lung cancer (NSCLC), classical Hodgkin lymphoma (cHL), head and neck squamous cell carcinoma (HNSCC), and urothcarcinoma (RCCa) [4].
However, due to the high heterogeneity of tumors, and many inhibitory immune checkpoint molecules jointly promote the immune escape of tumor cells, the use of a single target immunotherapy strategy may leave a lot of space for tumor survival. At the same time, due to the different patients with different ICI reaction rate, make the broad spectrum of ICI has been challenged. ICI two-drug combination therapy can make up for the shortcomings of monotherapy. When the drug appears immune tolerance, it can play a synergistic anti-tumor effect by interfering with other targets, so ICI two-drug combination therapy may become a development direction of tumor immunotherapy in the future. Moreover, for patients with chemotherapy contraindications, the combination of two ICI therapy has more advantages than chemotherapy combined with ICI therapy. At present, ICI two-drug combination therapy has shown a certain effect in various cancers such as melanoma, lung cancer, gastric cancer and mesothelioma. However, the number of large-scale clinical trials is currently small, and the toxic and side effects caused by ICI by promoting T cell activation and autoimmune reactions are still controversial. We performed a meta-analysis to analyze the efficacy and safety of anti-CTLA-4 combined with anti-PD-1/PD-L1 and ICI monotherapy in patients with advanced cancer.
Material and methods
Search strategy
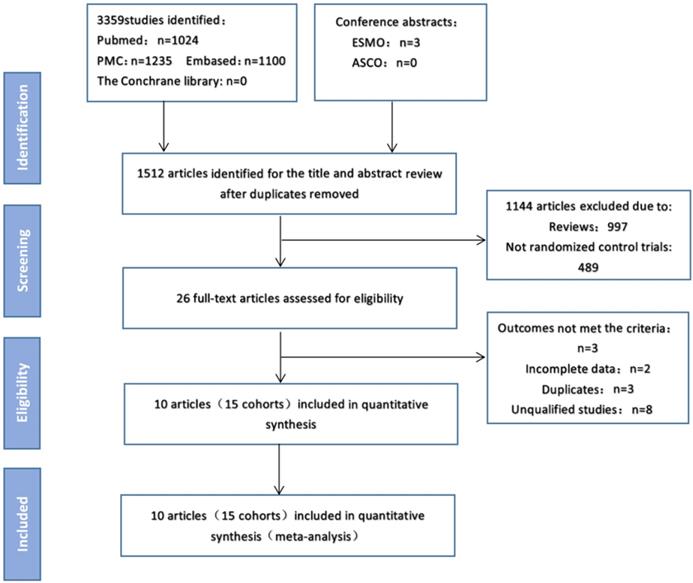
As of April 15, 2020, electronic searches were performed on PubMed, PMC, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), American Society of Clinical Oncology (ASCO), European Medical Oncology (ESMO), http://www.clinicaltrials.gov/. The detailed search strategy is shown in Figure 1.The search term was as follows: (“Nivolumab” OR “Pembrolizumab” OR “Atezolizumab” OR “Ipilimumab” OR “Tremelimumab” OR “Durvalumab” OR “Lambrolizumab” OR “Avelumab” OR “immune checkpoint inhibitors” OR “immune checkpoint inhibitor” OR “immuno checkpoint inhibitors” OR “immuno checkpoint inhibitor” OR “ICI” OR “ICIs” ) AND (“Neoplasia” OR “Neoplasias” OR “Neoplasm” OR “Tumors” OR “Tumor” OR “Cancer” OR “Cancers” OR “Malignancy” OR “Malignancies”) AND (“randomized controlled trial” OR “randomized” OR “placebo”). We searched all potentially relevant studies and reviewed the references in the final included articles to find possible missing studies.
Inclusion criteria
The included studies should meet the following inclusion criteria: (1) Advanced esophageal and gastric junction cancer/stomach cancer, lung cancer, melanoma, head and neck squamous cell carcinoma diagnosed by cytology or histology pathology; (2) Randomized controlled trial (RCT) comparing ICI combined with chemotherapy and chemotherapy alone; (3) Outcome indicators included the number of people who achieved objective response rate (ORR) and disease control rate (DCR), and the hazard ratio (HR) of progression-free survival (PFS) and OS, as well as their 95% confidence interval (95% CI), between the experimental group and the control group. (4) The number of people achieving ORR was the sum of complete response (CR) and partial response (PR), and the number of people achieving DCR was the sum of CR, PR and stable disease (SD).
Data extraction
Two independent researchers extracted data from the included studies based on the preferred report project (PRISMA) for systematic evaluation and meta-analysis. All inconsistencies were resolved with the unanimous consent of all researchers. Information collected from these studies includes title, first author, year of publication, number of patients, median age, treatment plan, medication dose, number of people achieving ORR and DCR, HR and 95% CI of PFS and OS.
Quality assessment
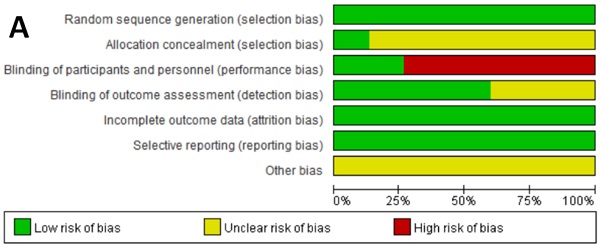
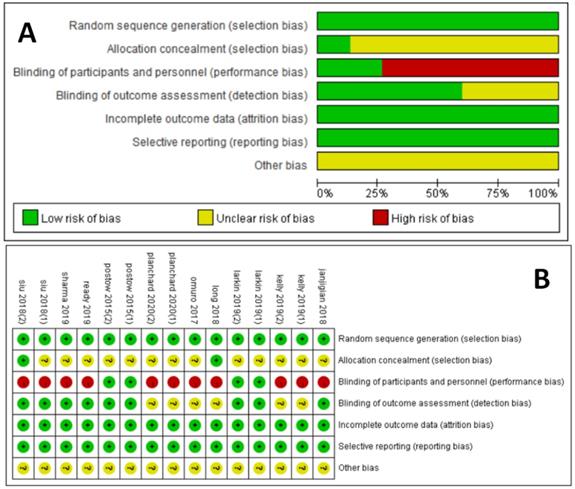
The Cochrane collaboration tool was used to assess the risk of bias for each study. This included the following assessment scopes: random sequence generation, allocation hiding, blindness of participants and researchers, blindness of outcome evaluators, incomplete outcome data, selective reporting, and other biases. According to the matching degree between the extracted information and the evaluation criteria, the risks in each field were classified as high risk, uncertain risk or low risk. GRADE was used to assess the level of evidence for all analysis results, which were classified as high quality, medium quality, low quality and very low quality.
Statistical analysis
Statistical analysis was performed using Review Manager 5.3 and Forest plots were made. The main end point of the meta-analysis was to compare the efficacy of ICI combination therapy and ICI monotherapy alone, and the evaluation indicators were risk ratio (RR) and its 95% CI of ORR and DCR, and HR and its 95% CI of PFS and OS. State 12.0 was used to evaluate publication bias based on Begg's and Egger's tests. Heterogeneity between studies was represented by Cochrane's X2 statistics and the inconsistency statistic (I2). We considered I2 <50% as low-level heterogeneity and I2 > 50% as significant heterogeneity. When I2 < 50%, the fixed effect model was used. When I2 > 50%, the random effects model was used. In addition, we used sensitivity analysis to test the stability of the results. In order to further explore the efficacy of ICI combination therapy, subgroup analysis was performed by line of therapy, ICI drugs in the control group, and tumor type. In all included studies, p < 0.05 was considered statistically significant.
Results
Characteristics of the included studies
Figure 1 shows the flow chart of this study. A total of 3362 records were retrieved through a database search. 1512 articles remaining after excluding duplicates, of which 138 were eliminated after reading the titles and abstracts. After excluding articles that did not meet the requirements, the full text of the remaining 26 articles was then reviewed, and 10 studies (15 cohorts) [5,6,7,8,9,10,11,12,13,14] involving 2410 patients were finally included in the meta-analysis. In these studies, the sample size of the included trials ranged from 19 to 945. Of these, three were phase III trials, seven were phase I/II trials, and three were first-line treatments, seven were second and above line treatments. Table 1 and sTable 1 summarize the characteristics of the included studies.
Study flow diagram.
Characteristics of the included studies
| Study | Region | Tumor | Phase | Line | Treatment arms | No. of patients | ORR (%) | DCR (%) | Progression-free survival | Overall survival |
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||||||||
| Jianjigian 2018 | North America, Europe | Esophagogastric cancer | 1/2 | 2nd | Nivolumab+ipilimumab | 101 | 12 (12) | 42 (42) | Not given | Not given |
| Nivolumab | 59 | 4 (7) | 22 (37) | |||||||
| Kelly 2019 | North America, Europe, Asia | Gastric/esophagogastric cancer | 2 | 2nd | Durvalumab+tremelimumab | 27 | 2 (7) | 7 (26) | Not given | Not given |
| Durvalumab | 24 | 0 (0) | 3 (1) | |||||||
| Tremelimumab | 12 | 1 (8) | 3 (25) | |||||||
| Larkin 2019 | South America, Europe, Asia, Africa | Melanoma | 3 | 1st | Nivolumab+ipilimumab | 314 | 183 (58) | 221 (70) | A:0.79 (0.64-0.96) | A:0.83 (0.67-1.03) |
| Nivolumab | 316 | 141 (45) | 171 (54) | B:0.42 (0.35-0.51) | B:0.52 (0.42-0.64) | |||||
| Ipilimumab | 315 | 60 (19) | 129 (41) | |||||||
| Long 2018 | Australia | Melanoma (brain metastases) | 2 | Not given | Nivolumab + ipilimumab | 35 | 17 (49) | 21 (60) | Not given | Not given |
| Nivolumab | 25 | 6 (24) | 8 (32) | |||||||
| Omuro 2017 | North America | Glioblastoma | 1 | ≥2nd | Nivolumab + ipilimumab | 10 | 3 (30) | 5 (50) | Not given | Not given |
| Nivolumab | 9 | 5 (56) | 7 (78) | |||||||
| Planchard 2020 | North America, South America, Europe, Asia, Australia | NSCLC | 3 | ≥3rd | Durvalumab+tremelimumab | 174 | 25 (14) | Not given | A:0.87 (0.68-1.12) | A:0.98 (0.74-1.30) |
| Durvalumab | 117 | 18 (15) | Not given | B:0.67 (0.49-0.92) | B:0.78 (0.56-1.11) | |||||
| Tremelimumab | 60 | 4 (7) | Not given | |||||||
| Postow 2015 | North America, Europe | Melanoma (BRAF-) | 1 | 1st | Nivolumab + ipilimumab | 72 | 44 (61) | 53 (74) | 0.40 (0.23-0.68) | Not given |
| Ipilimumab | 37 | 4 (11) | 17 (46) | |||||||
| Melanoma (BRAF+) | 1 | 1st | Nivolumab + ipilimumab | 23 | 12 (52) | 15 (65) | 0.38 (0.15-1.00) | Not given | ||
| Ipilimumab | 10 | 1 (10) | 2 (20) | |||||||
| Ready 2019 | North America, Europe | SCLC | 1/2 | ≥2nd | Nivolumab + ipilimumab | 96 | 21 (22) | 37 (39) | Not given | Not given |
| Nivolumab | 147 | 17 (12) | 42 (29) | |||||||
| Sharma 2019 | North America, Europe | Urothelial carcinoma | 1/2 | ≥2nd | Nivolumab + ipilimumab | 92 | 34 (37) | 58 (63) | Not given | Not given |
| Nivolumab | 78 | 16 (21) | 43 (55) | |||||||
| Siu 2018 | North America, Europe, Asia, Australia | HNSCC | 2 | 2nd | Durvalumab+tremelimumab | 129 | 16 (12) | 17 (13) | A:1.13 (0.82-1.56) | A:0.99 (0.69-1.43) |
| Durvalumab | 65 | 6 (9) | 10 (15) | B:0.73 (0.53-1.01) | B:0.72 (0.51-1.03) | |||||
| Tremelimumab | 63 | 1 (2) | 1 (2) |
Quality assessment of included studies
We critically evaluated the methodological quality of the included studies based on the Cochrane Collaborative Bias Risk Tool. Because of the authors' detailed description of the randomization principle, all included trials were rated as having a low risk of randomization bias. Other sources of bias were not identified. The graphical results of methodological quality are shown in Figure 2. The risk of bias items for each included study is presented in Figure 2.
Overall response rate (ORR) and disease control rate (DCR)
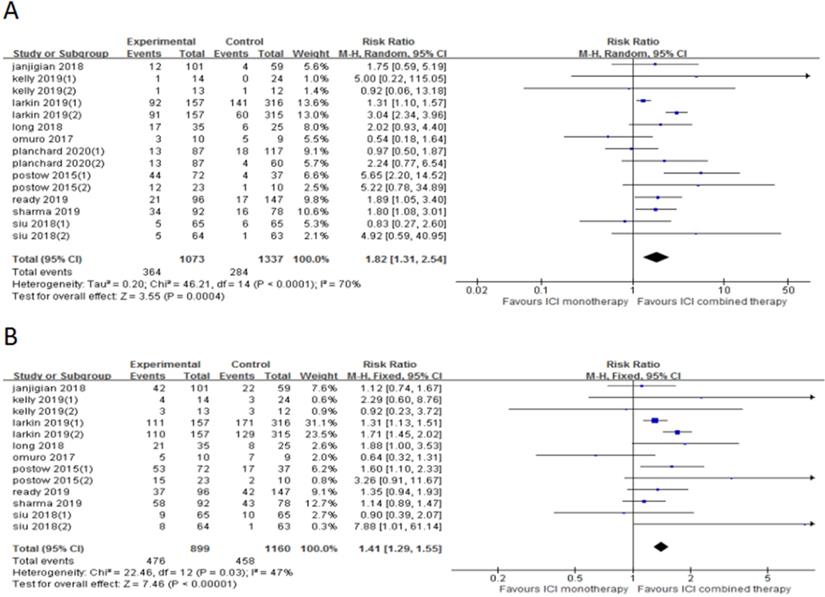
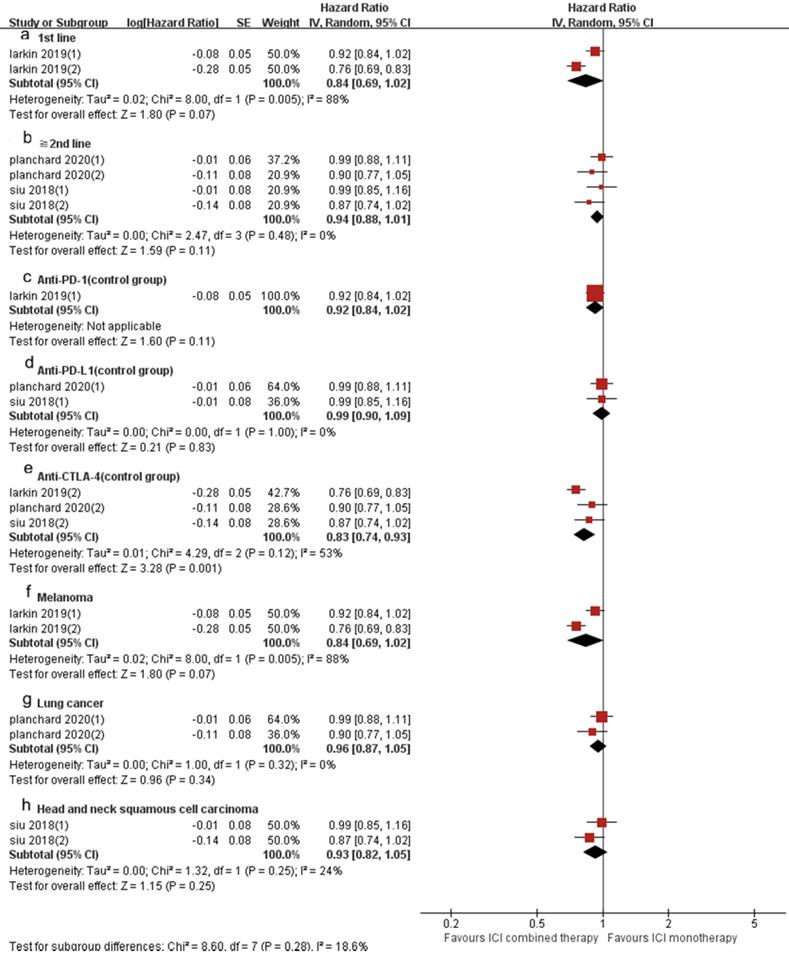
10 trials (15 cohorts) included reported ORR, and 9 trials (13 cohorts) reported DCR. The ORR range of the ICI combination therapy group was 7-61%, and the DCR range was 13-74%. The combined data showed that ORR was 34% and 21% in ICI combination therapy group and ICI monotherapy group, respectively, and DCR was 53% and 39% in ICI combination therapy group and ICI monotherapy group, respectively. The ORR of ICI combination therapy was significantly higher than that of ICI monotherapy (RR 1.82, 95% CI 1.31-2.54, p = 0.0004, Fig. 3A), and the heterogeneity was greater (p <0.0001, I2 = 70%). Therefore, we did a sensitivity analysis. After excluding the Kelly's study with the smallest weight (1.0%), there was no significant change in heterogeneity. After excluding the Larkin's study with the largest weight (13.6%), the heterogeneity decreased (I2 = 53%, p = 0.0002), but the statistical results were relatively stable and unchanged. To further explore the sources of heterogeneity, we conducted subgroup analysis based on line of therapy, ICI drugs in the control group, and tumor type (Fig. 4). The results showed that in 1st line (RR 2.77, 95% CI 1.33-5.77, p = 0.006, Fig. 4a), “≥ 2nd line” (RR 1.48, 95% CI 1.10-1.99, p = 0.01, Fig. 4b), anti-PD-1 (control group) (RR 1.48, 95% CI 1.15-1.89, p = 0.002, Fig. 4c), anti-CTLA-4 (control group) (RR 3.14, 95% CI 2.46-4.00, p < 0.00001, Fig. 4e) and melanoma (RR 2.57, 95% CI 1.38-4.79, p = 0.003, Fig. 4f) groups, the ORR of ICI combination therapy was significantly higher than that of ICI monotherapy. In anti-PD-L1 (control group) (RR 0.99, 95% CI 0.56-1.73, p = 0.96, Fig. 4d), gastric or esophagogastric cancer (RR 1.78, 95% CI 0.68-4.63, p = 0.24, Fig. 4g), lung cancer (RR 1.51, 95% CI 0.92-2.49, p = 0.10, Fig. 4h) and head and neck squamous cell carcinoma (RR 1.61, 95% CI 0.29-8.94, p = 0.58, Fig. 4i) groups, there was no statistically significant difference in ORR between ICI combination therapy and ICI monotherapy. According to the subgroup analysis, we found great heterogeneity in 1st line (p < 0.00001, I2 = 92%) and melanoma (p < 0.00001, I2 = 89%) groups. Heterogeneity of head and neck squamous cell carcinoma (p =0.08, I2 = 51%) group was relatively large. Other groups showed less heterogeneity.
Assessment of risk of bias. (A) Risk of bias summary. (B) Risk of bias graph.
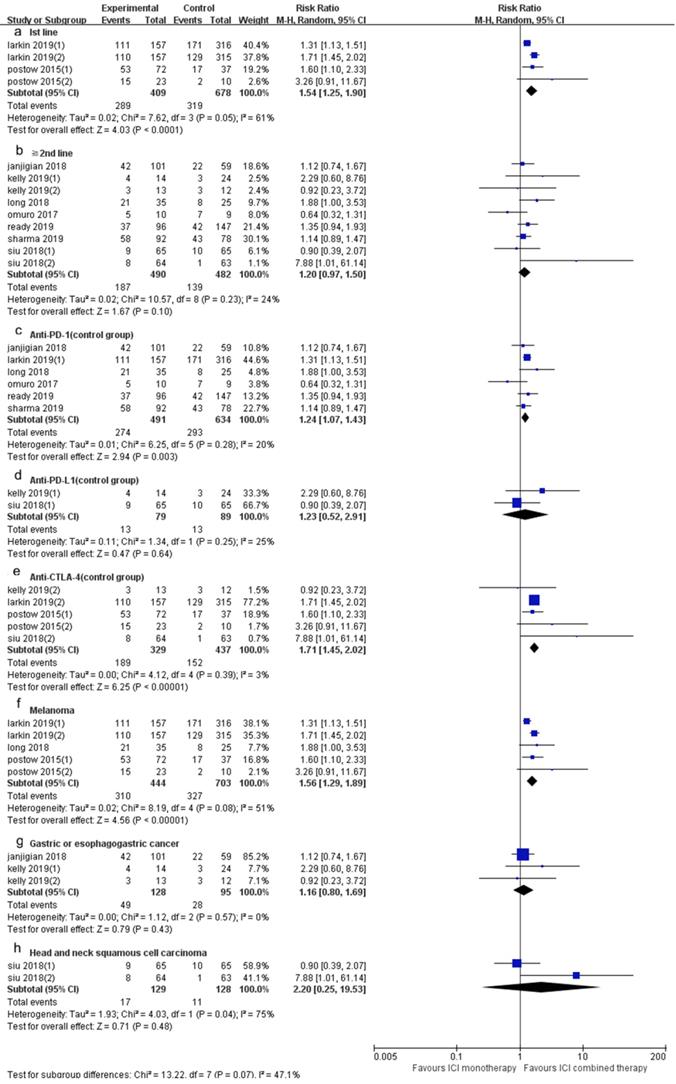
The DCR of ICI combination therapy was significantly higher than that of ICI monotherapy (RR 1.41, 95% CI 1.29-1.55, p < 0.0001, Fig. 3B), and there was heterogeneity (p = 0.03, I2 = 47%). Sensitivity analysis indicated that after excluding the Siu's study with the smallest weight (0.3%) and the Larkin's study with the largest weight (31.1%), there was no significant change in heterogeneity and statistical results. A subgroup analysis (Fig. 5) was also conducted, and the results showed that in 1st line (RR 1.54, 95% CI 1.25-1.90, p < 0.0001, Fig. 5a), anti-PD-1 (control group) (RR 1.24, 95% CI 1.07-1.43, p = 0.003, Fig. 5c), anti-CTLA-4 (control group) (RR 1.71, 95% CI 1.45-2.02, p < 0.00001, Fig. 5e) and melanoma (RR 1.56, 95% CI 1.29-1.89, p < 0.00001, Fig. 5f) groups, the DCR of ICI combination therapy was significantly higher than that of ICI monotherapy. In “≥ 2nd line” (RR 1.20, 95% CI 0.97-1.50, p = 0.10, Fig. 5b), anti-PD-L1 (control group) (RR 1.23, 95% CI 0.52-2.91, p = 0.64, Fig. 5d), gastric or esophagogastric cancer (RR 1.16, 95% CI 0.80-1.69, p = 0.43, Fig. 4g) and head and neck squamous cell carcinoma (RR 2.20, 95% CI 0.25-19.53, p = 0.48, Fig. 5h), there was no significant change in DCR of ICI combination therapy and ICI monotherapy. According to the subgroup analysis, we found great heterogeneity in 1st line (p = 0.05, I2 = 61%) and head and neck squamous cell carcinoma (p = 0.04, I2 = 75%) groups. Heterogeneity of melanoma (p = 0.08, I2 = 51%) group was relatively large. Other groups showed less heterogeneity.
Progression-free survival (PFS) and overall survival (OS)
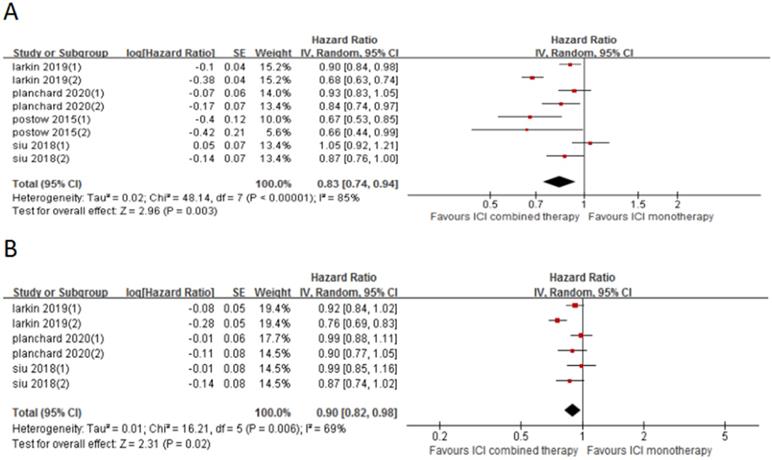
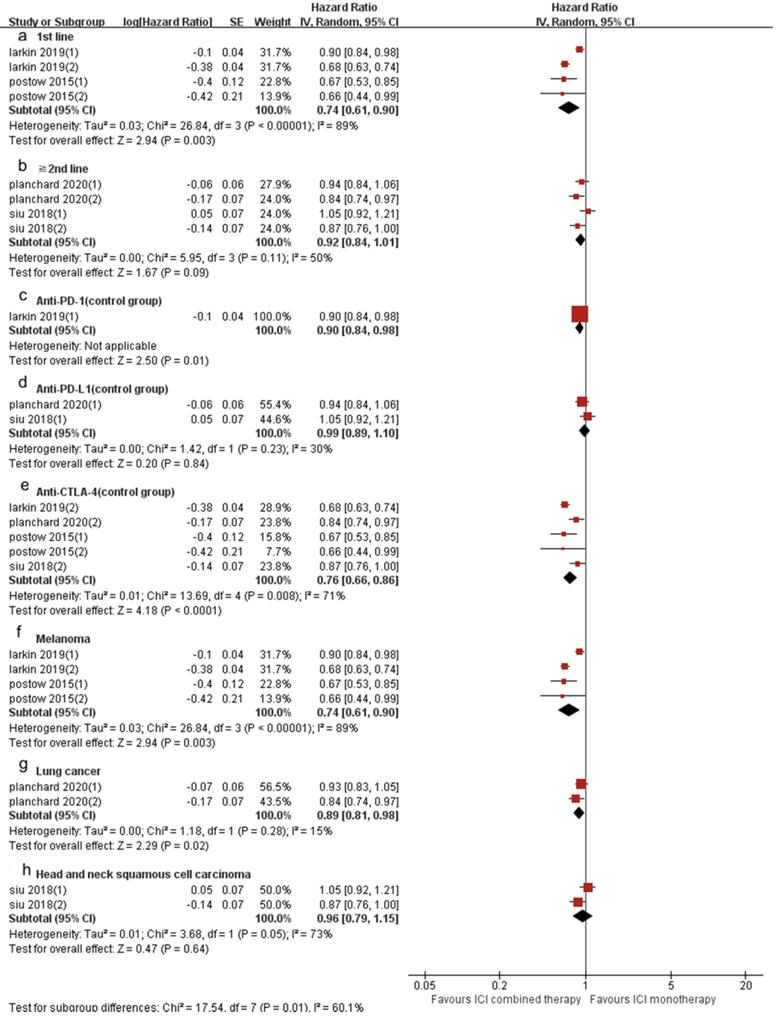
4 included trials (8 cohorts) reported PFS, and 3 trials (6 cohorts) reported OS. The combined results showed that ICI combination therapy has significantly longer PFS than ICI monotherapy (HR 0.83, 95% CI 0.74-0.94, p = 0.003, Fig. 6A), and the heterogeneity was greater (p < 0.0001, I2 = 85%). Therefore, we did a sensitivity analysis. After excluding the Postow's study with the smallest weight (5.6%) and the Larkin's study with the largest weight (15.1%), there was no significant change in heterogeneity and statistical results. To further explore the sources of heterogeneity, we conducted a subgroup analysis (Fig. 7). The results show that in 1st line (HR 0.74, 95% CI 0.61-0.90, p = 0.003, Fig. 7a), anti-PD-1 (control group) (HR 0.90, 95% CI 0.84-0.98, p = 0.01, Fig. 7c), anti-CTLA-4 (control group) (HR 0.76, 95% CI 0.66-0.86, p < 0.0001, Fig. 7e), melanoma (HR 0.74, 95% CI 0.61-0.90, p = 0.003, Fig. 7f) and lung cancer (HR 0.89, 95% CI 0.81-0.98, p = 0.02, Fig. 7g) groups, the PFS of ICI combination therapy was significantly longer than that of ICI monotherapy. In “≥2nd line” (HR 0.92, 95% CI 0.84-1.01, p = 0.09, Fig. 7b), anti-PD-L1 (control group) (HR 0.99, 95% CI 0.89-1.10, p = 0.84, Fig. 7d) and head and neck squamous cell carcinoma (HR 0.96, 95% CI 0.79-1.15, p = 0.64, Fig. 7h) groups, there was no significant difference in PFS between ICI combination therapy and ICI monotherapy. According to the subgroup analysis, we found that great heterogeneity in 1st line (p < 0.00001, I2 = 89%), anti-CTLA-4 (control group) (p = 0.008, I2 = 71%), melanoma (p < 0.00001, I2 = 89%), head and neck squamous cell carcinoma (p =0.05, I2 = 73%) groups. Other groups showed less heterogeneity.
Similarly, the OS of ICI combination therapy was significantly longer than that of ICI monotherapy (HR 0.90, 95%CI 0.82-0.98, p = 0.02, Fig. 6B), and the heterogeneity was greater (p = 0.006, I2 = 69%). Sensitivity analysis showed that after excluding the Siu's study or Phanchard's study with the smallest weight (15.4%), there was no significant change in heterogeneity and statistical results. After excluding the Larkin's study with the largest weight (19.4%), the heterogeneity disappeared (p = 0.63, I2 = 0%). A subgroup analysis (Fig. 8) was also conducted. And the results showed that in anti-CTLA-4 (control group) (HR 0.83, 95%CI 0.74-0.93, p = 0.001, Fig. 8e) group, the PFS of ICI combination therapy was significantly prolonged compared with that of ICI monotherapy. In 1st line (HR 0.84, 95% CI 0.6-1.02, p = 0.07, Fig. 8a), “≥2nd line” (HR 0.94, 95% CI 0.88-1.01, p = 0.11, Fig. 8b), anti-PD-1 (control group ) (HR 0.92, 95% CI 0.84-1.02, p = 0.21, Fig. 8c), anti-PD-L1 (control group) (HR 0.99, 95% CI 0.90-1.09, p = 0.83, Fig. 8d), melanoma (HR 0.74 , 95% CI 0.61-0.90, p = 0.003, Fig. 8f), lung cancer (HR 0.96, 95% CI 0.87-1.05, p = 0.34, Fig. 8g) and head and neck squamous cell carcinoma (HR 0.93, 95% CI 0.82-1.05, p = 0.25, Fig. 8h) groups, there was no significant difference in OS between ICI combination therapy and ICI monotherapy. According to the subgroup analysis, we found great heterogeneity in 1st line (p = 0.005, I2 = 88%) and melanoma (p = 0.005, I2 = 88%) groups. In the anti-CTLA-4 (control group) (p = 0.12, I2 = 53%) group, the heterogeneity was relatively large. Other groups showed less heterogeneity.
Forest plot and pooled RR and 95% CI for ORR (A) and DCR (B): “ICI combination therapy” versus “ICI monotherapy”. RR, relative ratio; CI, confidence interval; ORR, objective response rate; DCR, objective response rate; ICI, immune checkpoint inhibitor.
Forest plot and pooled RR and 95% CI for subgroup ORR: “ICI combination therapy” versus “ICI monotherapy”. (a: ORR of 1st line; b: ORR of “≥2nd line”; c: ORR of anti-PD-1 therapy in control group; d: ORR of anti-PD-L1 therapy in control group; e: ORR of anti-CTLA4 therapy in control group; f: ORR of subgroup of melanoma; g: ORR of gastric or esophagogastric cancer; h: ORR of lung cancer; i: ORR of head and neck squamous cell carcinoma) RR, risk ratios; CI, confidence intervals; ORR, objective response rate; ICI, immune checkpoint inhibitor.
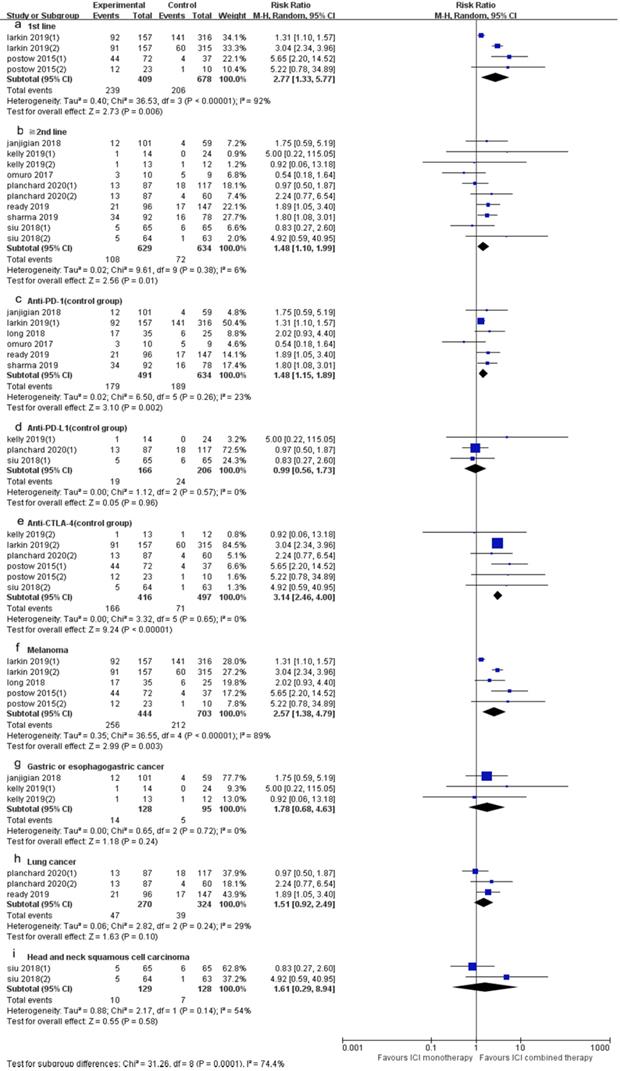
Forest plot and pooled RR and 95% CI for subgroup DCR: “ICI combination therapy” versus “ICI monotherapy”. (a: DCR of 1st line; b: DCR of “≥2nd line”; c: DCR of anti-PD-1 therapy in control group; d: DCR of anti-PD-L1 therapy in control group; e: DCR of anti-CTLA4 therapy in control group; f: DCR of melanoma; g: DCR of gastric or esophagogastric cancer; h: DCR of head and neck squamous cell carcinoma) RR, risk ratios; CI, confidence intervals; DCR, objective response rate; ICI, immune checkpoint inhibitor.
Forest plot and pooled HR and 95% CI for PFS (A) and OS (B): “ICI combination therapy” versus “ICI monotherapy”. HR, hazard ratio; CI, confidence interval; PFS, progression free survival; OS, progression free survival; ICI, immune checkpoint inhibitor.
Toxicity comparison between anti-PD-1 and anti-PD-L1
| Toxicity | Anti-PD-1 (p value) | Anti-PD-L1 (p value) |
|---|---|---|
| Anemia | - | 0.20 |
| Asthenia | 0.12 | 0.20 |
| Colitis | <0.0001 | 0.76 |
| Decreased appetite | 0.13 | 0.79 |
| Diarrhea | <0.0001 | 0.75 |
| Dyspnea | 0.05 | - |
| Fatigue | <0.00001 | 0.51 |
| Hyperthyroidism | 0.36 | 0.54 |
| Hypothyroidism | 0.09 | - |
| Increased ALT | <0.00001 | 0.79 |
| Increased amylase | 0.05 | 0.54 |
| Increased AST | <0.00001 | 0.28 |
| Increased lipase | 0.01 | 0.99 |
| Nausea | 0.03 | 0.79 |
| Pruritus | 0.02 | - |
| Rash | 0.0004 | 0.79 |
| Vomiting | 0.49 | - |
| <0.00001 | 0.34 |
Safety outcomes
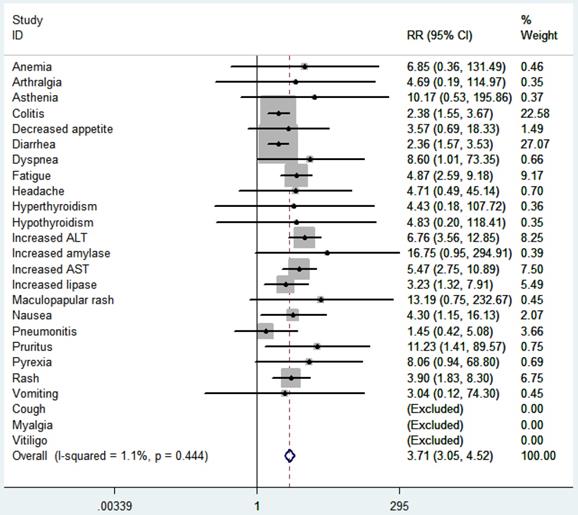
According to the common toxicity criteria of the National Cancer Institute, the side effects (≥3) included in the study were evaluated (Fig. 9). In general, skin toxicity, gastrointestinal toxicity, liver toxicity, and lung toxicity are more common among patients treated with ICI. Among patients treated with ICI combination therapy, fatigue (RR 5.11, 95% CI 2.68-9.76, p < 0.00001), nausea (RR 4.35, 95% CI 1.15-16.48, p = 0.03), diarrhea (RR 2.48, 95% CI 1.62-3.79, p < 0.0001), colitis (RR2.52, 95% CI 1.59-3.98, p < 0.0001), rash (RR 4.02, 95% CI 1.86-8.66, p = 0.0004), pruritus (RR 11.36 , 95% CI 1.42-91.03, p = 0.02), elevated ALT (RR 7.28, 95% CI 3.77-14.04, p < 0.00001), elevated AST (RR 5.76, 95% CI 2.85-11.63, p < 0.00001), elevated lipase (RR 3.44, 95% CI 1.36-8.74, p = 0.009) were higher than ICI monotherapy. We further compared the safety of anti-CTLA-4 combined with anti-PD-1 or anti-PD-L1 (Table 2). The results showed that the side effects of anti-CTLA-4 combined with anti-PD-1 was higher than that of monotherapy (p < 0.00001), including fatigue (p < 0.00001), nausea (p = 0.02), diarrhea (p < 0.0001), colitis (p < 0.0001), rash (p = 0.0004), elevated ALT (p < 0.00001), elevated AST (p < 0.00001), elevated lipase (p = 0.01) significantly increased, but no significant difference between the side effects of anti-CTLA-4 combined with anti-PD-L1 and monotherapy (p = 0.34).
Publication bias
Evaluation was performed by Egger's test and Begg's test. In Begg's test, z = 0.40 (continuity corrected), Pr > | z | = 0.692. In Egger's test, t = 0.53, p = 0.602. The results showed no publication bias.
Evidence level
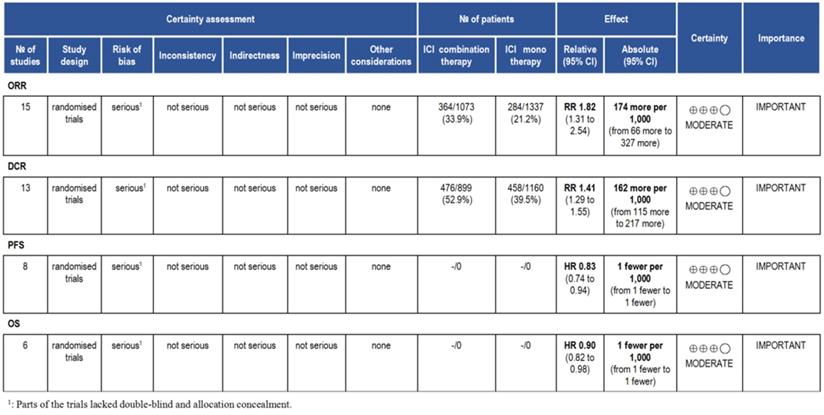
According to the GRADE, we analyzed the evidence level of the results, and the results showed that the evidence level of ORR, DCR, PFS and OS were moderate (Fig. 10).
Forest plot and pooled HR and 95% CI for subgroup PFS: “ICI combination therapy” versus “ICI monotherapy”. (a: PFS of 1st line; b: PFS of “≥2nd line”; c: PFS of anti-PD-1 therapy in control group; d: PFS of anti-PD-L1 therapy in control group; e: PFS of anti-CTLA4 therapy in control group; f: PFS of melanoma; g: PFS of lung cancer; h: PFS of head and neck squamous cell carcinoma) HR, hazard ratios; CI, confidence intervals; PFS, progression free survival; ICI, immune checkpoint inhibitor.
Forest plot and pooled HR and 95% CI for subgroup OS: “ICI combination therapy” versus “ICI monotherapy”. (a: OS of 1st line; b: OS of “≧2nd line”; c: OS of anti-PD-1 therapy in control group; d: OS of anti-PD-L1 therapy in control group; e: OS of anti-CTLA4 therapy in control group; f: OS of melanoma; g: OS of lung cancer; h: OS of head and neck squamous cell carcinoma) HR, hazard ratios; CI, confidence intervals; OS, progression free survival; ICI, immune checkpoint inhibitor.
Discussion
ICI is a type of therapy method that can improve the anti-tumor immune response by regulating the activity of T cells. Commonly referred to as the immune checkpoint is actually an inhibitory pathway in the immune system, their initial role is to avoid excessive immunity, but in cancer patients immune checkpoint is closely related to the immune escape of the tumor. Combined immunotherapy is the current treatment trend, including chemotherapy combined with ICI therapy and two combined ICI therapy. For patients with chemotherapy contraindications, the ICI two-drug combination therapy has more advantages than chemotherapy combined with ICI therapy. Existing clinical trials have shown that the ICI two-drug combination therapy has certain survival benefits [15,16]. The results of this meta-analysis showed that the ORR, DCR, PFS and OS of anti-CTLA-4 combined with anti-PD-1/PD-L1 were significantly better than that of ICI monotherapy.
RR of high grade adverse events in cancer patients with treated with ICI combination. RR, risk ratios; ICI, immune checkpoint inhibitor.
Summary of GRADE on evidences of outcomes.
Some clinical trials have been published in recent years. The CheckMate 227 trial included 793 untreated stage IV or relapsed NSCLC patients. The results showed that the ORR (45.3%) of nivolumab combined with ipilimumab in first-line treatment of NSCLC was higher than that of chemotherapy alone (26.9%), and the mPFS of nivolumab combined with ipilimumab and chemotherapy alone was 7.2 months versus 5.5 months (p < 0.001), mOS was 17.1 months versus 14.9 months (p = 0.007), and was independent of the expression level of PD-L1 [17]. The CheckMate 204 trial included 94 untreated patients with advanced melanoma brain metastases. The results showed that the intracranial clinical benefit rate was 57% and the extracranial clinical benefit rate was 56% [18]. In the non-contrast MAPS-II trial, patients with melanoma or NSCLC were randomly divided into nivolumab monotherapy group and nivolumab combined with ipilimumab treatment group. The results showed that both groups had clinical benefits, with DCR of 40% and 52%, and ORR of 19% and 28%, respectively, and the mPFS of nivolumab combined with ipilimumab treatment group was better than that of nivolumab monotherapy group (5.6 months versus 4.0 months) [19]. This meta-analysis included 10 randomized controlled studies with a total of 15 cohorts. The results of the analysis showed that the ORR, DCR, PFS and OS of ICI combination therapy were significantly better than that of ICI monotherapy. The sensitivity analysis of ORR, DCR and PFS all indicated that the statistical results were relatively stable. In the sensitivity analysis of OS, the heterogeneity disappeared after the exclusion of Larkin's study. In order to further explore heterogeneity, we performed subgroup analysis, which was divided into groups from line of therapy, ICI drugs in the control group and tumor type. We found that in 1st line and melanoma groups, the ORR, DCR and PFS of ICI combination therapy were superior to ICI monotherapy, but there was no significant difference in OS. In “≥2nd line” group, the ORR of ICI combination therapy was superior to ICI monotherapy, but there were no difference in those of DCR, PFS and OS. In the anti-PD-1 (control group) group, ORR, DCR and PFS of ICI combination therapy were superior to ICI monotherapy, but there was no difference in OS. In anti-PD-L1 (control group) group, there was no difference in ICI combination therapy and ICI monotherapy of ORR, DCR, PFS and OS. In anti-CTLA-4 (control group) group, ORR, DCR, PFS and OS of ICI combination therapy were superior to ICI monotherapy. In lung cancer group, the PFS of ICI combination therapy was superior to ICI monotherapy, but there were no significant difference in those of ORR and OS. In gastric or esophagogastric group, there were no significant difference in ICI combination therapy and ICI monotherapy of ORR and DCR. In head and neck squamous cell cancer group, there were no significant difference in ICI combination therapy and ICI monotherapy of PFS and OS.
In recent years, the research of ICI drugs is more and more extensive. CTLA-4 (CD-125) has a high homology with the co-stimulus receptor CD28 on the surface of T cells. It is also expressed on the surface of T cells and is a negative regulator of T cell activity. It can competitively bind to CD80/CD86 and block CD28/B7 costimulatory signals, thereby inhibiting the immune activity of T cells. PD-1 (CD-279), a member of the CD28 family, is also mainly expressed on the surface of T cells, and can also be expressed on the surface of B cells and monocytes. It is an inhibitory receptor that, when activated by binding with its ligands PD-L1 (B7-H1 /CD274) and PD-L2 (B7-H2/CD273), can cause T cell incapacitation, exhaustion or death [20-22]. Although both are negative signals for T cell activation, their location and timing are different. CTLA-4 is expressed on T cells, while PD-1 is more widely expressed on a variety of cells. Normally, CTLA-4 suppresses T cells in the early stages of the immune cycle in lymph nodes, while PD-1 regulates the immune response in peripheral tissues or tumor sites [21,23]. At present, there have been a lot of reports on anti-CTLA-4, anti-PD-1 and anti-PD-L1. Studies [24] have shown that in the tumor microenvironment, anti-CTLA-4 stimulates the activation of surrounding T cells by blocking the binding of CTLA-4 on the surface of T cells to the ligand CD80/CD86 on APCs, but does not activate T cells. Anti-PD-1/PD-L1 may play an anti-tumor effect by activating T lymphocytes. Reck also believes that because anti-CTLA-4 only plays a role in the immune activation stage, and lacks subsequent immune effectors, it cannot effectively stimulate sufficient anti-tumor immune responses [25]. Pardoll's study demonstrated that anti-PD-1 targeting tumor infiltrating lymphocytes (TIL) can complement the anti-tumor activity of anti-CTLA-4 through non-redundant pathways [26]. Our meta-analysis showed that the ORR, DCR, PFS and OS of anti-CTLA-4 combined with anti-PD-1 / PD-L1 were significantly better than those of anti-CTLA-4 monotherapy, which also verified the above view. We also considered the difference between anti-PD-1 and anti-PD-L1. Anti-PD-1 is not only bound to PD-L1, but also to PD-L2. PD-L2 also belongs to the B7 family of ligands. In addition to being selectively expressed on some tumor cells, it is mainly expressed on APCs. It participates in the formation of its own peripheral immune tolerance by inhibiting T cell proliferation and cytokine production [27]. Studies have shown that the affinity of PD-L2 and PD-1 is 3-4 times greater than PD-L1 [28,29]. Anti-PD-L1 only blocks the PD-1 ~ PD-L1 pathway and does not affect the PD-1 ~ PD-L2 pathway. Experiments have proved that the expression of PD-L2 in different tumors varies greatly. In melanoma tissue samples, only a few tumor cells expressed PD-L2; in gastric cancer, the expression of PD-L2 increased; while in more than half of the HNSCC samples expressed PD-L2 [30]. According to our subgroup analysis of tumor type, the conclusions were also consistent with the above views. In recent years, ICIs have gradually moved from second-line therapy to first-line therapy. In patients who have not received chemotherapy and other treatments, the immune cells in their bodies are in a relatively complete and active state, so that the combination therapy of anti-CTLA-4 and anti-PD-1/anti-PD-L1 can play a synergistic role on the two pathways [23]. For patients who have received more than one line of chemotherapy, systemic chemotherapy is usually considered to be immunosuppressive. Some chemotherapy drugs may reduce the effect of immunotherapy by inducing immunogenic danger signals in dying cancer cells, thus stimulating protective anti-tumor immunity [31]. In our subgroup analysis, the effect of ICI combination therapy in first-line therapy was more significant than that in second and above line therapy, which further validated the above views. Malignant melanoma is one of the most sensitive malignant tumors for immune regulation, and often shows good antigenicity and immunogenicity [31,32]. Most patients with melanoma cells have relatively high numbers of mutations in their DNA due to ultraviolet radiation and melanobiology. These mutations lead to changes in the protein sequence that are easily recognized by T cell responses [33,34]. Studies have shown that strong lymphocyte infiltration often occurs in the primary site of melanoma patients [35]. Our subgroup analysis also confirmed that melanoma was more effective in ICI combination therapy than other solid tumors.
The main concern about the combined application of anti-CTLA-4 and anti-PD-1/PD-L1 is the safety and tolerability of treatment. Immune-related toxic and side effects are managed in accordance with safety guidelines, and most level 2-4 immune-related events can be effectively managed. In this meta-analysis, the side effects (≥ 3) in the ICI combination therapy group were higher than those in the ICI monotherapy group, in which fatigue, nausea, diarrhea, colitis, rash, pruritus, elevated transaminase and lipase were significantly increased. However, there was no significant difference in two groups about decreased appetite, vomiting, maculopapular rash, dyspnea, pneumonia, anemia, asthenia, arthralgia, headache, pyrexia, hyperthyroidism and hypothyroidism. Most side effects are easier to control, and no grade 5 toxicity has been observed. In the systematic evaluation of the safety of ICI combined treatment, Abdelhafeez [2] indicated that fatigue, nausea, diarrhea, rash, elevated AST and lipase in ICI combination therapy group was larger than that in ICI monotherapy group, while there was no significant difference in other side effects, which was basically consistent with our analysis results. In addition, the combination of lower doses of ipilimumab (1 mg/kg) and standard doses of nivolumab or pembrolizumab were shown to reduce the incidence of higher levels of toxicity in some single-arm studies and randomized phase II trials [36]. But more research is needed to prove the effects of reduced doses. We further compared the safety of anti-CTLA-4 combined with anti-PD-1 or anti-PD-L1. The results showed that the side effects of anti-CTLA-4 combined with anti-PD-1 was higher than those of monotherapy (p < 0.00001), but there was no significant difference in side effects between anti-CTLA-4 combined with anti-PD-L1 and monotherapy (p = 0.34). This reminds us that while using anti-PD-L1 treatment, adding anti-CTLA-4 may not increase the toxic and side effects of the drug. In addition, studies have shown that PD-L1 not only binds to PD-1, but also binds to CD80, and PD-L1/CD80 interaction also transmits inhibitory signals in T cells [37,38]. This suggests that anti-PD-L1 can improve immune effects through other pathways, which may provide a new idea for the therapeutic mechanism of anti-PD-L1 combined with anti-CTLA-4. However, due to the limited research we have included and the small number of cases of anti-PD-L1 combined with anti-CTLA-4 treatment, no positive conclusion has been drawn.
Finally, we analyzed the quality of the included studies and the level of evidence for the outcome indicators discussed. The inclusion of relatively high-quality studies suggests the reliability of the meta-analysis. GRADE indicated that the level of evidence for ORR, DCR, PFS and OS were moderate, and further clinical studies are needed to confirm it.
Limitations
The current meta-analysis is limited by several aspects. First, we included 10 RCTs, but the sample size varied between studies, resulting in a smaller sample size among different subgroups, which may be the main source of affecting the quality of meta-analysis. Second, differences in patient statistical quality, follow-up time, course of treatment, and ethnicity lead to heterogeneity. Finally, this was an experimental meta-analysis based on data from studies rather than individual patients. We still lack patient's complications, ECOG score, disease progression and other factors affecting prognosis, which may play important roles in the efficacy of ICI combination therapy.
Conclusions
ICI two-drug combination therapy was superior to ICI monotherapy in ORR, DCR, PFS and OS, but the side effects of ICI combination therapy were higher than ICI monotherapy and most of the side effects were easier to control. Further clinical trials are needed to confirm the effect of dose reduction on efficacy and adverse reactions in order to improve clinical efficacy and patient prognosis.
Acknowledgements
Authors'contributions
XTM and JY contributed to the conception and design this study. YJZ and SW carried out the development of the methodology. XTM and HMW analyzed and interpreted the data. XTM wrote the manuscript. JY and HMW modified and approved it. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 81774221 and No. 81904102), The Capital Health Research and Development of Special (No. 2018-2-1113), Beijing Natural Science Foundation (No. 7184203), Basic-Clinical Cooperation Program from Capital Medical University (No. 17JL14), Research Foundation of Beijing Friendship Hospital, Capital Medical University (No. yyqdkt2016-4), Beijing Municipal 215 High-level Health Person Foundation Project (No. 2014-3-004).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Harris TJ, Drake CG. Primer on tumor immunology and cancer immunotherapy. J Immunother Cancer. 2013;1:12
2. Abdelhafeez AAM, Shohdy KS, Ibrahim W. Safety of Combination Immune Checkpoint Inhibitors Compared to Monotherapy; A Systematic Review and Meta-Analysis. Cancer Invest. 2020;38(3):150-157
3. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565-1570
4. Simsek M, Tekin SB, Bilici M. Immunological Agents Used in Cancer Treatment. Eurasian J Med. 2019;51(1):90-94
5. Janjigian YY, Bendell J, Calvo E. et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol. 2018;36(28):2836-2844
6. Kelly RJ, Lee J, Bang YJ. et al. Safety and Efficacy of Durvalumab and Tremelimumab Alone or in Combination in Patients with Advanced Gastric and Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res. 2020;26(4):846-854
7. Larkin J, Chiarion-Sileni V, Gonzalez R. et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535-1546
8. Long GV, Atkinson V, Lo S. et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672-681
9. Omuro A, Vlahovic G, Lim M. et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674-686
10. Planchard D, Reinmuth N, Orlov S. et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol. 2020;31(5):609-618
11. Postow MA, Chesney J, Pavlick AC. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006-2017
12. Sharma P, Siefker-Radtke A, de Braud F. et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J Clin Oncol. 2019;37(19):1608-1616
13. Siu LL, Even C, Mesia R. et al. Safety and Efficacy of Durvalumab With or Without Tremelimumab in Patients With PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol. 2019;5(2):195-203
14. Ready NE, Ott PA, Hellmann MD. et al. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J Thorac Oncol. 2020;15(3):426-435
15. Chen Y, Zhou Y, Tang L. et al. Immune-Checkpoint Inhibitors as the First Line Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. J Cancer. 2019;10(25):6261-6268
16. Dafni U, Tsourti Z, Vervita K. et al. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer. 2019;134:127-140
17. Hellmann MD, Paz-Ares L, Bernabe Caro R. et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2019;381(21):2020-2031
18. Tawbi HA, Forsyth PA, Algazi A. et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med. 2018;379(8):722-730
19. Scherpereel A, Mazieres J, Greillier L. et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, openlabel, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20(2):239-253
20. Hurst JH. Cancer immunotherapy innovator James Allison receives the 2015 Lasker~DeBakey Clinical Medical Research Award. The Journal of clinical investigation. 2015;125(10):3732-3736
21. Simsek M, Tekin SB, Bilici M. Immunological Agents Used in Cancer Treatment. Eurasian J Med. 2019;51(1):90-94
22. Sansom DM. Moving CTLA-4 from the trash to recycling. Science. 2015;349(6246):377-378
23. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561-584
24. Horn L, Mansfield AS, Szczesna A. et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379(23):2220-2229
25. Reck M, Luft A, Szczesna A. et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol. 2016;34(31):3740-3748
26. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264
27. Chae YK, Arya A, Iams W. et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J Immunother Cancer. 2018;6(1):39
28. Youngnak P, Kozono Y, Kozono H. et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307(3):672-677
29. Cheng X, Veverka V, Radhakrishnan A. et al. Structure and interactions of the human programmed cell death 1 receptor. J Biol Chem. 2013;288(17):11771-11785
30. Yearley JH, Gibson C, Yu N. et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin Cancer Res. 2017;23(12):3158-3167
31. Dudek-Peric AM, Ferreira GB, Muchowicz A. et al. Antitumor immunity triggered by melphalan is potentiated by melanoma cell surface-associated calreticulin. Cancer Res. 2015;75(8):1603-1614
32. Twyman-Saint Victor C, Rech AJ, Maity A. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373-377
33. Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84(3):539-549
34. Faramarzi S, Ghafouri-Fard S. Melanoma: a prototype of cancer-testis antigen-expressing malignancies. Immunotherapy. 2017;9(13):1103-1113
35. Quaglino P, Marenco F, Osella-Abate S. et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21(2):409-414
36. Weiss SA, Wolchok JD, Sznol M. Immunotherapy of Melanoma: Facts and Hopes. Clin Cancer Res. 2019;25(17):5191-5201
37. Park JJ, Omiya R, Matsumura Y. et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116(8):1291-1298
38. Talay O, Shen CH, Chen L. et al. B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proc Natl Acad Sci U S A. 2009;106(8):2741-2746
Author contact
![]() Corresponding authors: Cancer Center, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong An Road, Xicheng District, Beijing, 100050, China. Phone: +86-10-63139326, Fax: +86-10-63139326. E-mail: yujing026edu.cn (Jing Yu); Department of Traditional Chinese Medicine, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong An Road, Xi Cheng District, Beijing, 100050, China. Phone: +86-10-63139097, Fax: +86-10-63169326, E-mail: weihuamin6255com (Huamin Wei).
Corresponding authors: Cancer Center, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong An Road, Xicheng District, Beijing, 100050, China. Phone: +86-10-63139326, Fax: +86-10-63139326. E-mail: yujing026edu.cn (Jing Yu); Department of Traditional Chinese Medicine, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong An Road, Xi Cheng District, Beijing, 100050, China. Phone: +86-10-63139097, Fax: +86-10-63169326, E-mail: weihuamin6255com (Huamin Wei).

 Global reach, higher impact
Global reach, higher impact