Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(6):1532-1541. doi:10.7150/jca.37390 This issue Cite
Research Paper
Prognosis for different patterns of distant metastases in patients with uterine cervical cancer: a population-based analysis
1. International Medical Center, Tianjin First Central Hospital, Tianjin, China.
2. Nursing department, Tianjin First Central Hospital, Tianjin, China.
Received 2019-6-7; Accepted 2019-12-1; Published 2020-1-14
Abstract
Background: Uterine cervical cancer (UCC) is a common malignant tumor in women. We conducted this work to provide a though description on the patterns of distant metastases and to investigate the relevant factors for prognosis of UCC patients based on a large population.
Patients and methods: UCC patients with FIGO stage IVB, being the study group, were identified from the Surveillance, Epidemiology, and End Result (SEER) database from 2010 to 2016. UCC patients with same inclusion criteria, being validation group, were identified from Tianjin First Central Hospital from 2004 to 2017. Patterns of distant metastases were described according to the number of metastatic sites. Survival of different patterns were calculated and prognostic factors were investigated by Cox hazard regression analysis.
Results: Distant metastases were recorded in 1448 UCC patients among whom 295 patients (30.8%) developed metastases in two or more organs. Compared with the median OS of 8 (95%CI, 7.07-8.93) months in the patients with the single site, worse survival of 5 (95%CI, 4.29-5.71) months was noticed in the patients with multiple metastases. Age ≥ 65 years, black race, higher grade, higher T stage and more metastatic sites were proved to be the prognostic factors for all the patients in the advanced stage and the results were partly validated in the validation cohort. Moreover, black race and higher T-stage for the patients with the single metastatic sites and age ≥ 65, uninsured status, surgical treatment and metastatic pattern for the patients with two metastatic sites were prognostic factors. No independent factor was found in patients with three or more metastases.
Conclusion: Around 70% of the patients suffered one site distant metastasis in UCC patients with IVB stage while 30% with multiple metastases and a significantly reduced survival. Different survivals and prognostic factors were noticed for different patterns of distant metastases.
Keywords: Uterine cervical cancer, SEER, distant metastases, prognostic factor
Introduction
Uterine cervical cancer (UCC) is a common cancer in women. The incidence decreased in the developed countries in recent decades due to the HPV prevention strategy and cervical cancer screening [1]. However, as previously reported, the incidence of UCC increased by 2.6% per year (1.1%, 4.2%) in Japan after 1997 [2]. It was also thought to be a crucial problem in other countries [1,3] and the UCC patients lost workdays annually up to 8 years after diagnosis [4].
Distant metastasis was accepted to be one of the significant characteristics in the advanced cancer. Brain metastases were reported in a series of cases after primary diagnosis of UCC [5] or as the initial presentation [6]. Such distant metastases were further reported in the studies with the limited samples [7,8]. Bone metastases [9,10] and lung metastases [11] were also descripted based on the relatively large population. As to the effect of distant metastases on the survival of patients, around 13% of UCC patients were diagnosed at an advanced stage, leading to the decreased survival [12]. The median survival after bone metastases was reported to be only 5 months [13].
Prognostic factors in the cancer patients with distant metastases have been widely studied [14-16]. In the recent study, some independent factors resulting in poor survival were found in UCC patients including the extra-skeletal metastases, performance status (level 3 to 4), radiation and/or chemotherapy, multiple bone metastases, and a bone metastases-free interval of <12 months [13]. Another study, analyzing 56 UCC patients with lung metastases, revealed the prolonged overall survival (OS) in patients with less lung lesions or after surgical resection [11]. These studies described the survival trend in UCC patients with different distant metastases. However, limited sample size and different data resource made the results difficult to be integrated to analyze the performance of different patterns of distant metastases.
Thus, we conducted the present study based on the Surveillance, Epidemiology, and End Results (SEER) database and our own institution. The patterns of distant metastases were described and the prognostic factors were investigated in patients based on the number and patterns of metastatic sites.
Materials and Methods
The patterns of distant metastases for UCC patient in both groups were described. The data of the study group were extracted from National Cancer Institute Surveillance, Epidemiology and End Results (SEER) database (https://seer.cancer.gov/), which approximately covers 28% of population in the USA. Since the details of metastases were not available before 2010, UCC patients with International Federation of Gynecology and Obstetrics (FIGO) stage IVB who were diagnosed between 2010 and 2016 in SEER database were collected. The data of the validation group were collected from Tianjin First Central Hospital, diagnosed between 2004 and 2017. The exclusion criteria were as follows: patients diagnosed at autopsy or via death certificate, without detailed information about site-specific metastases (including the bone, brain, liver, and lung). The flow-chart of the selection for study group was listed in Figure 1. After getting the patterns and the prognostic factors in study group, we validated the results based on our own institute data. The procedure for the selection of validation cohort was described as Figure 2.
Flowchart of the patient selection in the study group.
Flowchart of the patient selection in the validation group.
Statistical analysis
The following demographic and clinicopathological variables were collected in the study group: age, race, insurance recode, marital status, tumor grade, histological subtype (including adenocarcinoma, squamous cell carcinoma and other subtypes), T stage, N stage, number of distant metastases and surgical treatment. Aforementioned variables except race and insurance recode were collected in our validation group.
The differences in the incidence of demographic and clinicopathological variables were analyzed by the chi-squared (χ2) test. The primary outcome of the survival analysis was the overall survival (OS), which was defined from the time of UCC diagnosis to death. Survival duration was obtained using the Kaplan-Meier method, the differences between the curves were tested by Log-rank test. Cox proportional hazard regression analysis was performed for revealing the prognostic factors in UCC patients with FIGO stage IVB. Variables with P < 0.05 in the univariate Cox regression analysis were then further analyzed using a multivariate regression analysis.
Ethics statement
The SEER database is a free database, and the data released by the SEER database do not require informed patient consent, because cancer is a reportable disease in every state of the USA. The present study complied with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Characteristics of the patients
According to the aforementioned inclusion criteria, a total of 1448 UCC patients with FIGO stage IVB were included in the study group. The mean age was 57.53 ± 14.35 years, most patients were white race (72.4%), and unmarried (60.6%). A total of 1305 (90.1%) patients got medical insurance. After excluding 35.4% of the patients whose information on grade were unrecorded, the main grades of primary tumor were grade III - IV (44.8%). The most common histological subtype was squamous cell carcinoma (58.3%). About 51.1% of the patients were diagnosed with T3 and T4 stage, while 29.5% with T1-2 stage and 19.4% with unknown T stage. Besides, only 8.4% of the patients received surgical treatment. Baseline demographic and clinical characteristics of patients in study group were shown in Table 1.
Sites of distant metastases
In the study group, lung was the most common site of distant metastases (n= 941, 65.0%), followed by bone (n=520, 35.9%), liver (n=466, 32.2%), and brain (n=81, 5.6%). A total of 1002 patients (69.2%) showed single distant metastatic site while 446 (30.8%) patients showed two or more distant metastatic sites. The distributions of the sites of distant metastases in study group and validation group are shown in Table 2.
Survival outcomes in different patterns of metastases
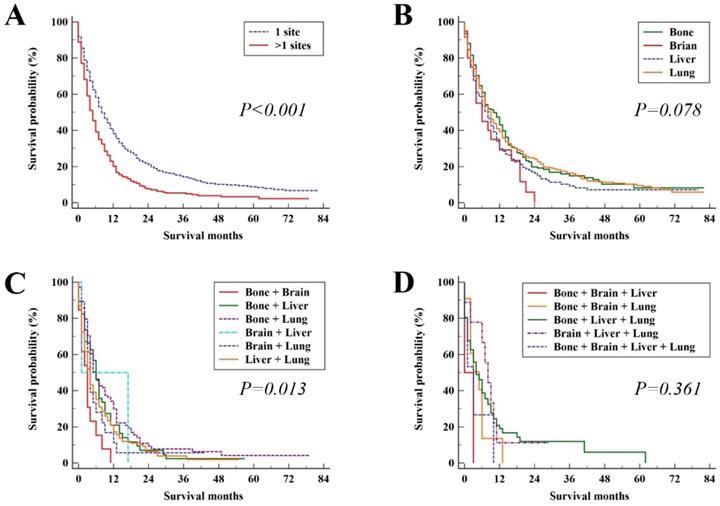
In the study group, the median survival time was 7 months (95%CI, 6.38 - 7.62). The median OS and 95% CI for patients with different metastatic patterns were shown in Table 2. The median OS for patients with the single metastatic site and multiple metastatic sites was 8 (95%CI, 7.07 - 8.93) and 5 (95%CI, 4.29 - 5.71) months, respectively (P < 0.001, Figure 3A). For patients with only bone, brain, liver and lung metastasis, the median OS was 10 (95%CI, 7.78 - 12.22), 6 (95%CI, 1.64 - 10.36), 8 (95%CI, 6.11 - 9.98) and 9 (95%CI, 7.85 - 10.15) months, respectively (P = 0.078, Figure 3B). In patients with two distant metastatic sites, there were significant difference among metastatic pattern (P = 0.013, Figure 3C). No significant difference among metastatic pattern was found in the patients with three or four distant metastatic sites (P = 0.361, Figure 3D).
Prognostic factors for UCC patients in different patterns of metastases
Prognostic factors for total UCC patients
In the study group, patient with age ≥ 65 years old (HR = 1.35; 95% CI, 1.03 -1.77; P = 0.031), black race (HR = 1.27; 95% CI, 1.03 -1.56; P = 0.028), higher grade (HR = 1.28; 95% CI, 1.08 - 1.53; P = 0.006), higher T-stage (HR = 1.27; 95% CI, 1.08 - 1.51; P = 0.005), and two or more metastatic sites were significantly associated with OS in the Cox regression analysis. The HR and 95%CI for patients with two or more than two metastatic sites were 1.65 (95% CI, 1.37 - 1.99; P < 0.001) and 1.68 (95% CI, 1.19 - 2.39; P = 0.004), respectively. Trend chi-square test confirmed the worse OS in patients with more than two metastatic sites compared with those with two metastatic sites.
Description of the SEER study population and validation group with uterine cervical cancer by distant metastases at diagnosis.
| Subject characteristics | Study group | Validation group | ||||||
|---|---|---|---|---|---|---|---|---|
| Bone metastases | Brain metastases | Liver metastases | Lung metastases | Bone metastases | Brain metastases | Liver metastases | Lung metastases | |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| (N=520, %) | (N=81, %) | (N=466, %) | (N=941, %) | (N=94, %) | (N=14, %) | (N=68, %) | (N=152, %) | |
| Age | ||||||||
| ≤ 40 | 72 (40.7) | 12 (6.8) | 67 (37.9) | 103 (58.2) | 8 (34.8) | 2 (8.7) | 4 (17.4) | 18 (78.3) |
| 41-64 | 307 (37.2) | 54 (6.5) | 260 (31.5) | 535 (64.8) | 65 (49.2) | 9 (6.8) | 47 (35.6) | 85 (64.4) |
| ≥ 65 | 141 (31.7) | 15 (3.4) | 139 (31.2) | 303 (68.1) | 21 (29.2) | 3 (4.2) | 17 (23.6) | 49 (68.1) |
| Race | ||||||||
| White | 384 (36.6) | 61 (5.8) | 318 (30.3) | 685 (65.3) | NA | NA | NA | NA |
| Black | 87 (32.7) | 12 (4.5) | 100 (37.6) | 164 (61.7) | NA | NA | NA | NA |
| Others | 49 (37.1) | 7 (5.3) | 48 (36.4) | 92 (69.7) | NA | NA | NA | NA |
| Unknown | 0 (0) | 1 (100.0) | 0 (0) | 0 (0) | NA | NA | NA | NA |
| Insurance Record | ||||||||
| Uninsured | 38 (33.3) | 8 (7.0) | 42 (36.8) | 62 (54.4) | NA | NA | NA | NA |
| Insured | 474 (36.3) | 72 (5.5) | 416 (31.9) | 858 (65.7) | NA | NA | NA | NA |
| Unknown | 8 (27.6) | 1 (3.4) | 8 (27.6) | 21 (72.4) | NA | NA | NA | NA |
| Marital status | ||||||||
| Unmarried | 307 (35.0) | 49 (5.6) | 271 (30.9) | 574 (65.4) | 53 (40.5) | 8 (6.1) | 43 (32.8) | 84 (64.1) |
| Married | 191 (37.9) | 26 (5.2) | 165 (32.7) | 322 (63.9) | 38 (44.2) | 4 (4.7) | 22 (25.6) | 58 (67.4) |
| Unknown | 22 (33.3) | 6 (9.1) | 30 (45.5) | 45 (68.2) | 3 (30.0) | 2 (20.0) | 3 (30.0) | 10 (100.0) |
| Grade | ||||||||
| Grade I-II | 94 (32.8) | 13 (4.5) | 67 (23.3) | 184 (64.1) | 18 (38.3) | 3 (6.4) | 10 (21.3) | 33 (70.2) |
| Grade III-IV | 241 (37.1) | 47 (7.2) | 209 (32.2) | 438 (67.5) | 42 (38.9) | 9 (8.3) | 35 (32.4) | 73 (67.6) |
| Unknown | 185 (36.1) | 21 (4.1) | 190 (37.1) | 319 (62.3) | 34 (47.2) | 2 (2.8) | 23 (31.9) | 46 (63.9) |
| Histology | ||||||||
| AC | 87 (36.0) | 11 (4.5) | 82 (33.9) | 157 (64.9) | 15 (41.7) | 4 (11.1) | 11 (30.6) | 26 (72.2) |
| SCC | 301 (35.7) | 51 (6.0) | 230 (27.3) | 566 (67.1) | 52 (39.7) | 7 (5.3) | 32 (24.4) | 89 (67.9) |
| Others | 132 (36.5) | 19 (5.2) | 154 (42.5) | 218 (60.2) | 27 (45.0) | 3 (5.0) | 25 (41.7) | 37 (61.7) |
| T stage | ||||||||
| T1-2 | 155 (36.3) | 24 (5.6) | 147 (34.4) | 267 (62.5) | 30 (40.0) | 5 (6.7) | 23 (30.7) | 53 (70.7) |
| T3-4 | 267 (36.1) | 28 (3.8) | 221 (29.9) | 482 (65.1) | 49 (44.1) | 4 (3.6) | 32 (28.8) | 68 (61.3) |
| Unknown | 98 (34.9) | 29 (10.3) | 98 (34.9) | 192 (68.3) | 15 (36.6) | 5 (12.2) | 13 (31.7) | 31 (75.6) |
| N stage | ||||||||
| N0 | 133 (32.0) | 16 (3.8) | 140 (33.7) | 260 (62.5) | 24 (33.3) | 1 (1.4) | 23 (31.9) | 45 (62.5) |
| N1 | 325 (39.3) | 46 (5.6) | 250 (30.2) | 539 (65.1) | 61 (47.3) | 11 (8.5) | 36 (27.9) | 87 (67.4) |
| Unknown | 62 (30.4) | 19 (9.3) | 76 (37.3) | 142 (69.6) | 9 (34.6) | 2 (7.7) | 9 (34.6) | 20 (76.9) |
| Surgical treatment | ||||||||
| No Surgery | 486 (36.7) | 73 (5.5) | 424 (32.0) | 866 (65.3) | 86 (42.4) | 13 (6.4) | 63 (31.0) | 136 (67.0) |
| Surgery | 34 (28.1) | 8 (6.6) | 42 (34.7) | 74 (61.2) | 8 (33.3) | 1 (4.2) | 5 (20.8) | 16 (66.7) |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (100.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Abbreviations: SEER = Surveillance, Epidemiology, and End Result; AC = adenocarcinoma; SCC = squamous cell carcinoma.
Kaplan-Meier analysis of overall survival for UCC patients in the study group (A), single site of distant metastases (B), two sites of distant metastases (C), and patients with three or more sites of distant metastases (D).
Patterns of distant metastases and overall survival time for uterine cervical cancer patients in the study and the validation group.
| Sites of distant metastases | Study group | Validation group | ||
|---|---|---|---|---|
| N (%) | Median OS (95%CI) | N (%) | Median OS (95%CI) | |
| One site of distant metastases | ||||
| Bone | 236 (16.3) | 10 (7.78 - 12.22) | 39 (17.2) | 11 (5.30 - 16.70) |
| Brain | 20 (1.4) | 6 (1.64 - 10.36) | 2 (0.9) | 6 (-) |
| Liver | 177 (12.2) | 8 (6.11 - 9.89) | 20 (8.8) | 8 (1.97 - 14.04) |
| Lung | 569 (39.3) | 9 (7.85 - 10.15) | 90 (39.6) | 8 (5.24 - 10.76) |
| Two sites of distant metastases | ||||
| Bone + Brain | 13 (0.9) | 3 (1.37 - 4.63) | 3 (1.3) | 4 (2.40 - 5.60) |
| Bone + Liver | 57 (3.9) | 6 (4.71 - 7.29) | 10 (4.4) | 3 (0 - 6.10) |
| Bone + Lung | 114 (7.9) | 6 (4.43 - 7.57) | 20 (8.8) | 9 (3.52 - 14.48) |
| Brain + Liver | 2 (0.1) | 1 | 0 | - |
| Brain + Lung | 19 (1.3) | 4 (2.99 - 5.01) | 1 (0.4) | 2 (-) |
| Liver + Lung | 132 (9.1) | 4 (3.06 - 4.94) | 17 (7.5) | 3 (0 - 6.36) |
| Three sites of distant metastases | ||||
| Bone + Brain + Liver | 2 (0.1) | - | 1 (0.4) | 0 (-) |
| Bone + Brain + Lung | 11 (0.8) | 5 (0.48 - 9.52) | 4 (1.8) | 6 (5.15 - 6.85) |
| Bone + Liver + Lung | 82 (5.7) | 4 (2.04 - 5.96) | 17 (7.5) | 8 (4.22 -11.78) |
| Brain + Liver + Lung | 9 (0.6) | 8 (5.08 - 10.92) | 3 (1.3) | 8 (0 - 20.80) |
| Four sites of distant metastases | ||||
| Bone + Brain + Liver + Lung | 5 (0.3) | 3 (0.51 - 5.49) | 0 | - |
Abbreviations: FIGO=International Federation of Gynecology and Obstetrics, OS=Overall Survival
However, the factor of two metastatic sites was the only independent prognostic factor for all patients in the validation group. The aforementioned prognostic factors were shown in Table 3.
Patients with single distant metastatic site
As shown in Table 4, for patients with one metastatic site, univariate Cox regression analysis showed that the following factors were significantly associated with overall survival: patient with age ≥ 65 (HR = 1.61; 95% CI, 1.25 - 2.07; P < 0.001), black race (HR = 1.28; 95% CI, 1.07 - 1.53; P = 0.006), married status (HR = 0.81; 95% CI, 0.69 - 0.94; P = 0.007), higher grade (HR = 1.23; 95% CI, 1.02 - 1.49; P = 0.029), higher T-stage (HR = 1.44; 95% CI, 1.21 - 1.70; P < 0.001), surgical treatment (HR = 0.60; 95% CI, 0.45 - 0.79; P < 0.001). Although the variable of metastatic site was not significant in univariate analysis, it was included in further multivariate analysis. The multivariate analysis identified the following variables as independent factors for prognosis: black race and higher T-stage. Metastatic site was not the independent prognostic factor in patients with the single distant metastatic site. In the validation group, N-stage was the only independent prognostic factor for patients with one site metastasis. The aforementioned prognostic factors were shown in Table 4.
Univariate and Multivariate Cox regression analysis of prognostic factors for all FIGO stage IVB UCC patients in study and validation group.
| Variable | Study group | Validation group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age | ||||||||||||
| ≤ 40 | 1 | 1 | 1 | - | ||||||||
| 41-64 | 1.05 | 0.87 - 1.27 | 0.614 | 0.97 | 0.75 - 1.25 | 0.794 | 0.75 | 0.46 - 1.23 | 0.256 | - | - | - |
| ≥ 65 | 1.51 | 1.23 - 1.84 | <0.001 | 1.35 | 1.03 - 1.77 | 0.031 | 1.42 | 0.85 - 2.37 | 0.185 | - | - | - |
| Race | ||||||||||||
| White | 1 | 1 | NA | |||||||||
| Black | 1.25 | 1.07 - 1.44 | 0.004 | 1.27 | 1.03 - 1.56 | 0.028 | NA | NA | NA | NA | NA | NA |
| Others | 0.87 | 0.70 - 1.07 | 0.177 | 0.97 | 0.72 - 1.30 | 0.813 | NA | NA | NA | NA | NA | NA |
| Insurance Recode | ||||||||||||
| Uninsured | 1 | - | NA | NA | ||||||||
| Insured | 0.87 | 0.70 - 1.08 | 0.201 | - | - | - | NA | NA | NA | NA | NA | NA |
| Marital status | ||||||||||||
| Unmarried | 1 | 1 | 1 | - | ||||||||
| Married | 0.86 | 0.76 - 0.97 | 0.014 | 0.89 | 0.75 - 1.05 | 0.160 | 0.93 | 0.68 - 1.27 | 0.635 | - | - | - |
| Grade | ||||||||||||
| Grade I- II | 1 | 1 | 1 | - | ||||||||
| Grade III- IV | 1.31 | 1.12 - 1.53 | 0.001 | 1.28 | 1.08 - 1.53 | 0.006 | 0.951 | 0.65 - 1.39 | 0.796 | - | - | - |
| Histology | ||||||||||||
| AC | 1 | - | 1 | - | ||||||||
| SCC | 1.01 | 0.86 - 1.18 | 0.945 | - | - | - | 0.694 | 0.46 - 1.05 | 0.081 | - | - | - |
| Others | 1.17 | 0.98 - 1.41 | 0.087 | - | - | - | 0.966 | 0.61 - 1.52 | 0.880 | - | - | - |
| T stage | ||||||||||||
| T1-2 | 1 | 1 | 1 | - | ||||||||
| T3-4 | 1.29 | 1.12 - 1.47 | <0.001 | 1.27 | 1.08 - 1.51 | 0.005 | 0.903 | 0.65 - 1.26 | 0.552 | - | - | - |
| N stage | ||||||||||||
| N0 | 1 | - | 1 | - | ||||||||
| N1 | 1.00 | 0.88 - 1.15 | 0.984 | - | - | - | 0.892 | 0.64 - 1.24 | 0.496 | - | - | - |
| Number of metastasis sites | ||||||||||||
| 1 | 1 | 1 | 1 | 1 | ||||||||
| 2 | 1.56 | 1.36 - 1.79 | <0.001 | 1.65 | 1.37 - 1.99 | <0.001 | 1.508 | 1.07 - 2.13 | 0.021 | 1.48 | 1.05 - 2.10 | 0.026 |
| ≥ 3 | 1.70 | 1.36 - 2.13 | <0.001 | 1.68 | 1.19 - 2.39 | 0.004 | 1.542 | 0.96 - 2.47 | 0.070 | 1.46 | 0.91 - 2.34 | 0.119 |
| Surgical treatment | ||||||||||||
| No Surgery | 1 | 1 | 1 | 1 | ||||||||
| Surgery | 0.63 | 0.50 - 0.79 | <0.001 | 0.76 | 0.58 - 1.00 | 0.053 | 0.592 | 0.35 - 1.01 | 0.053 | 0.62 | 0.37 - 1.06 | 0.083 |
Patients with two or more distant metastatic sites
As shown in Table 5, univariate Cox regression analysis suggested that the following factors being significantly associated with the survival of patients who developed two distant metastatic sites: age, race, insurance, metastatic pattern and surgical treatment. The multivariate analysis suggested that age ≥ 65, uninsured status, surgical treatment and metastatic pattern were the independent prognostic factors in patients with two distant metastatic sites. In the validation group, N-stage was the only factor associated with overall survival in patients with two distant metastatic sites.
For patients with three or four distant metastatic sites in study group, no factors were significantly associated with overall survival and metastatic site was not the independent prognostic factor in patients with three or more distant metastatic sites. In the validation group, marital status was the only factor associated with overall survival. The results of Univariate Cox regression analysis for patients with three or more distant metastatic sites in both study group and validation group were shown in Table 6.
Validation of the survival and for prognostic factors in the validation group
In the validation group, from 2004 to 2017 in Tianjin First Central Hospital, 227 UCC patients were included. The baseline demographic and clinical characteristics for patients in validation group were shown in Table 1.
In our validation cohort, lung was also the most common site of distant metastases (n=152, 67.0%), followed by bone (n=94, 41.4%), liver (n=68, 30.0%), and brain (n=14, 6.2%). A total of 151 (66.5%) patients showed single distant metastatic site while 76 (33.5%) patients showed two or three distant metastatic sites. No patient showed both lung, bone, liver and brain metastasis at the same time. The distribution of the distant metastatic sites for two groups was listed in Table 2.
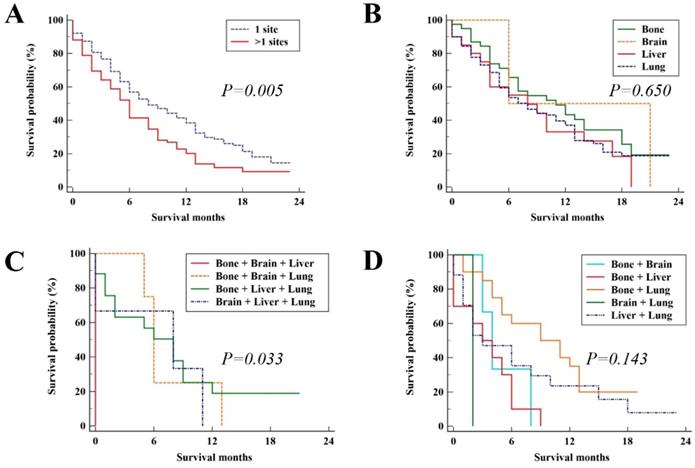
In the validation group, the median survival time was 7 months (95%CI, 5.70 - 8.30). The median OS for patients with the single metastatic site and multiple metastatic sites was 8 (95%CI, 5.43 - 10.57) and 6 (95%CI, 4.72 - 7.28), respectively with significant difference (P = 0.005, Figure 4A). The survival outcome of validation group was similar to the results in the study group. For patients with single metastatic site and patients with three distant metastatic sites, there was no significant difference among metastatic pattern (P = 0.650, Figure 4B and P = 0.143, Figure 4D, respectively). While for patients with two distant metastatic sites, there were significant differences among metastatic pattern (P = 0.033, Figure 4C).
Discussion
In the present study with the large population, to our knowledge, it was the first time to perform a thorough investigation on both the survival and prognostic factors for different patterns of distant metastases in initial UCC patients at the FIGO stage ⅣB. Around 30% of all the patients suffered multiple distant metastases at the end of their lives. Higher incidence of multiple metastases was reported in another study [8]. The present study revealed that involvement of extra metastases significantly reduced patient's survival. Meanwhile, different prognostic factors were found in patients with various metastatic patterns.
Most of the patients suffered single distant metastasis among which lung was the most common site in the present study, followed by bone, liver, and brain. A similar metastatic distribution was reported in another study including metastases to lung in 11 patients, bone in 7 patients and liver in 4 patients [17]. However, a much larger sample size in this study provided us a chance to evaluate the diversities in metastatic site-specific survival. Cox analysis found no significant difference in patients with any one metastasis in this study. UCC patients with only bone metastasis showed a trend of better survival than the patients with other three types of distant metastases. Comparable median OS of 8 months in patients with lung metastasis was confirmed in both previous and the present study. Comparable median survival of 10 months [9] and worse survival (5 months) [13] after diagnosis of bone metastasis were shown in other studies. Brain metastasis in UCC patients showed the worst median OS of only 4 months which was consistent with the reported result [8]. Besides, the multivariate analysis identified the higher grade and higher T-stage as prognostic factors for UCC patients with the single site of distant metastases.
Univariate and Multivariate Cox regression analysis of prognostic factors for FIGO stage IVB UCC patients with single distant metastatic site in the study group and the validation group.
| Variable | Study group | Validation group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age | ||||||||||||
| ≤ 40 | 1 | 1 | 1 | - | ||||||||
| 41-64 | 1.00 | 0.79 - 1.28 | 0.972 | 0.90 | 0.65 - 1.25 | 0.538 | 0.67 | 0.36 - 1.26 | 0.212 | - | - | - |
| ≥ 65 | 1.61 | 1.25 - 2.07 | <0.001 | 1.38 | 0.98 - 1.94 | 0.062 | 1.41 | 0.75 - 2.67 | 0.286 | - | - | - |
| Race | ||||||||||||
| White | 1 | 1 | NA | |||||||||
| Black | 1.28 | 1.07 - 1.53 | 0.006 | 1.37 | 1.07 - 1.75 | 0.012 | NA | NA | NA | NA | NA | NA |
| Others | 0.83 | 0.62 - 1.10 | 0.186 | 0.96 | 0.64 - 1.44 | 0.835 | NA | NA | NA | NA | NA | NA |
| Insurance Recode | ||||||||||||
| Uninsured | 1 | - | NA | NA | ||||||||
| Insured | 0.93 | 0.72 - 1.21 | 0.595 | - | - | - | NA | NA | NA | NA | NA | NA |
| Marital status | ||||||||||||
| Unmarried | 1 | 1 | 1 | - | ||||||||
| Married | 0.81 | 0.69 - 0.94 | 0.007 | 0.90 | 0.73 - 1.11 | 0.318 | 0.74 | 0.50 - 1.10 | 0.133 | - | - | - |
| Grade | ||||||||||||
| Grade I- II | 1 | 1 | 1 | - | ||||||||
| Grade III- IV | 1.23 | 1.02 - 1.49 | 0.029 | 1.22 | 0.99 - 1.51 | 0.057 | 0.88 | 0.55 - 1.41 | 0.596 | - | - | - |
| Histology | ||||||||||||
| AC | 1 | - | 1 | - | ||||||||
| SCC | 0.95 | 0.78 - 1.15 | 0.573 | - | - | - | 0.71 | 0.40 - 1.25 | 0.232 | - | - | - |
| Others | 1.05 | 0.84 - 1.32 | 0.661 | - | - | - | 0.87 | 0.46 - 1.62 | 0.650 | - | - | - |
| T stage | ||||||||||||
| T1-2 | 1 | 1 | 1 | - | ||||||||
| T3-4 | 1.44 | 1.21 - 1.70 | <0.001 | 1.43 | 1.16 - 1.77 | 0.001 | 1.03 | 0.67 - 1.58 | 0.904 | - | - | - |
| N stage | ||||||||||||
| N0 | 1 | - | 1 | 1 | ||||||||
| N1 | 0.96 | 0.82 - 1.13 | 0.622 | - | - | - | 0.67 | 0.45 - 0.99 | 0.046 | 0.65 | 0.43 - 0.99 | 0.043 |
| Metastatic sites | ||||||||||||
| Bone | 1 | 1 | 1 | 1 | ||||||||
| Brain | 1.54 | 0.96 - 2.48 | 0.073 | 1.66 | 0.91 - 3.03 | 0.102 | 1.01 | 0.24 - 4.29 | 0.992 | 0.95 | 0.22 - 4.10 | 0.945 |
| Liver | 1.22 | 0.98 - 1.52 | 0.083 | 1.00 | 0.73 - 1.38 | 0.987 | 1.39 | 0.75 - 2.58 | 0.301 | 1.29 | 0.67 - 2.49 | 0.443 |
| Lung | 1.03 | 0.86 - 1.23 | 0.749 | 0.91 | 0.71 - 1.15 | 0.415 | 1.27 | 0.81 - 1.99 | 0.289 | 1.27 | 0.78 - 2.07 | 0.330 |
| Surgical treatment | ||||||||||||
| No Surgery | 1 | 1 | 1 | 1 | ||||||||
| Surgery | 0.60 | 0.45 - 0.79 | <0.001 | 0.78 | 0.56 - 1.09 | 0.145 | 0.52 | 0.27 - 1.00 | 0.051 | 0.51 | 0.26 - 1.03 | 0.059 |
Univariate and Multivariate Cox regression analysis of prognostic factors for FIGO stage ⅣB UCC patients with two sites of distant metastases in the study and the validation group.
| Variable | Study group | Validation group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | |||||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age | |||||||||
| ≤ 40 | 1 | 1 | 1 | ||||||
| 41-64 | 1.23 | 0.86 - 1.74 | 0.255 | 0.99 | 0.69 - 1.44 | 0.970 | 0.99 | 0.38 - 2.58 | 0.984 |
| ≥ 65 | 1.79 | 1.21 - 2.65 | 0.004 | 1.72 | 1.14 - 2.59 | 0.010 | 2.60 | 0.89 - 7.60 | 0.080 |
| Race | |||||||||
| White | 1 | 1 | NA | ||||||
| Black | 1.39 | 1.04 - 1.85 | 0.027 | 1.237 | 0.92 - 1.67 | 0.165 | NA | NA | NA |
| Others | 0.78 | 0.54 - 1.13 | 0.195 | 0.775 | 0.53 - 1.14 | 0.191 | NA | NA | NA |
| Insurance Recode | |||||||||
| Uninsured | 1 | 1 | NA | ||||||
| Insured | 0.51 | 0.33 - 0.79 | 0.002 | 0.522 | 0.34 - 0.81 | 0.004 | NA | NA | NA |
| Marital status | |||||||||
| Unmarried | 1 | - | 1 | ||||||
| Married | 0.83 | 0.65 - 1.05 | 0.117 | - | - | - | 1.18 | 0.63 - 2.20 | 0.603 |
| Grade | |||||||||
| Grade I- II | 1 | - | 1 | ||||||
| Grade III- IV | 1.35 | 0.97 - 1.87 | 0.071 | - | - | - | 1.09 | 0.52 - 2.30 | 0.815 |
| Histology | |||||||||
| AC | 1 | - | 1 | ||||||
| SCC | 1.27 | 0.90 - 1.78 | 0.174 | - | - | - | 0.81 | 0.40 - 1.63 | 0.550 |
| Others | 1.40 | 0.97 - 2.03 | 0.074 | - | - | - | 0.98 | 0.45 - 2.16 | 0.959 |
| T stage | |||||||||
| T1-2 | 1 | - | 1 | ||||||
| T3-4 | 1.05 | 0.80 - 1.37 | 0.740 | - | - | - | 0.87 | 0.45 - 1.69 | 0.675 |
| N stage | |||||||||
| N0 | 1 | - | 1 | ||||||
| N1 | 1.12 | 0.85 - 1.47 | 0.420 | - | - | - | 2.63 | 1.13 - 6.11 | 0.025 |
| Metastatic sites | |||||||||
| Bone + Brain | 1 | 1 | 1 | ||||||
| Bone + Liver | 0.47 | 0.25 - 0.86 | 0.015 | 0.44 | 0.24 - 0.83 | 0.011 | 1.27 | 0.35 - 4.62 | 0.721 |
| Bone + Lung | 0.40 | 0.22 - 0.72 | 0.002 | 0.36 | 0.20 - 0.65 | 0.001 | 0.40 | 0.11 - 1.42 | 0.158 |
| Brain + Liver | 0.46 | 0.10- 2.06 | 0.312 | 0.99 | 0.21 - 4.63 | 0.984 | NA | NA | NA |
| Brain + Lung | 0.57 | 0.28 - 1.18 | 0.133 | 0.56 | 0.27 - 1.17 | 0.123 | 2.14 | 0.22 - 21.19 | 0.517 |
| Liver + Lung | 0.56 | 0.31 - 0.99 | 0.047 | 0.50 | 0.28 - 0.89 | 0.018 | 0.64 | 0.18 - 2.27 | 0.485 |
| Surgical treatment | |||||||||
| No Surgery | 1 | 1 | 1 | ||||||
| Surgery | 0.64 | 0.42 - 0.99 | 0.046 | 0.509 | 0.32 - 0.81 | 0.005 | 0.74 | 0.26 - 2.07 | 0.560 |
Univariate Cox regression analysis of prognostic factors for FIGO stage IVB UCC patients with three or four sites of distant metastases in the study and the validation group.
| Variable | Study group | Validation group | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age | ||||||
| ≤ 40 | 1 | 1 | ||||
| 41-64 | 1.23 | 0.62 - 2.43 | 0.551 | 0.51 | 0.11 - 2.28 | 0.375 |
| ≥ 65 | 0.91 | 0.43 - 1.93 | 0.814 | 0.74 | 0.12 - 4.47 | 0.738 |
| Race | ||||||
| White | 1 | NA | ||||
| Black | 0.66 | 0.33 - 1.33 | 0.249 | NA | NA | NA |
| Others | 0.77 | 0.40 - 1.51 | 0.450 | NA | NA | NA |
| Insurance Recode | ||||||
| Uninsured | 1 | NA | ||||
| Insured | 0.94 | 0.38 - 2.33 | 0.897 | NA | NA | NA |
| Marital status | ||||||
| Unmarried | 1 | 1 | ||||
| Married | 1.21 | 0.76 - 1.94 | 0.429 | 4.00 | 1.36 - 11.8 | 0.012 |
| Grade | ||||||
| Grade I- II | 1 | 1 | ||||
| Grade III- IV | 0.84 | 0.33 - 2.16 | 0.717 | 0.76 | 0.20 - 2.93 | 0.691 |
| Histology | ||||||
| AC | 1 | 1 | ||||
| SCC | 1.00 | 0.55 - 1.80 | 0.990 | 0.85 | 0.18 - 3.96 | 0.840 |
| Others | 1.46 | 0.77 - 2.75 | 0.249 | 2.76 | 0.57 - 13.37 | 0.206 |
| T stage | ||||||
| T1-2 | 1 | 1 | ||||
| T3-4 | 0.87 | 0.53 - 1.42 | 0.576 | 0.71 | 0.25 - 2.01 | 0.521 |
| N stage | ||||||
| N0 | 1 | 1 | ||||
| N1 | 0.64 | 0.37 - 1.13 | 0.123 | 0.38 | 0.12 - 1.16 | 0.088 |
| Metastatic sites | ||||||
| Bone + Brain + Liver | 1 | 1 | ||||
| Bone + Brain + Lung | 0.44 | 0.09 - 2.05 | 0.294 | 0.13 | 0.01 - 1.49 | 0.101 |
| Bone + Liver + Lung | 0.34 | 0.08 - 1.43 | 0.143 | 0.12 | 0.01 - 1.17 | 0.068 |
| Brain + Liver + Lung | 0.28 | 0.06 - 1.37 | 0.116 | 0.16 | 0.01 - 1.87 | 0.142 |
| Bone + Brain + Liver + Lung | 0.54 | 0.10 - 2.99 | 0.483 | NA | NA | NA |
| Surgical treatment | ||||||
| No Surgery | 1 | 1 | ||||
| Surgery | 1.35 | 0.55 - 3.36 | 0.513 | 8.01 | 0.83 - 76.94 | 0.072 |
With the exception of the single site of metastasis, 295 of 979 patients suffered metastases in two or more organs. Different configurations of two metastatic sites showed different patients' survivals. Compared with the patient with metastases to bone plus brain, better survivals were noticed in patients with metastases to bone plus liver, bone plus lung, and liver plus lung. No significant difference was noticed between patients with brain plus bone, liver or lung. Therefore, more attention should be paid for the screening of brain metastases even though the relevant incidence was low. As to the factor's analyses, only the uninsured status was found to be the independent prognostic factor. For patients with three or more metastatic sites, no significant difference was found among metastatic patterns. The late stage without any effective treatments may be the main reason why no difference or prognostic factors were found. Timely screening and proper treatment should be performed in order to slow down the progress into multiple metastases and to improve the survival.
Kaplan-Meier analysis of overall survival for UCC patients in the validation group (A), single site of distant metastases (B), two sites of distant metastases (C), and patients with three or more sites of distant metastases (D).
Treatment for patients with distant metastases was accepted to be the important prognostic factor and was investigated in the previous studies. A study reported a significantly longer 5-year survival in patients after definitive radiotherapy and chemoradiotherapy [18]. Surgical resections of metastatic pulmonary lesions were performed in 12 patients with longer survival than those who did not receive the surgery [11]. A review concluded that multimodal therapy can guarantee a better survival [12]. Only 7.9% of the UCC patients received surgery of primary site in our study and no significant impact of surgery on the survival was found. The records on the surgery on the metastatic lesion in the SEER database were not available. Besides, no information of radiotherapy or chemotherapy can be pooled, making it impossible to evaluate the influence on survival. All the treatments should be further investigated based on larger population.
Although the present study identified the largest cohort of single and multiple distant metastases in UCC patients with FIGO stage ⅣB, the limitations should be revealed. In this study, we only analyzed the distant metastases to organs of lung, bone, liver and brain. The metastases to distant lymph nodes and peritoneal spread were not investigated because of no available data in the SEER database. The sequence of each metastasis in patients with multiple sites may be the important confusing factor to survival and should be further investigated in the larger population with related information. Limited number of patients in some patterns of metastases may result in bias in survival calculation and comparation. Moreover, some important factors including human papillomavirus (HPV) infection and special gene expression should be investigated in future study. Moreover, due to the limited sample size in the validation group, not all the prognostic factors were validated, and more studies with larger sample size were needed in future.
In summary, the lung, bone, liver, and brain were the common metastatic sites in patients with UCC at FIGO stage ⅣB, among whom 30% patients suffered multiple metastases. Decreased survival was confirmed in patients with increased number of metastatic sites. Higher grade, higher T stage, black race, unmarried status and more sites were risk factors in all the patients with distant metastases. Different performance was showed in survival and prognostic factors among the patterns of distant metastases.
Acknowledgements
The Authors would like to thank SEER public database to make the uterine cervical cancer patients' data available.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Vaccarella S, Lortet-Tieulent J, Plummer M. et al. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49(15):3262-3273
2. Utada M, Chernyavskiy P, Lee WJ. et al. Increasing risk of uterine cervical cancer among young Japanese women: Comparison of incidence trends in Japan, South Korea and Japanese-Americans between 1985 and 2012. Int J Cancer. 2019;144(9):2144-2152
3. Hernandez-Hernandez DM, Apresa-Garcia T, Patlan-Perez RM. Epidemiological overview of uterine cervical cancer. Rev Med Inst Mex Seguro Soc. 2015:53 Suppl 2(S154-S161
4. Everhov AH, Ekberg S, Hirschberg AL. et al. Lost workdays in uterine cervical cancer survivors compared to the general population: impact of treatment and relapse. J Cancer Surviv. 2016;10(3):514-523
5. Park SH, Ro DY, Park BJ. et al. Brain metastasis from uterine cervical cancer. J Obstet Gynaecol Res. 2010;36(3):701-704
6. Sato Y, Tanaka K, Kobayashi Y. et al. Uterine cervical cancer with brain metastasis as the initial site of presentation. J Obstet Gynaecol Res. 2015;41(7):1145-1148
7. Ikeda S, Yamada T, Katsumata N. et al. Cerebral metastasis in patients with uterine cervical cancer. Jpn J Clin Oncol. 1998;28(1):27-29
8. Hwang JH, Yoo HJ, Lim MC. et al. Brain metastasis in patients with uterine cervical cancer. J Obstet Gynaecol Res. 2013;39(1):287-291
9. Yoon A, Choi CH, Kim HJ. et al. Contributing factors for bone metastasis in uterine cervical cancer. Int J Gynecol Cancer. 2013;23(7):1311-1317
10. Zhang Y, Guo X, Wang G. et al. Real-World Study of the Incidence, Risk Factors, and Prognostic Factors Associated with Bone Metastases in Women with Uterine Cervical Cancer Using Surveillance, Epidemiology, and End Results (SEER) Data Analysis. Med Sci Monit. 2018:24 (6387-6397
11. Ki EY, Lee KH, Park JS. et al. A Clinicopathological Review of Pulmonary Metastasis from Uterine Cervical Cancer. Cancer Res Treat. 2016;48(1):266-272
12. Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016;27(4):e43
13. Matsumiya H, Todo Y, Okamoto K. et al. A prediction model of survival for patients with bone metastasis from uterine cervical cancer. J Gynecol Oncol. 2016;27(6):e55
14. Zhang C, Wang G, Han X. et al. Comparison of the therapeutic effects of surgery combined with postoperative radiotherapy and standalone radiotherapy in treating spinal metastases of lung cancer. Clin Neurol Neurosurg. 2016:141 (38-42
15. Guo X, Zhang C, Guo Q. et al. The homogeneous and heterogeneous risk factors for the morbidity and prognosis of bone metastasis in patients with prostate cancer. Cancer Manag Res. 2018:10 (1639-1646
16. Guo Q, Zhang C, Guo X. et al. Incidence of bone metastasis and factors contributing to its development and prognosis in newly diagnosed renal cell carcinoma: a population-based study. Cancer Manag Res. 2018:10 (2935-2944
17. Hata M, Omura M, Miyagi E. et al. The role of radiation therapy for uterine cervical cancer with distant metastasis. Oncology. 2012;83(2):67-74
18. Wang YM, Wang CJ, Fang FM. et al. Differences in the outcomes and complications between elderly and younger uterine cervical cancer patients treated by definitive radiotherapy - A propensity score-matched study. Gynecol Oncol. 2017;145(2):277-283
Author contact
![]() Corresponding author: Dr. Xiaofei Wang (wangxiaofeiedu.cn), address: Tianjin First Central Hospital, 24 Fukang Road, Nankai area, Tianjin 300192, China.
Corresponding author: Dr. Xiaofei Wang (wangxiaofeiedu.cn), address: Tianjin First Central Hospital, 24 Fukang Road, Nankai area, Tianjin 300192, China.

 Global reach, higher impact
Global reach, higher impact