Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(16):3173-3182. doi:10.7150/jca.20523 This issue Cite
Research Paper
Arecoline Increases Glycolysis and Modulates pH Regulator Expression in HA22T/VGH Hepatoma Cells, Leading to Increase of Intracellular Ca2+, Reactive Oxygen Species, and Anoikis
1. Department of Biochemistry, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80708, Taiwan;
2. Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 80756, Taiwan;
3. Department of Surgery, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80708, Taiwan;
4. Division of General and Digestive and Pancreatic Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University 80756, Taiwan;
5. Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80708, Taiwan;
6. Department of Medical Laboratory Science and Biotechnology, College of Health Sciences, Kaohsiung Medical University, Kaohsiung 80708, Taiwan;
7. Institute of Medical Science and Technology, College of Sciences, National Sun Yat-sen University, Kaohsiung 80424, Taiwan;
8. Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 80756, Taiwan.
Received 2017-4-11; Accepted 2017-8-9; Published 2017-9-15
Abstract
Background: Cancer cells proliferate rapidly and are resistant to cell death, relying on aggravated glycolysis to satisfy their increased demand for energy and biosynthetic precursors. However, this process may create unfavorable microenvironments, such as increased acidity, leading to cytotoxicity. Our previous study demonstrated that arecoline induces anoikis of HA22T/VGH hepatoma cells. The present study aimed to examine if arecoline induced anoikis is related to the glycolytic pathway and explore the underlying mechanisms.
Methods: HA22T/VGH cells were treated with arecoline and changes in the glycolytic end products lactate and ATP, glycolytic-related gene expression, intracellular and extracellular pH, pH-regulating gene expression, reactive oxygen species (ROS) levels, intracellular Ca2+ concentration ([Ca2+]i) and mitochondrial membrane potential were examined, relative to untreated cells. Cell viability and morphology were also assessed.
Results: Arecoline increased lactate and ATP production through induction of glycolytic genes, including glucose transporter 3 (Glut3), hexokinase 1 (HK1), hexokinase 2 (HK2), and pyruvate kinase (PK). The intracellular pH was not changed, despite increased lactate levels, implying that intracellular H+ was exported out of the cells. mRNA expression of pH regulators including monocarboxylate transporter 1 and 4 (MCT 1 and 4), sodium bicarbonate cotransporter 1 (NBC1), carbonic anhydrases (CA) IX and XII and vacuolar ATPase (V-ATPase) were down-regulated. Na+/H+ exchanger 1 (NHE1) mRNA levels remained unchanged while Na+/Ca2+ exchanger (NCX) was up-regulated and eventually [Ca2+]i was increased. ROS generation was increased and mitochondrial membrane potential was decreased followed by cell detachment and death. Addition of 2-deoxy-d-glucose, a glucose competitor that interferes with glycolysis, attenuated arecoline induction of lactate [Ca2+]i, ROS and cell detachment. Similarly, ROS scavengers could block the effects of arecoline.
Conclusions: This study demonstrated that arecoline induced glycolysis and modulated the mRNA expression of pH-regulator genes in HA22T/VGH cells. This phenomenon led to the elevation of [Ca2+]i, ROS generation, and subsequent cell detachment.
Keywords: Arecoline, Glycolysis, ROS, Calcium ion, Anoikis.
Introduction
Arecoline, the active component of the betel nut, comprises up to 0.12-0.24 % by weight of the ripe nut [1]. Arecoline has cholinergic, sialogogic, diaphoretic, and parasympathomimetic effects [2] and has been recognized as a possible cognition enhancer in Alzheimer's dementia [3, 4]. Our previous study showed that arecoline inhibited IL-6 production, induced apoptosis, and arrested cell cycle progress in basal carcinoma cells [5]. We also demonstrated that arecoline induced anoikis by inhibiting STAT3 and activating RhoA/Rock in HA22T/VGH hepatoma cells but it didn't effect on normal hepatocytes [6]. These results suggest that arecoline is a potential therapeutic agent for cancer.
Exacerbated glycolysis to meet the increased demand for energy and biosynthetic precursors of the most aggressive and invasive cancers is reported [7]. Consequently, these cancer cells need vigorous pH-regulating systems to handle the high levels of lactic and carbonic acid, which are byproducts of glycolysis. Tumor cells produce excess H+ due to their higher metabolic rate relative to normal cells [8, 9]. These alterations in the intracellular pH (pHi) affect the structures and activities of most enzymes, which drastically influence cell signaling and metabolic function [7], resulting in considerable cellular stress. According to the principle of Darwinian selection, growing tumors may create adverse microenvironments to facilitate cancer cell survival and compete with normal cells. This process favors highly selective microenvironments and genetic instability, which allow tumor cells to continuously adapt and expand [10]. Interrupting this process can potentially induce cancer cell-specific apoptosis.
Because intracellular acidification may lead to cell death, it is important to maintain intracellular homeostasis. Homeostasis is achieved by pHi-regulating proteins that facilitate H+ export. The monocarboxylate transporter (MCT) is a passive lactate-proton symporter [11]. MCT1-4 are located in the cell membrane. In tumor and stromal cells, MCT1 and the hypoxia-induced MCT4 are reported to be co‑transporters of lactate and H+ [11]. Carbonic anhydrases IX (CAIX) and CAXII participate in the reversible hydration of CO2, a cell-permeable acidic metabolic product. CO2 is exported and then undergoes CAIX- or CAXII-facilitated hydration to carbonic acid followed by dissociation into to bicarbonate and a proton [12]. The bicarbonate is imported by the sodium bicarbonate cotransporter (NBC) and further reacts with a proton intracellularly [13]. In several tumor cell types, vacuolar ATPase (V-ATPase) is expressed at the plasma membrane, acting as an ATP dependent pump [13]. In cancer cells, Na+/H+ exchanger 1 (NHE1) serves as a passive proton-sodium antiporter that exports free intracellular H+ when the intracellular proteins have exhausted their buffering capacities [14, 15]. In contrast, NHE1 is almost inactive in normal cells which have neutral pH. However, a proton-dependent NHE1 is activated when the pHi becomes acidic [16]. The plasma membrane Na+/Ca2+ exchanger (NCX) senses the Na+ electrochemical gradient and can exchange Ca2+ for Na+ through Ca2+-influx or Ca2+-efflux, depending on the intracellular Na+ concentration and the membrane potential [17]. An active Na+/Ca2+ exchange system is present in the hepatocyte plasma membrane [18], and an estimated 60 % of the Ca2+ entering the hepatocyte passes through this exchanger [19]. It has also been demonstrated that NCX significantly contributes to the Ca2+ increase seen in hepatocytes exposed to hypoxia, Na+ load, or oxidative stress [20-22].
Accordingly, it is rational to propose that interfering with H+ dynamics [both the pHi and the extracellular pH (pHe)] coupled with metabolic disruption may provide a new strategy for anticancer therapy. In the present study, we examined whether arecoline affects glycolysis and H+ dynamics in HA22T/VGH hepatoma cells. We also explored which kinds of pH-regulating proteins are involved in this process and whether glycolysis is related to the induction of anoikis. Herein, only HA22T/VGH hepatoma cells were used without normal hepatocyte as control since our previous study has demonstrated arecoline did not effects on normal hepatocyte [6].
Materials and Methods
Reagents
Arecoline hydrobromide (methyl 1-methyl-1, 2, 5, 6-tetrahydronicotinate hydrobromide) was obtained from Sigma-Aldrich (St. Louis, MO, USA); its purity was greater than 99.0 %. Rotenone, antimycin A, 2′,7′-dichlorofluorescin diacetate (H2DCF-DA), and BCECF AM ester were also from Sigma-Aldrich (St. Louis, MO, USA). Reduced L-glutathione was from Boehringer Mannheim GmbH (Mannheim, West Germany). Protein assay reagents were from Bio-Rad Laboratories (Hercules, CA, USA). Fluo-4 AM ester were from Molecular Probes (Eugene, OR, USA). TRIzol reagent was from Invitrogen Life Technologies (Carlsbad, CA, USA). All other chemicals, including calcium carbonate, 2-deoxy-d-glucose, and N-acetyl-L-cysteine, were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell line, cell culture, and arecoline treatment
HA22T/VGH (BCRC Number: 60168), a poorly differentiated human hepatoma cell line, was obtained from the Bioresource Collection and Research Center (BCRC) in the Food Industry Research and Development Institute, Hsinchu, Taiwan. These cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Grand Island, NY, USA) containing 10 % fetal bovine serum (HyClone, Auckland, NZ), 2 mM L-glutamine, 0.1 mM non-essential amino acids, 100 units/ml of penicillin, and 100 μg/ml of streptomycin (all from Gibco BRL, Grand Island, NY, USA) at 37°C in a humidified chamber with 5 % CO2. Our previous study [6] showed that 100 μg/ml arecoline could induce anoikis in HA22T/VGH cells. 100 μg/ml arecoline was added to the culture medium for either 12 hours (h) for mRNA studies or 24 h for others and then the cells were harvested and analyzed. Cell morphological changes were observed under an inverted phase-contrast microscope (Olympus, Tokyo, Japan) and photographs were taken at 200× magnification.
Cell viability assay
After treatment, viable cells were counted using a dye exclusion technique. The cell suspension was centrifuged at 5000× g, the supernatant was discarded and the cell pellet resuspended in serum-free medium. One volume of 0.4 % Trypan blue (Gibco BRL, Grand Island, NY, USA) was added to one volume of cell suspension. After incubation at room temperature for 3 min, cells were counted in a hemocytometer. All counts were performed in triplicate.
ATP and lactate measurement
HA22T/VGH cells (2 × 105) were seeded in six-well plates (Corning, Inc.) incubated with 100 μg/ml arecoline for 24 h, scraped off and centrifuged. The cell pellet was resuspended in phosphate-buffered saline and sonicated. ATP levels were measured using a commercial bioluminescence kit (ENLITEN® ATP assay, Promega, Madison, WI, USA) and a luminometer (Hidex PLATE CHAMELEON, Turku, Finland). Lactate levels were measured using a colorimetric/fluorometric assay kit (BioVision Incorporated, Milpitas, CA, USA) and a microplate reader (BioTek® PowerWave XS2).
Quantitative real-time PCR analysis
Total RNA was isolated using TRIzol reagent according to the manufacturer's instructions. Real-time PCR was performed on a MiniOpticon™ real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA) using iQ™ SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) according to our published procedure [23]. The primers and the amplified gene products are shown in Table 1. Data were collected and analyzed using MJ Opticon Monitor Analysis software version 3.1 (Bio-Rad Laboratories, Hercules, CA, USA). Each reaction mixture was amplified in triplicate and the results calculated using the ΔΔCt method [24]. The cycle threshold (Ct) value for the test gene was normalized using the mean Ct value for the GAPDH gene. Relative gene expression was expressed as the fold change (2-ΔΔCt) relative to expression in the untreated control.
Primers and amplified products for each test gene used in real-time PCR.
| Genes | Forward | Reverse | Product (bp) |
|---|---|---|---|
| GAPDH | 5'-cgaccactttgtcaagctca-3' | 5'-aggggagattcagtgtggtg-3' | 203 |
| Glut1 | 5'-ccgcttcctgctcatcaacc-3' | 5'-catcatctgccgactctcttcc-3' | 124 |
| Glut3 | 5'-aactttgacggacaagggaaatgc-3' | 5'-ccaccagtgacagccaacagg-3' | 183 |
| HK1 | 5'-ctgaatagcacctgcgatgac-3' | 5'-ctggagaagtgtggatgaagc-3' | 194 |
| HK2 | 5'-cgagagcatcctcctcaagtg-3' | 5'-tcaccacagcaaccacatcc-3' | 131 |
| PFK1 | 5'-ttacaggtgccaacatcttc-3' | 5'-tgttcaggtgcgagtagg-3' | 108 |
| PK | 5'-tcgtctttgcctcctttgtg-3' | 5'-cttgatgccgtgtccttcc-3' | 83 |
| LDHA | 5'-gattcagcccgattccgttacc-3' | 5'-agagacaccagcaacattcattcc-3' | 135 |
| LDHB | 5'-tggattctgctagatttcgctacc-3' | 5'-aacacctgccacattcacacc-3' | 134 |
| MCT1 | 5'-gtgaccattgtggaatgctg-3' | 5'-cctttttctgctcgtttgct-3' | 199 |
| MCT4 | 5'-ggccctactccgtctacctc-3' | 5'-ccaatggcactggagaactt-3' | 199 |
| NBCN1 | 5'-gcaagcagccttgtgtgtta-3' | 5'-agtttcattgctggggtttg-3' | 201 |
| CAIX | 5'-gtctcgcttggaagaaatcg-3' | 5'-agagggtgtggagctgctta-3' | 200 |
| CAXII | 5'-tagggaatggcaggttcaag-3' | 5'-ccactgacaggaggttggat-3' | 201 |
| V-ATPase | 5'-gtgggcatgatcctgattct-3' | 5'-tgcgcatgtacaagaccaac-3' | 207 |
| NHE1 | 5'-actggaccttcgtcatcagc-3' | 5'-catggggaagtgcttcttgt-3' | 200 |
| NCX | 5'-cctggagcatctttgcctac-3' | 5'-taatcatccccctctgcttg-3' | 201 |
ROS and ΔΨm measurement
The method of ROS detection was described in our previous study [25]. 2',7'-Dichlorodihydrofluorescein diacetate (H2DCF-DA) (Molecular Probes, Eugene, OR, USA) was used to measure intercellular ROS production. The ΔΨm is defined as a change in the electrochemical gradient. The mitochondrial ΔΨm was measured using a fluorescent cationic dye, tetraethylbenzimidazolylcarbocyanine iodide (JC-1) (Molecular Probes). JC-1 dye exhibits potential-dependent accumulation in mitochondria and is detected by a fluorescence emission shift from green (~529 nm) to red (~590 nm). The mitochondrial depolarization is indicated by a decrease in the red/green fluorescence intensity ratio. HA22T/VGH cells were seeded in six-well plates (Corning, Inc., Corning, NY, USA) and incubated overnight, then underwent treatment for 24 h before being incubated with 2 μM JC-1 dye for 15 min at 37°C. The cells were subsequently trypsinized and resuspended for analysis by FACScan flow cytometry (Beckman Coulter-Epics XL) [26]. Data were analyzed using WinMDI 2.8 software (Scripps Research Institute, La Jolla, CA, USA), and a minimum of 1×104 cells per sample were evaluated.
Measurement of the intracellular pH (pHi) and intracellular calcium levels ([Ca2+]i)
HA22T/VGH cells were treated with or without 100 μg/ml arecoline for 24 h, trypsinized and centrifuged. Next, the cell pellet was resuspended in culture medium and labeled with fluorescence dye for 30 minutes. Intracellular calcium level was measured using 10 μM fluo-4 AM ester at room temperature, and the fluorescence was detected using a Coulter Epics XL cytometer with an excitation wavelength of 488 nm and an emission wavelength of 520 nm. The intracellular pH (pHi) was measured with 10 μM BCECF AM ester at 37℃, and the fluorescence was detected using a Coulter Epics XL cytometer with an excitation wavelength of 488 nm and an emission wavelength of 525/620 nm. The data were analyzed using WinMDI 2.8 software (Scripps Research Institute, La Jolla, CA, USA), and a minimum of 1×104 cells per sample were evaluated.
Statistical analysis
All data are presented as the mean ± SD (standard deviation). The differences between the treated and control group were analyzed using either Student's t test or ANOVA followed by Fisher's exact test. Statistical analyses were performed using SAS version 6.011 (SAS Institute Inc, Cary, NC). A p-value <0.05 was considered to be statistically significant.
Results
Arecoline increases glycolysis
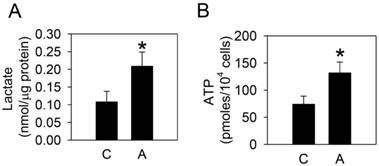
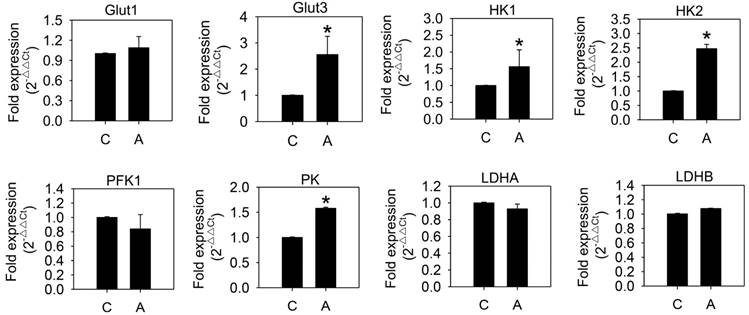
To examine whether arecoline affected glycolysis in HA22T/VGH cells, the glycolytic end products, lactate and ATP, were measured after treatment with 100 μg/ml arecoline for 24 h. Both lactate and ATP levels increased significantly (Fig. 1), which indicated that arecoline augmented glycolysis in HA22T/VGH cells. We further assessed the mRNAs expression of glycolytic pathway components, including glucose transporter 1 and 3 (Glut1 and Glut3), hexokinase 1 and 2 (HK1 and HK2), phosphofructokinase 1 (PFK1), pyruvate kinase (PK), and lactate dehydrogenase A and B (LDHA and LDHB). Arecoline significantly enhanced the mRNA expression of Glut3, HK1, HK2, and PK, while it had no impact on Glut1, PFK1, LDHA, or LDHB (Fig. 2). Glut facilitates the glucose transport across the plasma membranes in mammalian cells. Both hexokinase and pyruvate kinase are rate-limiting enzymes of glycolytic pathway. These results indicated that arecoline augmented glycolysis in HA22T/VGH cells by modulating expression of glycolysis-related genes.
Arecoline increases lactate and ATP production. HA22T/VGH cells were treated with or without 100 μg/ml of arecoline for 24 h, then the expression levels of (A) lactate and (B) ATP were measured. The data are the mean ± S.D. for three independent experiments. *: p<0.05 compared to the untreated control group. C: control; A: arecoline-treated.
Arecoline decreases the pHe, but not the pHi
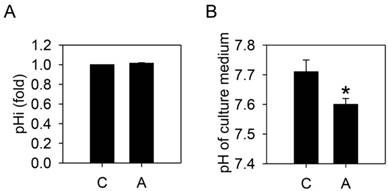
To test whether arecoline-induced lactate production would alter the homeostasis of HA22T/VGH cells, the intracellular and extracellular pH values were measured. After treatment with 100 μg/ml arecoline for 24 h, the extracellular pH was significantly decreased but the intracellular pH remained unchanged (Fig. 3). This suggested that the intracellular H+ derived from the increased lactate was being exported.
Effects of arecoline on the expression of genes coding for pH-regulating proteins
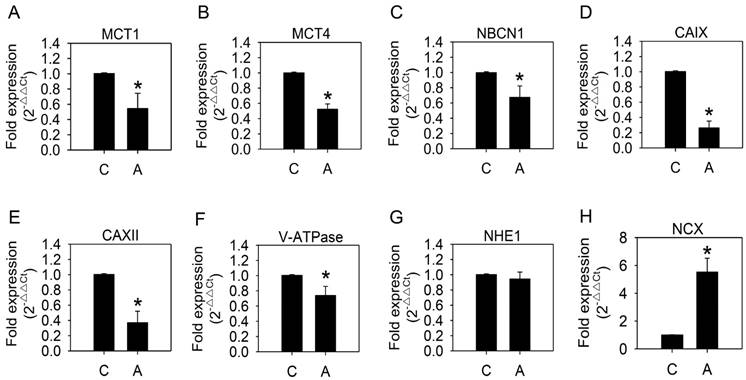
We speculated that the cellular pH-regulating systems might be altered to maintain an appropriate intracellular pH. Therefore, we examined the mRNA expression of pH-regulating genes. As shown in Fig. 4, MCT1, MCT4, NBCN1, CAIX, CAXII, and V-ATPase expression was significantly reduced, while NHE1 remained unaffected, after arecoline treatment. Down-regulation of pH-regulating genes was unexpected in a context where intracellular pH was unaltered. Since mRNA expression of NHE1 was the only unchanged pH-regulating gene that we tested, we speculated that NHE1 has a greater influence on H+ export than the others. Moreover, we proposed there might be other factors that coordinate with NHE1 in maintaining ion and pH balance between intracellular and extracellular environments.
Arecoline increases levels of mRNAs coding for glycolysis-related proteins. HA22T/VGH cells were treated with or without 100 μg/ml of arecoline for 12 h, then the levels of mRNAs coding for glycolysis-related proteins were quantified by quantitative real-time PCR analysis. C: control; A: arecoline-treated; Glut1 and Glut3: glucose transporter 1 and 3; HK1 and HK2: hexokinase 1 and 2; PFK1: phosphofructokinase 1; PK: pyruvate kinase; LDHA and LDHB: lactate dehydrogenase A and B. The data are shown as the fold change compared to the untreated control group. The results are the mean ± S.D. for three independent experiments. *: p<0.05 compared to the untreated control group.
Arecoline increases NCX mRNA expression and the [Ca2+]i
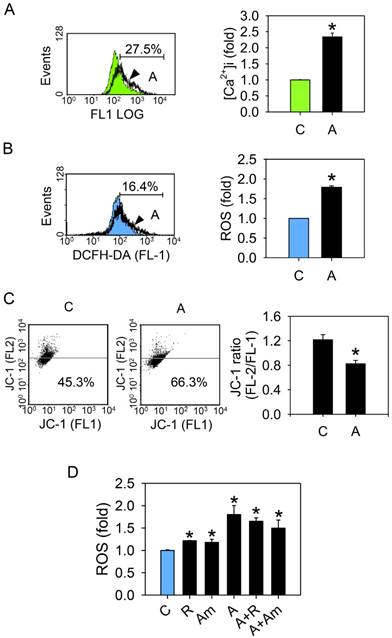
Under normal conditions, the NHE on the plasma membrane equilibrates internal and external Na+ and H+ levels and the NCX maintains a constant cytosolic Ca2+ concentration. During stress conditions, such as ischemia or lactic acidosis, NHE exports H+ and imports Na+. This leads to cytosolic Na+ overload, and the NCX is forced to export excess Na+, which accompanied by import of Ca2+, and eventually results in elevation of cytosolic Ca2+ concentration [27]. To determine whether the NCX coordinates with NHE1 in maintaining the intracellular pH value of the arecoline-treated HA22T/VGH cells, we analyzed NCX mRNA expression and intracellular Ca2+ levels. This finding revealed that NCX mRNA expression (Fig. 4H) was significantly increased as was the intracellular Ca2+ (Fig. 5A), suggesting that the intracellular pH was maintained by both NHE1 and NCX and accompanied by increased intracellular Ca2+ levels.
Arecoline stimulates ROS generation and interferes with mitochondrial function
To determine whether arecoline affected mitochondrial function either directly or indirectly, mitochondrial membrane potential (ΔΨm) and ROS production of arecoline-treated HA22T/VGH cells were measured. ROS was markedly elevated (Fig. 5B), while ΔΨm was decreased (Fig. 5C). Since disturbance of mitochondrial electron transfer chain leads to electron leakage and ROS generation [28, 29], we suspected that arecoline-induced ROS production originated from the mitochondrial electron transfer chain. To test this, the mitochondria complex I (rotenone, 0.3 μM) and complex III (antimycin A, 0.3 μg/ml) inhibitors were added to HA22T/VGH cells after treatment with arecoline. Both rotenone and antimycin A significantly increased ROS levels in the cells without arecoline treatment, but these inhibitors did not further enhance ROS generation in the arecoline-treated cells (Fig. 5D). This suggested that arecoline interferes with the mitochondrial electron transfer chain, resulting in ROS generation.
The glucose competitor 2-deoxy-d-glucose reduces the effects of arecoline
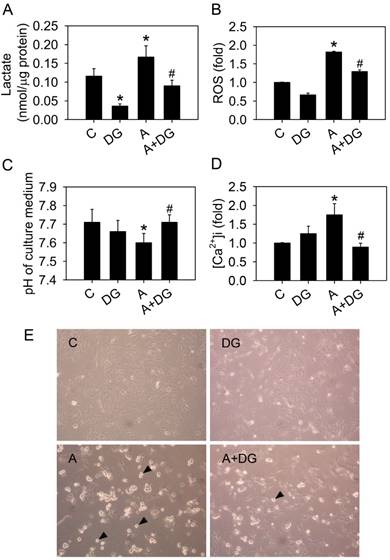
We previously demonstrated that arecoline induced anoikis in HA22T/VGH hepatoma cells [6]. We hypothesized that glycolysis triggered arecoline-induced anoikis. To test this hypothesis, 10 mM 2-deoxy-d-glucose (DG) was used to inhibit the glycolytic pathway in the HA22T/VGH cells treated with arecoline. DG is a glucose analog that inhibits glycolysis through its actions on hexokinase. Arecoline-induced lactate and ROS overproduction were significantly reduced and normal extracellular pH levels were restored in the presence of DG (Fig. 6A, 6B and 6C). DG also reduced intracellular Ca2+ concentration in the HA22T/VGH cells treated with arecoline (Fig. 6D). Cell morphology showed that arecoline-induced cell detachment was inhibited by DG (Fig. 6E). These results demonstrated that arecoline contributed to cell detachment and subsequent anoikis by trigging the glycolytic pathway.
Effects of arecoline on levels of mRNAs coding for pH-regulating proteins. HA22T/VGH cells were treated with or without 100 μg/ml of arecoline for 12 h, then the expression of mRNAs coding for pH-regulating proteins were quantified by quantitative real-time PCR analysis. C: control; A: arecoline-treated; MCT1 and MCT4: monocarboxylate transporter 1 and 4; NBCN1: sodium bicarbonate cotransporter 1; CAIX and CAXII: carbonic anhydrase IX and XII; V-ATPase: vacuolar ATPase; NHE1: Na+/H+ exchanger 1; NCX: Na+/Ca2+ exchanger. The results are shown as the fold change compared to the untreated control and are the mean ± S.D. for three independent experiments. *: p<0.05 compared to the untreated control group.
Arecoline decreases the pHe, but not the pHi. HA22T/VGH cells were treated with or without 100 μg/ml of arecoline for 24 h, then (A) the intracellular pH was measured by flow cytometry and (B) the pH of the culture medium was measured using a pH meter. The data are the mean ± S.D. for three independent experiments. *: p<0.05 compared to the untreated control group. C: control; A: arecoline-treated.
The ROS scavenger reduces arecoline-induced cell death
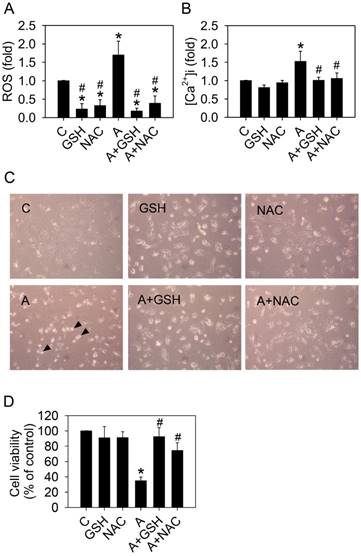
Next, we sought to determine whether ROS was directly involved in arecoline-induced cell detachment and anoikis. Addition of the ROS scavengers, 10 mM glutathione (GSH) or 10 mM N-acetylcysteine (NAC), in the arecoline-treated HA22T/VGH cells significantly inhibited ROS generation and reduced the elevation of intracellular calcium (Fig. 7A and 7B). Both GSH and NAC prevented HA22T/VGH cells from arecoline-induced detachment and death (Fig. 7 C and 7D). These results demonstrated that ROS played an important role in the elevation of intracellular calcium concentration and arecoline-induced cell detachment.
Discussion
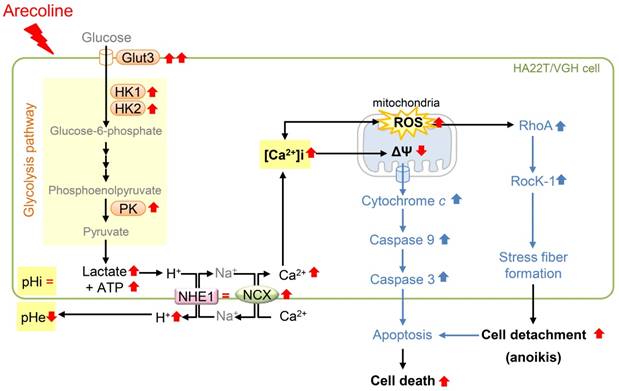
A summary of this study is illustrated in Fig. 8. This study showed that arecoline enhanced glycolysis by inducing mRNA expression of the Glut3 and glycolytic genes, including HK1, HK2, and PK, and induced ROS generation in HA22T/VGH cells. Unexpectedly, the intracellular pH was not changed even though lactate, a glycolytic end product, was increased and the pH of the extracellular medium was declined; this implied that intracellular H+ was exported out of the cell. We found that mRNA expression of most pH regulators (MCT1, MCT4, NBCN1, CAIX, CAXII, and V-ATPase) were reduced, while NHE1 levels were unchanged. We therefore hypothesized that NHE1 plays an important role in maintaining intracellular H+ levels. This finding raised the question of whether NHE1-mediated H+ export could lead to intracellular Na+ overload. To answer this question, we measured NCX mRNA levels and found that they were markedly increased. This finding suggested that excess Na+ could be exported while extracellular Ca2+ was imported, resulting in an increase in the [Ca2+]i concentrations and ROS and a decrease in ΔΨm. Furthermore, we demonstrated that the arecoline-induced effects were reversed by DG, a glucose competitor that acts as inhibitor of glycolysis. These data suggested that the effects of arecoline are dependent on the induction of glycolysis. Addition of ROS scavengers to HA22T/VGH cells almost completely eliminated the arecoline-induced ROS generation, significantly inhibited the arecoline-induced elevation of intracellular calcium concentration, and prevented HA22T/VGH cells from cell detachment, suggesting that ROS plays important role in the process of arecoline-induced cell death.
Arecoline causes an increase in the [Ca2+]i, stimulates ROS production, and interferes with the mitochondrial membrane potential. HA22T/VGH cells were treated with or without 100 μg/ml of arecoline for 24 h, then (A) the [Ca2+]i was measured by using flow cytometry. The green filled area is the untreated control and those delimited by the thick black lines is the treated group. (B) ROS levels (H2DCF-DA fluorescence) and (C) the mitochondrial membrane potential (ΔΨm) (JC-1 fluorescence) were measured by flow cytometry. The blue filled area is the untreated control and those delimited by the thick black lines is the treated group. (D) or were treated with 0.3 μM rotenone or 0.3 μg/ml of antimycin A alone or together with 100 μg/ml of arecoline for 24 h, then ROS levels were measured. The quantified data are the percentage of stained cells (gate %) and the fold induction compared to the untreated control represented as the mean ± S.D. for three independent experiments; *: p<0.05 compared to the untreated control group. C: control; A: arecoline-treated; R: rotenone; Am: antimycin A.
Cancer cells proliferate rapidly, creating a demand for increased glycolysis. This produces more lactate and H+ and may create an unfavorable microenvironment, leading to cancer cell-specific apoptosis. Tumor cells may produce excess H+ compared to normal cells leading to growth arrest or apoptosis if pH-regulating capabilities are compromised [7]. Targeting pH-regulating proteins, such as the NHE, CAs, MCTs, and H+ pumps may impair tumor progression [7]. Thus, agents able to disturb intracellular pH regulation or cellular metabolism have the potential to be developed as targeted cancer therapies. In the present study, we showed that arecoline enhanced glycolysis and decreased the expression of mRNAs for pH-regulating proteins, suggesting it may be developed as an anti-cancer therapeutic agent.
The glucose competitor, 2-deoxy-d-glucose, blocks all of the effects of arecoline. HA22T/VGH cells were treated with or without 100 μg/ml of arecoline in the presence of 10 mM 2-deoxy-d-glucose or not for 24 h, then (A) the production of lactate, (B) ROS levels, (C) the pH of the culture medium and (D) the intracellular Ca2+ concentration were measured. (E) Cell detachment was observed by phase-contrast microscopy at 200×. The black arrowheads indicated detached cells. In Fig. 6A-D, the data are the mean ± S.D for three independent experiments. *: p<0.05 compared to the untreated control group; #: p<0.05 compared to arecoline-treated cells. C: control group; A: arecoline-treated; DG: 2-deoxy-d-glucose.
Glutathione or N-acetylcysteine blocks the effects of arecoline on HA22T/VGH cells. HA22T/VGH cells were treated with or without 100 μg/ml arecoline in the presence of 10 mM glutathione or 10 mM N-acetylcysteine for 24 h, then (A) ROS generation, (B) the intracellular Ca2+ concentration, and (D) cell viability were analyzed. (C) Cell detachment was observed by phase-contrast microscopy at 200×. The black arrowheads indicated detached cells. In Fig. 7A, B, and D, the data are the mean ± S.D. for three independent experiments. *: p<0.05 compared to the untreated control group; #: p<0.05 compared to the arecoline-treated group. C: control group; A: arecoline-treated; GSH: glutathione; NAC: N-acetylcysteine.
Schematic diagram of how arecoline affects glycolysis and the signaling pathway of anoikis in HA22T/VGH cells. Arecoline induces glycolytic gene expression (Glut3, HK1, HK2, and PK), which in turn increases the production of lactate and ATP. Arecoline also enhanced the ROS generation. However, the intracellular pH (pHi) is not changed and the extracellular pH (pHe) is declined. The pH-regulating protein NHE1 expression is unchanged and NCX is up-regulated. This suggests that intracellular H+ is exported via NHE1 and NCX and intracellular Ca2+ is increased during this ion exchange process. The increase in [Ca2+]i and respiratory chain of mitochondria leads to generate more ROS and cause mitochondrial dysfunction. These results demonstrate that arecoline enhances aerobic glycolysis in HA22T/VGH cells and modulates the mRNA expression of pH regulators, resulting in an elevated [Ca2+]i, ROS generation, and subsequent cell detachment. RedÇ: increased (shown in this study); RedÈ: decreased (shown in this study); Red =: not changed (shown in this study); BlueÇ: enhanced (shown in a previous studies) (see Cheng et al., 2010) [6].
Ca2+ can enhance ROS production by stimulating the tricarboxylic acid cycle and oxidative phosphorylation, which makes mitochondria function faster and consume more oxygen [30]. Indeed, mitochondrial ROS generation correlates with metabolic rate [30], suggesting that a faster metabolism can cause more electron leakage in the respiratory chain, leading to increased ROS levels. The present study showed that arecoline decreased the mitochondrial membrane potential and increased ROS production.
Ca2+ has long been known to play an important role in regulation of necrosis, apoptosis, anoikis and autophagic cell death [31]. It has been shown that ROS can activate RhoA to induce stress fiber formation [32, 33], as well as control apoptosis through stimulation of the intrinsic mitochondrial apoptotic pathway [34]. Our previous study demonstrated that arecoline induced anoikis of HA22T/VGH cells by increasing RhoA/Rock activation [6]. This study showed that ROS scavengers attenuated the arecoline-induced ROS generation and cell detachment of HA22T/VGH. Taken together, these data demonstrated that the arecoline-induced increase of ROS contributes to its effects on HA22T/VGH cells.
Conclusions
In this study, we demonstrated that arecoline enhanced aerobic glycolysis in HA22T/VGH cells and modulated the mRNA expression of pH regulators, resulting in elevated [Ca2+]i, ROS generation and subsequent cell detachment. Therefore, arecoline-induced cell detachment and anoikis is dependent on glycolysis.
Abbreviations
pHi: intracellular pH; pHe: extracellular pH; [Ca2+]i: intracellular Ca2+; Glut1 and Glut3: glucose transporter 1 and 3; HK1 and HK2: hexokinase 1 and 2; PK: pyruvate kinase; PFK1: phosphofructokinase 1; LDHA and LDHB: lactate dehydrogenase A and B; MCT: monocarboxylate transporter; CAIX and XII: carbonic anhydrase IX and XII; NBC1: sodium bicarbonate cotransporter 1; V-ATPase: vacuolar ATPase; NHE1: Na+/H+ exchanger 1; NCX: Na+/Ca2+ exchanger; DG: 2-deoxy-d-glucose; H2DCF-DA: 2',7'-dichlorofluorescin diacetate; ROS: reactive oxygen species; ΔΨm: mitochondrial membrane potential; GSH: glutathione; NAC: N-acetylcysteine.
Acknowledgements
This study was supported by the National Science Council, Executive Yuan (NSC 101-2320-B-037-045-MY3; NSC 101-2811-B-037-027; NSC 102-2811-B-037-021) and the Ministry of Science and Technology (MOST 103-2811-B-037-015) of Taiwan. These funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jayalakshmi A, Mathew AG. The Arecanut Palm: Chemical composition and processing. Keralaw, India: Central Plantation Crops Research Institutes. 1982
2. Nery R. The metabolic interconversion of arecoline and arecoline 1-oxide in the rat. Biochem J. 1971;122:503-8
3. Malviya M, Kumar YC, Mythri RB, Venkateshappa C, Subhash MN, Rangappa KS. Muscarinic receptor 1 agonist activity of novel N-aryl carboxamide substituted 3-morpholino arecoline derivatives in Alzheimer's presenile dementia models. Bioorg Med Chem. 2009;17:5526-34
4. Malviya M, Kumar YC, Asha D, Chandra JN, Subhash MN, Rangappa KS. Muscarinic receptor 1 agonist activity of novel N-arylthioureas substituted 3-morpholino arecoline derivatives in Alzheimer's presenile dementia models. Bioorg Med Chem. 2008;16:7095-101
5. Huang LW, Hsieh BS, Cheng HL, Hu YC, Chang WT, Chang KL. Arecoline decreases interleukin-6 production and induces apoptosis and cell cycle arrest in human basal cell carcinoma cells. Toxicol Appl Pharmacol. 2012;258:199-207
6. Cheng HL, Su SJ, Huang LW, Hsieh BS, Hu YC, Hung TC. et al. Arecoline induces HA22T/VGH hepatoma cells to undergo anoikis - involvement of STAT3 and RhoA activation. Mol Cancer. 2010;9:126
7. Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611-23
8. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-33
9. Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364-73
10. Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer. 2012;12:487-93
11. Halestrap AP. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life. 2012;64:1-9
12. Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene. 2010;29:6509-21
13. Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol. 2011;2:49
14. Spugnini EP, Sonveaux P, Stock C, Perez-Sayans M, De Milito A, Avnet S. et al. Proton channels and exchangers in cancer. Biochim Biophys Acta. 2015;1848:2715-26
15. Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T. et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13:89
16. Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M. et al. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000;14:2185-97
17. Khananshvili D. Sodium-calcium exchangers (NCX): molecular hallmarks underlying the tissue-specific and systemic functions. Pflugers Arch. 2014;466:43-60
18. Bernstein J, Santacana G. The Na+/Ca2+ exchange system of the liver cell. Res Commun Chem Pathol Pharmacol. 1985;47:3-34
19. Urcelay E, Butta N, Cipres G, Martin-Requero A, Ayuso MS, Parrilla R. Functional coupling of Na+/H+ and Na+/Ca2+ exchangers in the alpha 1-adrenoreceptor-mediated activation of hepatic metabolism. J Biol Chem. 1994;269:860-7
20. Carini R, Bellomo G, Dianzani MU, Albano E. Evidence for a sodium-dependent calcium influx in isolated rat hepatocytes undergoing ATP depletion. Biochem Biophys Res Commun. 1994;202:360-6
21. Carini R, de Cesaris MG, Bellomo G, Albano E. Role of Na+/Ca2+ exchanger in preventing Na+ overload and hepatocyte injury: opposite effects of extracellular and intracellular Ca2+ chelation. Biochem Biophys Res Commun. 1997;232:107-10
22. Kim JA, Kang YS, Lee SH, Lee YS. Inhibitors of Na+/Ca2+ exchanger prevent oxidant-induced intracellular Ca2+ increase and apoptosis in a human hepatoma cell line. Free Radic Res. 2000;33:267-77
23. Hu YC, Cheng HL, Hsieh BS, Huang LW, Huang TC, Chang KL. Arsenic trioxide affects bone remodeling by effects on osteoblast differentiation and function. Bone. 2012;50:1406-15
24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-8
25. Hung TC, Huang LW, Su SJ, Hsieh BS, Cheng HL, Hu YC. et al. Hemeoxygenase-1 expression in response to arecoline-induced oxidative stress in human umbilical vein endothelial cells. Int J Cardiol. 2011;151:187-94
26. Hsieh BS, Huang LW, Su SJ, Cheng HL, Hu YC, Hung TC. et al. Combined arginine and ascorbic acid treatment induces apoptosis in the hepatoma cell line HA22T/VGH and changes in redox status involving the pentose phosphate pathway and reactive oxygen and nitrogen species. J Nutr Biochem. 2011;22:234-41
27. Karmazyn M. The role of the myocardial sodium-hydrogen exchanger in mediating ischemic and reperfusion injury. From amiloride to cariporide. Annals of the New York Academy of Sciences. 1999;874:326-34
28. Bagkos G, Koufopoulos K, Piperi C. A new model for mitochondrial membrane potential production and storage. Med Hypotheses. 2014;83:175-81
29. Michelakis ED. Mitochondrial medicine: a new era in medicine opens new windows and brings new challenges. Circulation. 2008;117:2431-4
30. Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark Ed). 2009;14:1197-218
31. Orrenius S, Gogvadze V, Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun. 2015;460:72-81
32. Aghajanian A, Wittchen ES, Campbell SL, Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One. 2009;4:e8045
33. Chi AY, Waypa GB, Mungai PT, Schumacker PT. Prolonged hypoxia increases ROS signaling and RhoA activation in pulmonary artery smooth muscle and endothelial cells. Antioxid Redox Signal. 2010;12:603-10
34. Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free radical biology & medicine. 2010;48:749-62
35. Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M. et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32:1893-907
Author contact
![]() Corresponding author: Kee-Lung Chang, Ph.D., Department of Biochemistry, School of Medicine, College of Medicine, Kaohsiung Medical University, No.100, Shih-Chuan 1st Road, Kaohsiung 80708, Taiwan Tel: +886-7-312-1101 ext 2306 ext 11; Fax: +886-7-322-3075 E-mail: Chang.KeeLunghinet.net; keeluchedu.tw
Corresponding author: Kee-Lung Chang, Ph.D., Department of Biochemistry, School of Medicine, College of Medicine, Kaohsiung Medical University, No.100, Shih-Chuan 1st Road, Kaohsiung 80708, Taiwan Tel: +886-7-312-1101 ext 2306 ext 11; Fax: +886-7-322-3075 E-mail: Chang.KeeLunghinet.net; keeluchedu.tw

 Global reach, higher impact
Global reach, higher impact