Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(15):4415-4425. doi:10.7150/jca.123992 This issue Cite
Research Paper
Isoginkgetin Induces Caspase Cascade Activation and Cell Apoptosis via JNK Signaling in Oral Cancer
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
2. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
3. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
4. Department of Otolaryngology, Chung Shan Medical University Hospital, Taichung, Taiwan.
5. Institute of Oral Sciences, Chung Shan Medical University, Taichung, Taiwan.
6. Department of Dentistry, Chung Shan Medical University Hospital, Taichung, Taiwan.
7. Whole-Genome Research Core Laboratory of Human Diseases, Chang Gung Memorial Hospital, Keelung, Taiwan.
8. Department of Medical Biotechnology and Laboratory Science, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
9. Program for Cancer Biology and Drug Discovery, China Medical University, Taichung, Taiwan.
10. School of Dentistry, Chung Shan Medical University, Taichung, Taiwan.
# These authors contributed equally to this work.
Received 2025-8-20; Accepted 2025-11-6; Published 2025-11-14
Abstract
Isoginkgetin (IGG), a naturally occurring biflavonoid found in the leaves of many medicinal plants, is known to inhibit pre-mRNA splicing and display anti-cancer characteristics. However, knowledge regarding the use of IGG on oral squamous cell carcinoma (OSCC) lags behind that on the other common malignancies. The aim of this study is to explore whether IGG hinders OSCC proliferation and further investigated its oncostatic actions. We demonstrated that exposure of OSCC cell lines (HSC-3 and SCC-9) to IGG significantly diminished cell viability and induced apoptotic cell death. Furthermore, levels of several tentative apoptosis suppressors (cIAP-1 and XIAP) were decreased in IGG-treated HSC-3 and SCC-9 cells, accompanied with increased cleavage of caspases. Of note, such activation of caspase cascades by IGG was reduced by pharmaceutical inhibition of c-Jun N-terminal kinase (JNK) via a specific kinase antagonist, suggesting a functional connection of JNK activity with caspase activation during IGG-induced oral cancer cell apoptosis. In conclusion, we exhibited that IGG hampered cell viability and stimulated apoptotic events in OSCC, driven by a JNK-dependent pathway of caspase activations. Our findings present new insights into applications of a natural biflavonoid compound in fighting oral carcinogenesis.
Keywords: oral squamous cell carcinoma, isoginkgetin, apoptosis, caspase, JNK
Introduction
Oral squamous cell carcinoma (OSCC) is the most common type of oral cancer, representing approximately 90% of oral malignancies [1]. In patients with OSCC, surgery in combination with radiotherapy or chemotherapy are the primary treatment of choice. In addition, the use of an antagonist for the epidermal growth factor receptor (EGFR) has improved disease outcomes in OSCC patients receiving radiotherapy [2]. Besides, immune checkpoint inhibitors that restore anti-cancer immunity have been demonstrated to extend patients' survival as combined with chemotherapy or radiotherapy [3-5]. Yet, these treatment strategies did not significantly leverage the survival rate (roughly 50%) [6], mainly due to tumor recurrence and metastasis. This emphasizes the imperative to investigate complementary therapeutic modalities that can tackle these challenges. In this regard, the exploration of phytoconstituents that manifest favorable responses but minimal undesirable effects for the management of OSCC has come to the fore as a pivotal filed of research.
Isoginkgetin (IGG), originally extracted from the leaves of Metasequoia glyptostroboides (Dawn redwood, family: Taxodiaceae), is a naturally occurring biflavonoid shown to suppress cell invasion and migration by inhibiting the production of the matrix metalloproteinase 9 (MMP-9) [7, 8]. Pharmacological investigations revealed that IGG functions as an effective inhibitor for transcription elongation [9] and pre-mRNA splicing [10], to some degree providing the mechanistic basis of its anti-cancer activity. Such inhibition of the splicing machinery by IGG in cancer cells enhanced the antigen presentation by MHC class I [11], thereby facilitating the adaptive immune response against tumor antigens [12]. In addition, IGG was found to disturb protein homeostasis, eventually leading to cancer cell death [13]. Intriguingly, IGG may induce cytotoxic autophagy in hepatocellular carcinoma via directly binding to the N terminus of cyclin-dependent kinase 6 (CDK6) and promoting its subsequent degradation [14]. Although these in vitro and in vivo results have unveiled distinct anti-cancer features of this natural biflavonoid, beneficial effects of IGG on oral tumorigenesis are mostly unknown. In this study, we aimed to investigate whether IGG hampers OSCC progression and further explored the underlying mechanisms. Our findings highlight potential avenues for the use of a natural compound in the management of OSCC.
Materials and methods
Cell culture and reagents
Two cell lines of OSCC, SCC-9 and HSC-3, were obtained from the American Type Culture Collection (Manassas, VA, USA) and propagated in MEM medium (Life Technologies, Grand Island, NY, USA) as described previously [15]. SCC-9 is derived from a human tongue squamous cell carcinoma and HSC-3, also originating from tongue carcinoma, is known for its highly aggressive behavior. Isoginkgetin (IGG) of HPLC grade with ≥ 98% purity was commercially acquired from Sigma-Aldrich. U0126 and JNK-IN-8 were purchased from Sigma-Aldrich (St. Louis, MO, USA), and SB203580 was obtained from Cell Signaling Technology (Danvers, MA, USA).
Assessment of cell viability
Viability of OSCC cells in response to IGG was measured with a MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay (Sigma-Aldrich) as described previously [16]. In brief, cells were cultured in the presence of IGG at various concentrations for 24 hr and assessed for cell viability by using MTT. Levels of cell proliferation/viability were evaluated according to the chemical yield of formazan following solubilization with isopropanol, which was spectrophotometrically measured at 563 nm.
Flow cytometric analysis
Apoptotic cell populations were analyzed by monitoring the levels of annexin V flipping via flow cytometry as previously described [17]. In brief, cells exposed to various concentrations of IGG for 24 hr were assessed for the levels of annexin V on the outer leaflet of the plasma membrane with an FITC-labeled Annexin-V/PI Apoptosis Detection kit (BD Biosciences, San Jose, CA, USA). The proportions of annexin V- or propidium iodide (PI)-positive cells were evaluated by using flow cytometry (Accuri C6 Plus flow cytometer, BD Biosciences, San Diego, CA, USA).
Profiling of apoptotic proteome
Apoptosis-related protein markers in IGG-treated OSCC cells were profiled through a Proteome Profiler Human Apoptosis Array Kit (R&D Systems, Minneapolis, MN, USA) [18]. This membrane-based antibody array allows the simultaneous detection of 35 human apoptosis-related proteins, including both pro-apoptotic and anti-apoptotic factors. Protein lysates of OSCC cell lines treated with and without IGG were collected and applied to the protein array analysis according to manufacturer's instructions. Pixel density for apoptotic markers was measured and normalized to that of reference array spots.
Western blot
Protein lysates of cells under various conditions were harvested and subjected to SDS-PAGE assays. Individual protein targets were detected via a series of specific primary antibodies. These include Anti-cleaved Caspase-3 (ab2302), Anti-cleaved Caspase-8 (ab25901), Anti-pro-caspase-3 (ab32150), Anti-pro-caspase-8 (ab108333), and Anti-β-actin (ab8226) antibodies from Abcam (Cambridge, UK), Anti-Caspase-9 (#9502), Anti-cleaved Caspase-9 (#9505), Anti-PARP (#9542), Anti-Phospho-Erk1/2 (#4370), Anti-Erk1/2 (#9102), and Anti-c-IAP1 (#7065) antibodies from Cell Signaling Technology (Danvers, MA, USA), as well as Anti-Phospho-JNK (sc-6254), Anti-JNK (sc-7345), Anti-phospho-p38 (sc-166182), Anti-p38 (sc-7972), and Anti-XIAP (sc-55550) antibodies from Santa Cruz Biotechnology (Dallas, TX, USA). Visualization was conducted by hybridization with HRP-conjugated secondary antibodies (Dako Corporation, Carpinteria, CA, USA). Densitometry of immunoblots was analyzed via the ImageJ software.
Immunofluorescence
Cells were grown on coverslips, treated with IGG for 24 hr, fixed, and permeabilized. Cell cultures were stained with a primary antibody against cleaved caspase 3 (#9661, Cell Signaling Technology, Danvers, MA, USA), visualized by hybridization with a fluorescence-labeled secondary antibody (#4412, Cell Signaling Technology), washed, fixed, and mounted in Fluoromount-G (Electron Microscopy Sciences) [19]. Images were acquired by Olympus IX73 inverted fluorescence microscope using cellSENS microscope imaging software.
Statistical analysis
Data represent mean ± standard deviation (SD) from at least two separate experiments. Significant difference was based on a p value of < 0.05 by Student's t-test.
Results
IGG stimulates cytotoxicity and apoptosis in OSCC cells
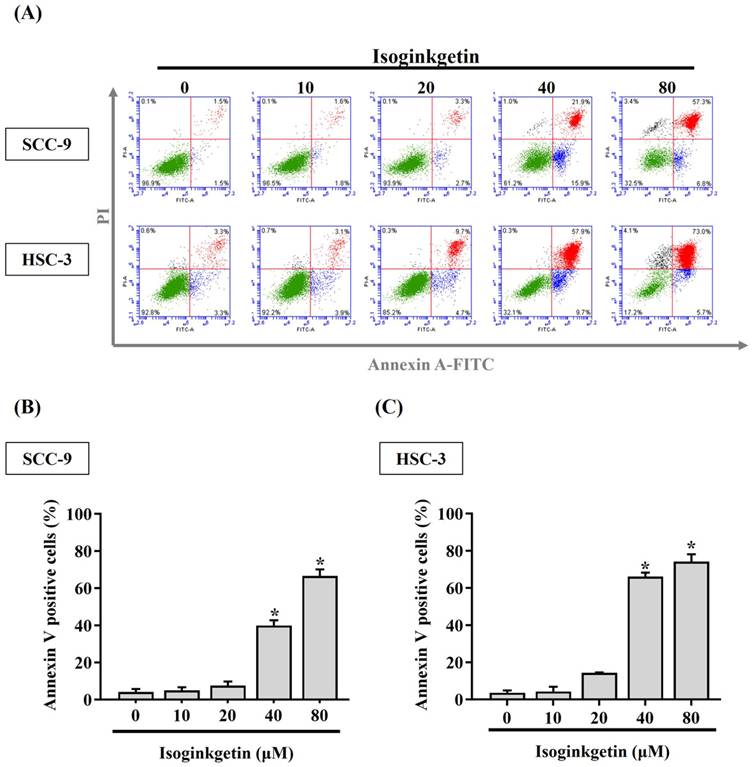
To clarify the potential of IGG on affecting OSCC progression, the viability of SCC-9 and HSC-3 cells treated with various concentrations of IGG (5 to 80 μM) was tested. We observed a reduction in cell viability of OSCC cells in response to 10 μM of IGG (Figure 1). Such cytotoxic effect appeared to be dose-dependent, as 40 and 80 μM of IGG profoundly interfered with cell proliferation of SCC-9 and HSC-3. This finding is in accordance with its anti-cancer properties noted in other tumor types [7, 12-14], unveiling a suppressive effect of IGG on oral carcinogenesis. Since a dose-dependent effect of IGG on influencing OSCC proliferation was demonstrated, we next examined whether IGG alters apoptotic responses in oral cancer. By monitoring the levels of annexin V flipping from the inner side to the outer leaflet of the plasma membrane, an increased proportion of apoptotic cell populations under the treatment of 40 and 80 μM IGG was detected in both cell lines (Figures 2A-C), revealing an elevation in OSCC apoptosis by IGG. These data suggest that the anti-cancer potential of IGG on oral tumorigenesis is attributed to induction of cytotoxicity and apoptosis.
IGG triggers cytotoxicity to OSCC cell lines. (A) Structural formula of isoginkgetin (IGG). (B-C) IGG is cytotoxic to OSCC cells. SCC-9 (B) and HSC-3 cells (C) were cultured in the presence of indicated concentrations of IGG for 24 hr and evaluated for cell viability. Data represent the average ± SD from three separate experiments. *p < 0.05, in comparison with untreated cells using Students t-test.
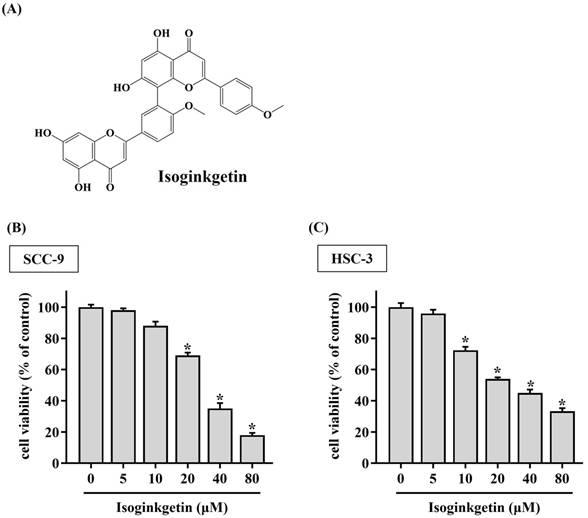
IGG triggers apoptotic events in OSCC. (A) SCC-9 and HSC-3 cells were maintained in the presence of indicated concentrations of IGG (10-80 μM) for 24 hr, stained with PI and annexin V, and assessed for apoptotic cell death by flow cytometry. Data are representative of three independent experiments. (B-C) Comparison of apoptotic responses among IGG-treated SCC-9 (B) and HSC-3 cells (C). The proportion of annexin V-positive cells was measured, and data represent the average ± SD from three independent experiments. *p < 0.05, in comparison with untreated cells using Students t-test.
IGG reshapes apoptosis-related proteome in OSCC
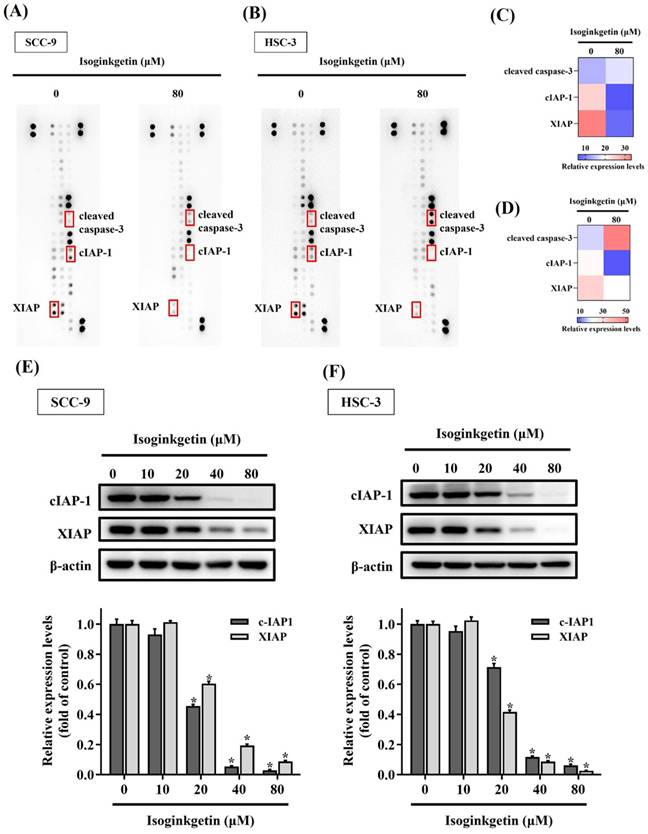
We subsequently aimed to investigate the profile of apoptosis-related proteins in IGG-treated OSCC cells by surveying 35 known protein markers of programmed cell death. A consistent shift in the expression levels of these markers was detected between two cell lines in response to IGG (Figures 3A-D). In particular, the levels of X-linked inhibitor of apoptosis protein (XIAP) and cellular inhibitor of apoptosis protein-1 (cIAP-1) in SCC-9 and HSC-3 cells were decreased under the treatment with IGG, whereas the signal intensities of cleaved caspase-3 were augmented in the same scenario. Further verification demonstrated that treatment with IGG downregulated XIAP and cIAP-1 in a dose-dependent manner (Figures 3E-F). These results indicate that IGG attunes the apoptotic proteome in OSCC cells, as manifested by downregulation of tentative apoptosis inhibitors.
IGG promotes proteolytic cleavage of caspase substrates in OSCC
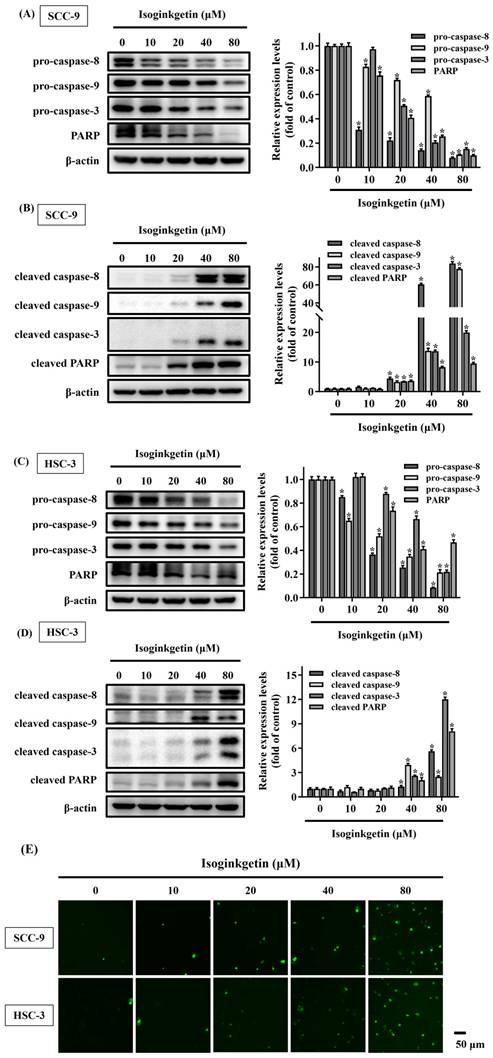
Cleavage of caspase substrates is a hallmark of cell apoptosis that generates many forms of active fragments to mediate cell membrane blebbing, cell body shrinkage, and DNA fragmentation [20]. Therefore, the effect of IGG on the cleavage of caspase substrates was explored in OSCC cells. We found that treatment of SCC-9 and HSC-3 cells with IGG, especially at 40 and 80 μM, reduced the levels of precursor (inactive) forms of caspase-3, -8, -9 and poly (ADP-ribose) polymerase-1 (PARP), accompanied with increased production of cleaved (active) forms of these apoptotic mediators in both lines (Figures 4A-D). In addition, activation of caspase 3 by IGG was further validated by immunofluorescence labeling of cleaved caspase 3 in SCC-9 and HSC-3 cells treated with IGG at different concentrations (Figure 4E). These results further support the observation that IGG acts as an inducer of apoptotic cell death in oral malignancy.
Profiling of apoptosis-related proteins in IGG-treated OSCC cells. (A-B) Representative dot plots corresponding to the levels of 35 apoptosis-related protein markers in IGG-untreated and -treated SCC-9 (A) and HSC-3 cells (B). Markers with differential expression proteins are marked, labelled, and further verified. (C-D) Heatmaps depicting relative expression of selected dots from SCC-9 (C) and HSC-3 samples (D). (E-F) Verification of apoptotic marker expression. SCC-9 (E) and HSC-3 cells (F) were maintained in the presence of indicated concentrations of IGG (10-80 μM) for 24 hr and assayed for the levels of indicated apoptosis markers by Western blot. Densitometric analyses of protein bands were conducted, normalized with internal controls (β-actin), compared, and shown underneath. Data represent the mean ± SD of three independent experiments. *p < 0.05, in comparison with untreated controls using Students t-test.
Effect of IGG on promoting proteolytic cleavage of caspase substrates in OSCC. SCC-9 and HSC-3 cells were maintained in the presence of indicated concentrations of IGG (10-80 μM) for 24 hr and assayed for the levels of precursor (A, C) and cleaved forms (B, D) of individual caspase substrates by Western blot. Densitometric analyses and signal comparisons are shown in the right. Data represent the mean ± SD of three separate experiments. *p < 0.05, compared with untreated controls using Students t-test. (E) Representative immunofluorescence images of cleaved caspase-3 in IGG-treated OSCCs. SCC-9 and HSC-3 cells were treated with IGG and stained with an antibody against cleaved forms of caspase-3. Bar = 50 μm.
JNK contributes to IGG-stimulated caspase activations in OSCC
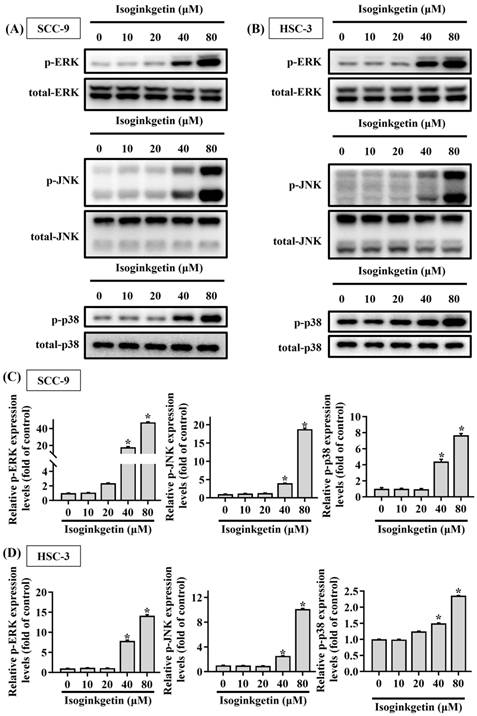
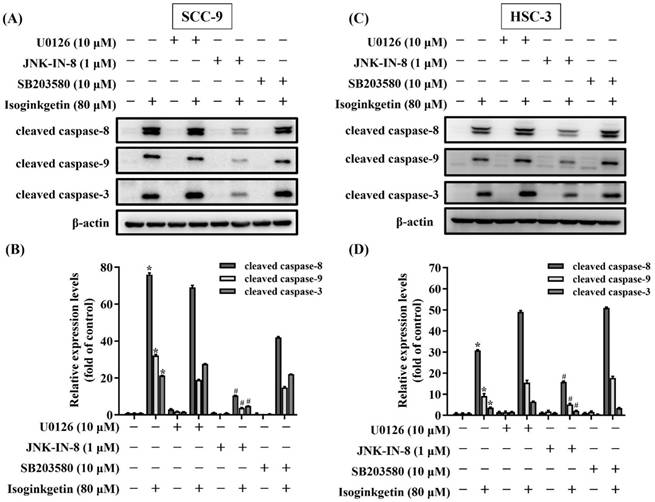
Mitogen-activated protein kinases (MAPKs) are known to function as a key regulator to direct apoptotic responses to various external stresses [21-23]. In the context of OSCC, dysregulation of MAPK signaling has been implicated in tumor progression and resistance to therapy [21, 24, 25]. We next tried to dissect whether MAPKs are functionally involved in the activation of caspase cascades in IGG-treated OSCC cells. Our survey of MAPK phosphorylation status revealed that JNK, ERK, and p38-MAPK were highly phosphorylated in both OSCC cell lines as treated with 40 and 80 μM IGG (Figures 5A-D), indicating a promotive effect of IGG on MAPKs activation in oral cancer. To further unravel whether there is a functional link between MAPK phosphorylation and caspase activation in the process of IGG-induced apoptosis, we tested the influence of kinase inhibitions on caspase cleavages in IGG-treated OSCC cells. Our results demonstrated that blockage of JNK activation with JNK-IN-8, a specific JNK inhibitor, significantly diminished the induction of cleaved (active) caspase-3, caspase-8, and caspase-9 in IGG-treated SCC-9 and HSC-3 cells (Figures 6A-D). Yet, pharmaceutical inhibition of ERK and p38-MAPK did not affect IGG-induced cleavage of pro-caspase-3, -8, and -9 in both cell lines. These findings connect JNK activity with activation of caspase cascades during IGG-stimulated oral cancer cell apoptosis.
Promotive effect of IGG on MAPK phosphorylation in OSCC cells. SCC-9 (A) and HSC-3 cells (B) were incubated with IGG at indicated concentrations and tested for the levels of phosphorylation on individual MAPKs via Western blot. (C-D) Quantification and comparison of relative phosphorylation status for ERK1/2 (ERK), JNK, and p38-MAPK in each condition of SCC-9 (C) and HSC-3 cells (D). The values represent the mean ± SD of three independent experiments. *p < 0.05, compared with untreated controls using Students t-test.
Functional connection of JNK to IGG-activated caspase cascades in OSCC. SCC-9 (A-B) and HSC-3 cells (C-D) were pretreated with individual MAPK antagonists for 2 hr and subsequently incubated with IGG for 24 hr, followed by assessment for the degree of caspase cleavage via Western blot. Densitometric analyses of SCC-9 (B) and HSC-3 data (D) were conducted, and relative expression levels were normalized to internal controls (β-actin). Data represent the mean ± SD of three separate experiments. *p < 0.05, compared with untreated controls using Students t-test. #p < 0.05, compared with IGG-treated cells using Students t-test.
Discussion
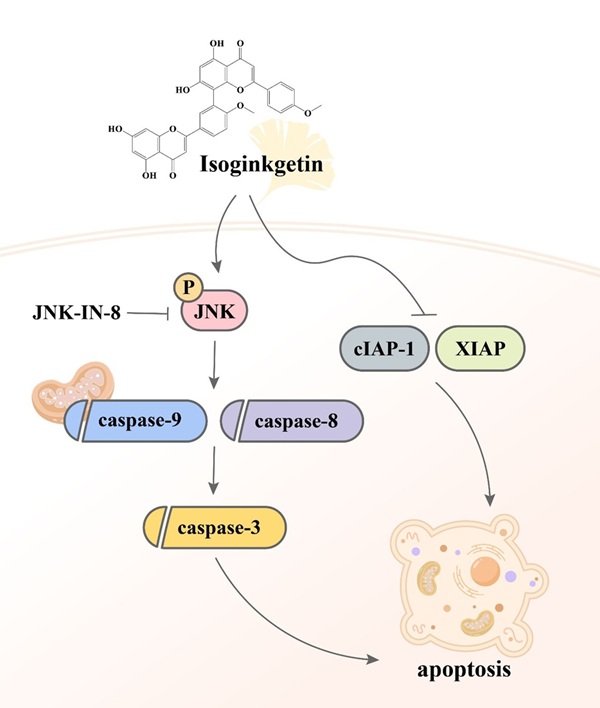
Despite the observations that contemporary therapeutic methods have generated favorable outcomes in patients with early-stage oral malignancy, the survival rate and prognostic response of patients bearing late-stage OSCC still present an enormous burden. The application of complementary treatment options, thus, is needed to deal with the clinical challenge. It is widely accepted that natural constituents isolated from herbal plants can be beneficial for cancer treatment as given in combination with other standard cares [26]. In our investigation, we demonstrated that IGG, a naturally occurring biflavonoid found in the leaves of ginkgo (Ginkgo biloba), effectively induced apoptotic responses in OSCC cell lines. Furthermore, the molecular mechanism underlying IGG-stimulated cell apoptosis involves downregulation of several apoptotic inhibitors and a JNK-dependent activation of caspase pathways (Figure 7). Our results highlight a potential of IGG in improving the management of OSCC.
Several studies of phytomedicine have documented a tumor-suppressive effect of IGG on various malignant diseases [7, 10, 13, 14, 27-29]. Such anti-cancer activity relies upon multiple cell type-specific or general mechanisms and affects a variety of molecular targets. Firstly, IGG is a general inhibitor of spliceosome to block pre-mRNA splicing, thereby interfering with tumor growth [10]. Unlike other anti-cancer natural compounds (e.g. pladienolide and spliceostatin A) that target the splicing factor SF3b to prevent the assembly of prespliceosome (A complex) [30, 31], the presence of IGG promotes accumulation of A complex and mediates its progression into B complex, a catalytically active form of spliceosome [10]. Although these spliceosome inhibitors have distinct core structures and affect different steps of splicing, disruption of mRNA splicing leads to generation of aberrant proteins and triggers apoptotic cell death [32, 33]. These findings are in accordance with our data showing a promotive effect of IGG on apoptotic responses in OSCC. Intriguingly, these spliceosome-targeted agents elicit double-stranded RNA responses via induction of widespread intron-retained transcripts to drive not only extrinsic apoptosis but also adaptive immune signaling in fighting cancer [34]. Consistently, inhibition of the splicing machinery by IGG in malignant cells augmented the antigen presentation by MHC class I [11], thereby boosting the downstream adaptive immune response against tumor antigens [12]. In addition to mRNA processing, IGG was also reported to inhibit transcription elongation, further highlighting its impact on regulation of gene expression [9]. Moreover, IGG has been shown to sensitize cancer cells to apoptosis via impairment of protein clearance through directly inhibiting activities of 20S proteosome [13]. The 20S proteosome simply degrades unfolded or misfolded proteins to maintain proper protein dynamics, and its inhibition could stimulate the unfolded protein response (UPR) [35], a signal transduction pathway that is activated by accumulation of excessive unfolded proteins and eventually renders the vulnerability of malignant cells to death [36]. These findings, together with our data, implicate the use of IGG as a promising OSCC therapy.
Proposed mechanism of IGG-induced cell apoptosis in OSCC.
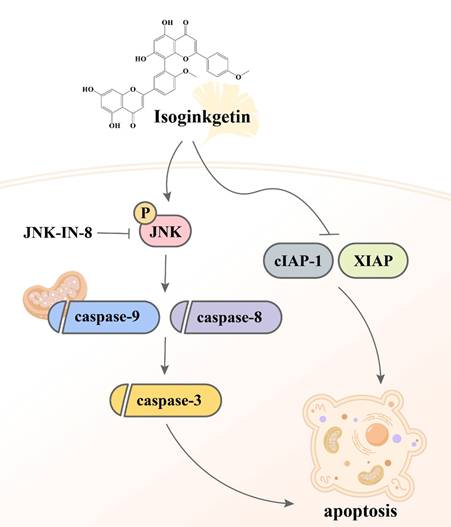
Beside caspase activation, IGG-induced OSCC apoptosis was accompanied with downregulation of several putative apoptosis inhibitors. One such example is XIAP (X-linked inhibitor of apoptosis protein), an E3 ubiquitin ligase that has been shown to promote resistance to therapy-induced apoptosis and confer poor outcome in cancer patients [37]. Different biological functions of XIAP localized at distinct cellular compartments, such as cytoplasm, mitochondria, and nucleus, have been documented. Cytoplasmic XIAP can physically interact with caspase-3, -7, and -9 and subsequently restrain activation of these caspases to counteract apoptosis [38, 39], whereas nuclear XIAP acts to modulate many oncogenic pathways [40-42], irrelevant to its caspase-inhibitory cytoplasmic function. Although cellular localization of XIAP was not investigated in our experiments, our observation that IGG downregulated XIAP and activated caspase cascades during OSCC apoptosis is largely in agreement with the anti-apoptotic function of cytotoxic XIAP. In addition, another IGG-downregulated proteins, cIAP-1 (cellular inhibitor of apoptosis protein 1), is also an E3 ubiquitin ligase that has a key role in regulating NF-κB signaling and programmed cell death via the ubiquitylation of major components of TNF receptor complexes [43]. cIAP-1 is a substrate for caspase-8, and its degradation by caspase-8 is associated with TNF-related apoptosis [44]. Furthermore, cIAP-1 binds to the apoptosome and sterically hinders the access of pro-caspase-3 to the catalytic center of the Apaf-1-caspase-9 complex, thereby inhibiting the processing and activation of pro-caspase-3 [45]. These IGG-downregulated apoptosis mediators appear to act upstream or downstream of multiple caspase cascades, cooperatively contributing to IGG-stimulated apoptotic events in OSCC.
JNK signaling is known to mediate the extrinsic apoptotic pathway initiated by death receptors as well as the intrinsic pathway initiated at the mitochondria [46]. In our study, IGG triggered apoptotic responses in OSCC, employing a JNK-dependent activation of caspase cascades. Consistently, such involvement of JNK activities in phytomedicine-induced caspase activation and apoptosis was observed in oral cancer [47, 48] and other cancer types [49-51]. Our findings reiterate that JNK signaling behaves as a critical hub in caspase-dependent apoptosis of IGG-treated oral cancer cells.
Even though anti-cancer effects of IGG on oral carcinogenesis were observed, there are some limitations to this study. One weakness is that both OSCC cell lines tested in our experiments originated from tongue cancer. It is proposed that cancers developed at distinct anatomical locations of the mouth (e.g. tongue, buccal mucosa, lip, and gingiva) tends to be correlated with different mutational signatures, oncogenic pathways, and survival rates [52]. Thus, the generalizability of this study can be reinforced if additional OSCC cell lines derived from other anatomical positions are examined to explore IGG's actions. Another concern is that the influence of this biflavonoid compound might be incongruous in animal studies, despite our in vitro finding that IGG rendered a tumor-suppressive effect on oral cancer progression. As IGG has exhibited promising oncostatic characteristics in mouse cancer models [12, 14], future experiments in in vivo settings could further clarify the impact of IGG on oral carcinogenesis.
In conclusion, we showed that IGG effectively triggered apoptotic cell death in OSCC, employing a JNK-dependent pathway of caspase activations. Our findings implicate this natural compound as a tentative therapeutic modality against oral malignancies.
Acknowledgements
This study was supported by Chung Shan Medical University Hospital (CSH-2023-D-004). This study was also funded by China Medical University, Taiwan (CMU107-TU-10).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63
2. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2006;354:567-78
3. Kujan O, van Schaijik B, Farah CS. Immune Checkpoint Inhibitors in Oral Cavity Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Cancers (Basel). 2020;12:1937
4. Su CW, Yang WE, Hsieh YH, Tang CH, Lin CW, Yang SF. CEACAM7 enhances oral cancer metastasis by upregulating CD317 expression. Life Sci. 2025;381:123998
5. Su SC, Lin CW, Chen MK, Lee YC, Su CW, Bai S. et al. Multimodal profiling of oral squamous cell carcinoma identifies genomic alterations and expression programs associated with betel quid chewing. Neoplasia. 2025;68:101218
6. Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4-14
7. Yoon SO, Shin S, Lee HJ, Chun HK, Chung AS. Isoginkgetin inhibits tumor cell invasion by regulating phosphatidylinositol 3-kinase/Akt-dependent matrix metalloproteinase-9 expression. Mol Cancer Ther. 2006;5:2666-75
8. Shao N, Feng Z, Li N. Isoginkgetin inhibits inflammatory response in the fibroblast-like synoviocytes of rheumatoid arthritis by suppressing matrix metallopeptidase 9 expression. Chem Biol Drug Des. 2022;99:923-9
9. Boswell SA, Snavely A, Landry HM, Churchman LS, Gray JM, Springer M. Total RNA-seq to identify pharmacological effects on specific stages of mRNA synthesis. Nat Chem Biol. 2017;13:501-7
10. O'Brien K, Matlin AJ, Lowell AM, Moore MJ. The biflavonoid isoginkgetin is a general inhibitor of Pre-mRNA splicing. J Biol Chem. 2008;283:33147-54
11. Apcher S, Millot G, Daskalogianni C, Scherl A, Manoury B, Fahraeus R. Translation of pre-spliced RNAs in the nuclear compartment generates peptides for the MHC class I pathway. Proc Natl Acad Sci U S A. 2013;110:17951-6
12. Darrigrand R, Pierson A, Rouillon M, Renko D, Boulpicante M, Bouyssie D. et al. Isoginkgetin derivative IP2 enhances the adaptive immune response against tumor antigens. Commun Biol. 2021;4:269
13. Tsalikis J, Abdel-Nour M, Farahvash A, Sorbara MT, Poon S, Philpott DJ. et al. Isoginkgetin, a Natural Biflavonoid Proteasome Inhibitor, Sensitizes Cancer Cells to Apoptosis via Disruption of Lysosomal Homeostasis and Impaired Protein Clearance. Mol Cell Biol. 2019 39
14. Yao J, Tang S, Shi C, Lin Y, Ge L, Chen Q. et al. Isoginkgetin, a potential CDK6 inhibitor, suppresses SLC2A1/GLUT1 enhancer activity to induce AMPK-ULK1-mediated cytotoxic autophagy in hepatocellular carcinoma. Autophagy. 2023;19:1221-38
15. Lin CW, Yang WE, Su CW, Lu HJ, Su SC, Yang SF. IGF2BP2 promotes cell invasion and epithelial-mesenchymal transition through Src-mediated upregulation of EREG in oral cancer. Int J Biol Sci. 2024;20:818-30
16. Chuang CY, Ho YC, Lin CW, Yang WE, Yu YL, Tsai MC. et al. Salvianolic acid A suppresses MMP-2 expression and restrains cancer cell invasion through ERK signaling in human nasopharyngeal carcinoma. J Ethnopharmacol. 2020;252:112601
17. Su SC, Chen YT, Hsieh YH, Yang WE, Su CW, Chiu WY. et al. Gambogic Acid Induces HO-1 Expression and Cell Apoptosis through p38 Signaling in Oral Squamous Cell Carcinoma. Am J Chin Med. 2022;50:1663-79
18. Su CW, Kao SH, Chen YT, Hsieh YH, Yang WE, Tsai MY. et al. Curcumin Analog L48H37 Induces Apoptosis in Human Oral Cancer Cells by Activating Caspase Cascades and Downregulating the Inhibitor of Apoptosis Proteins through JNK/p38 Signaling. Am J Chin Med. 2024;52:565-81
19. Hsin MC, Hsieh YH, Wang PH, Ko JL, Hsin IL, Yang SF. Hispolon suppresses metastasis via autophagic degradation of cathepsin S in cervical cancer cells. Cell Death Dis. 2017;8:e3089
20. Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821-46
21. Chien MH, Shih PC, Ding YF, Chen LH, Hsieh FK, Tsai MY. et al. Curcumin analog, GO-Y078, induces HO-1 transactivation-mediated apoptotic cell death of oral cancer cells by triggering MAPK pathways and AP-1 DNA-binding activity. Expert Opin Ther Targets. 2022;26:375-88
22. Lee CY, Chen PN, Kao SH, Wu HH, Hsiao YH, Huang TY. et al. Deoxyshikonin triggers apoptosis in cervical cancer cells through p38 MAPK-mediated caspase activation. Environ Toxicol. 2024;39:4308-17
23. Hsieh MJ, Lin CW, Su SC, Reiter RJ, Chen AW, Chen MK. et al. Effects of miR-34b/miR-892a Upregulation and Inhibition of ABCB1/ABCB4 on Melatonin-Induced Apoptosis in VCR-Resistant Oral Cancer Cells. Mol Ther Nucleic Acids. 2020;19:877-89
24. Yang WE, Chen YT, Su CW, Chen MK, Yeh CM, Chen YL. et al. Hispolon induces apoptosis in oral squamous cell carcinoma cells through JNK/HO-1 pathway activation. J Cell Mol Med. 2023;27:1250-60
25. Chien MH, Yang WE, Yang YC, Ku CC, Lee WJ, Tsai MY. et al. Dual Targeting of the p38 MAPK-HO-1 Axis and cIAP1/XIAP by Demethoxycurcumin Triggers Caspase-Mediated Apoptotic Cell Death in Oral Squamous Cell Carcinoma Cells. Cancers (Basel). 2020;12:703
26. Yin SY, Wei WC, Jian FY, Yang NS. Therapeutic applications of herbal medicines for cancer patients. Evid Based Complement Alternat Med. 2013;2013:302426
27. Oliva MA, Staffieri S, Sanchez M, Arcella A. Isoginkgetin-A Natural Compound to Control U87MG Glioblastoma Cell Growth and Migration Activating Apoptosis and Autophagy. Molecules. 2022;27:8335
28. van Zyl E, Tolls V, Blackmore A, McKay BC. Isoginkgetin leads to decreased protein synthesis and activates an ATF4-dependent transcriptional response. Biochim Biophys Acta Mol Cell Res. 2021;1868:119123
29. Mariano N, Wolf H, Vivekanand P. Isoginkgetin exerts apoptotic effects on A375 melanoma cells. MicroPubl Biol. 2024;2024:10.17912
30. Kaida D, Motoyoshi H, Tashiro E, Nojima T, Hagiwara M, Ishigami K. et al. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat Chem Biol. 2007;3:576-83
31. Kotake Y, Sagane K, Owa T, Mimori-Kiyosue Y, Shimizu H, Uesugi M. et al. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat Chem Biol. 2007;3:570-5
32. Larrayoz M, Blakemore SJ, Dobson RC, Blunt MD, Rose-Zerilli MJ, Walewska R. et al. The SF3B1 inhibitor spliceostatin A (SSA) elicits apoptosis in chronic lymphocytic leukaemia cells through downregulation of Mcl-1. Leukemia. 2016;30:351-60
33. Vanzyl EJ, Sayed H, Blackmore AB, Rick KRC, Fernando P, McKay BC. The spliceosome inhibitors isoginkgetin and pladienolide B induce ATF3-dependent cell death. PLoS One. 2020;15:e0224953
34. Bowling EA, Wang JH, Gong F, Wu W, Neill NJ, Kim IS. et al. Spliceosome-targeted therapies trigger an antiviral immune response in triple-negative breast cancer. Cell. 2021;184:384-403 e21
35. Zhou X, Xu R, Wu Y, Zhou L, Xiang T. The role of proteasomes in tumorigenesis. Genes Dis. 2024;11:101070
36. Gsottberger F, Meier C, Ammon A, Parker S, Wendland K, George R. et al. Targeted inhibition of protein synthesis renders cancer cells vulnerable to apoptosis by unfolded protein response. Cell Death Dis. 2023;14:561
37. Mendonca BS, Ferreira CA, Maia RC, Nestal de Moraes G. Subcellular localization of X-linked inhibitor of apoptosis protein (XIAP) in cancer: Does that matter? BBA Adv. 2022;2:100050
38. Scott FL, Denault JB, Riedl SJ, Shin H, Renatus M, Salvesen GS. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 2005;24:645-55
39. Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J. et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112-6
40. Ng VH, Hang BI, Sawyer LM, Neitzel LR, Crispi EE, Rose KL. et al. Phosphorylation of XIAP at threonine 180 controls its activity in Wnt signaling. J Cell Sci. 2018 131
41. Xu J, Hua X, Yang R, Jin H, Li J, Zhu J. et al. XIAP Interaction with E2F1 and Sp1 via its BIR2 and BIR3 domains specific activated MMP2 to promote bladder cancer invasion. Oncogenesis. 2019;8:71
42. Delbue D, Mendonca BS, Robaina MC, Lemos LGT, Lucena PI, Viola JPB. et al. Expression of nuclear XIAP associates with cell growth and drug resistance and confers poor prognosis in breast cancer. Biochim Biophys Acta Mol Cell Res. 2020;1867:118761
43. Graber TE, Holcik M. Distinct roles for the cellular inhibitors of apoptosis proteins 1 and 2. Cell Death Dis. 2011;2:e135
44. Guicciardi ME, Mott JL, Bronk SF, Kurita S, Fingas CD, Gores GJ. Cellular inhibitor of apoptosis 1 (cIAP-1) degradation by caspase 8 during TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Exp Cell Res. 2011;317:107-16
45. Burke SP, Smith L, Smith JB. cIAP1 cooperatively inhibits procaspase-3 activation by the caspase-9 apoptosome. J Biol Chem. 2010;285:30061-8
46. Nadel G, Maik-Rachline G, Seger R. JNK Cascade-Induced Apoptosis-A Unique Role in GqPCR Signaling. Int J Mol Sci. 2023;24:13527
47. Tung KL, Wu SZ, Yang CC, Chang HY, Chang CS, Wang YH. et al. Cordycepin Induces Apoptosis through JNK-Mediated Caspase Activation in Human OEC-M1 Oral Cancer Cells. Evid Based Complement Alternat Med. 2022;2022:1842363
48. Lan YY, Chen YH, Liu C, Tung KL, Wu YT, Lin SC. et al. Role of JNK activation in paclitaxel-induced apoptosis in human head and neck squamous cell carcinoma. Oncol Lett. 2021;22:705
49. Avisetti DR, Babu KS, Kalivendi SV. Activation of p38/JNK pathway is responsible for embelin induced apoptosis in lung cancer cells: transitional role of reactive oxygen species. PLoS One. 2014;9:e87050
50. Chinen T, Nagumo Y, Watanabe T, Imaizumi T, Shibuya M, Kataoka T. et al. Irciniastatin A induces JNK activation that is involved in caspase-8-dependent apoptosis via the mitochondrial pathway. Toxicol Lett. 2010;199:341-6
51. Zhang L, Kong SY, Zheng ZQ, Meng XX, Feng JL, Tan HS. et al. Nujiangexathone A, a Novel Compound Derived from Garcinia nujiangensis, Induces Caspase-Dependent Apoptosis in Cervical Cancer through the ROS/JNK Pathway. Molecules. 2016;21:1360
52. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92
Author contact
![]() Corresponding author: Shun-Fa Yang, Ph.D. and Yi-Tzu Chen, Ph.D. Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysfedu.tw (Shun-Fa Yang); chenyitzu0831com (Yi-Tzu Chen).
Corresponding author: Shun-Fa Yang, Ph.D. and Yi-Tzu Chen, Ph.D. Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysfedu.tw (Shun-Fa Yang); chenyitzu0831com (Yi-Tzu Chen).

 Global reach, higher impact
Global reach, higher impact