Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(15):4371-4377. doi:10.7150/jca.123900 This issue Cite
Research Paper
Validation of WHO 2017 Classification and Identification of Prognostic Factors in Patients with Pancreatic Neuroendocrine Neoplasms: A Real-World Experience in Taiwan
1. Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan.
2. College of Medicine, Chang Gung University, Taoyuan, Taiwan.
3. Department of Pathology, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan.
Received 2025-8-18; Accepted 2025-10-20; Published 2025-10-27
Abstract

Background: Pancreatic neuroendocrine tumors (PanNETs) are rare neoplasms with an increasing incidence. This study aims to validate the clinical relevance of the WHO 2017 classification system in the Taiwanese population and identify independent prognostic factors for patients with PanNETs.
Materials and methods: We conducted a retrospective analysis of 176 patients with PanNETs from the Chang Gung Medical Hospital at Linkou in Taiwan, spanning the years 2009 to 2022. Pathology reports were reassessed according to the WHO 2017 classification. Clinical characteristics, treatment patterns, and survival outcomes were documented, with subgroup analyses to compare grade 3 (G3) neuroendocrine tumors and neuroendocrine carcinomas (NEC).
Results: The overall 5-year survival rate was 58.7%, with median survival of 107.6 months. Survival rates showed clear stratification across WHO 2017 classifications: G1 (83.1%, median 141.0 months), G2 (55.0%, median 105.2 months), G3 (14.6%, median 21.5 months), and NEC (9.4%, median 19.6 months). Multivariate analysis identified five independent prognostic factors: age over 60 years (HR 1.70), tumor size >2cm (HR 1.893), lymph node involvement (HR 1.801), distant metastasis (HR 3.042), and NEC classification (HR 2.382). NEC demonstrated significantly higher lymph node involvement (81% vs 48%, p=0.026), higher Ki-67 index (69 vs 43.8, p<0.001), and higher rates of metastases compared with G3 NET.
Conclusions: Our findings validate the prognostic utility of the WHO 2017 classification, particularly in differentiating NET G3 from NEC. This refined classification system, combined with identified prognostic factors, provides valuable guidance for clinical decision-making and treatment selection in patients with PanNETs.
Keywords: pancreatic neuroendocrine tumor, neuroendocrine carcinoma, WHO 2017 classification, prognostic factors
Introduction
Pancreatic neuroendocrine tumor (PanNETs) are rare neoplasms, accounting for ≤1% of all pancreatic tumors [1, 2]. Significantly, recent years have seen a notable increase in the incidence of pancreatic neuroendocrine tumors [2, 3], a phenomenon that can largely be attributed to advancements in diagnostic imaging and techniques [4]. A previous epidemiological study conducted in Taiwan reported an incidence of 0.446 cases per 100,000 individuals diagnosed with neuroendocrine tumors, further emphasizing the growing incidence [5]. This rise underscores the necessity for a comprehensive understanding of PanNETs to improve their effective management.
PanNETs represent a heterogeneous group of neoplasms with diverse clinical manifestations, pathological characteristics, and long-term prognoses [6-8]. Notably, the updated World Health Organization (WHO) 2017 classification has provided a more refined framework for categorizing PanNETs, particularly by distinguishing well-differentiated Grade 3 (G3) neuroendocrine tumors (NET) from neuroendocrine carcinomas (NEC) [9-11], with significant implications for prognosis. The evolution of classification systems, particularly the transition from the WHO 2010 classification to the updated WHO 2017 classification, has significantly refined the framework for PanNETs categorization. The updated classification distinguishes well-differentiated Grade 3 (G3) neuroendocrine tumors from NEC, providing clearer differentiation between PanNETs subtypes [12] This enhanced clarity has critical implications for treatment strategies and prognostic assessments, enabling clinicians to better tailor interventions to the specific tumor characteristics of each patient [13].
To validate the clinical relevance and prognostic value of the WHO 2017 classification system in the Taiwanese population with PanNETs, we conducted a comprehensive retrospective analysis at Chang Gung Medical Hospital at Linkou, the largest medical center in Taiwan. Second, to identify independent prognostic factors that could guide clinical decision-making and treatment planning. Furthermore, this study seeks to identify key clinical and pathological prognostic factors that influence survival outcomes in patients with PanNETs. Through this analysis, we aimed to provide evidence-based insights that would enhance the management of patients with PanNETs in Taiwan and contribute to the broader understanding of these tumors in Asian populations.
Materials and Methods
A comprehensive retrospective analysis was conducted on 176 cases of PanNETs collected from Chang Gung Memorial Hospital at Linkou, Taiwan, encompassing data from January 2009 to December 2022. Patient included in this study had a prior histopathological diagnosis of PanNETs. Clinical characteristics, symptom presentation, and overall survival outcomes were comprehensively documented for all cases.
To ensure diagnostic precision and appropriate staging, pathology reports for all cases were meticulously re-evaluated by expert pathologists according to both the WHO 2010 and updated WHO 2017 classifications. Patients were classified into four groups based on the WHO 2017 classification: Grade 1 (G1), Grade 2 (G2), Grade 3 (G3), and NEC.
Survival outcomes, including median survival time and five-year survival rates, were calculated for the entire cohort and specific PanNETs subgroups. Kaplan-Meier survival curves were used for initial survival analyses, with subgroup stratification based on the WHO 2017 classification to explore survival trends. To identify independent prognostic factors, both univariate and multivariate Cox regression analyses were performed. Variables analyzed included age (≤60 vs >60 years), presence or absence of symptoms at diagnosis, tumor size (≤2cm vs >2cm), lymph node involvement, distant metastasis, Ki-67 index (≤55% vs >55%), and WHO 2017 classification groups. Statistical significance was set at a p-value of 0.05.
To investigate the potential differences in tumor biology, a subgroup analysis was performed to compare the G3 NET and NEC groups, focusing on tumor characteristics, Ki-67 index distribution, treatment patterns, and oncologic outcomes. Also, we utilized Chi-square analysis to investigate the relationship between KI-67 expression and high-grade pancreatic neuroendocrine neoplasms (NET G3 and NEC). Furthermore, we performed a subgroup analysis of high-grade neuroendocrine neoplasms based on differing levels of Ki-67 expression.
All statistical analyses were performed using SPSS version 26 software. Categorical variables were compared using chi-square or Fisher's exact tests as appropriate, while continuous variables were analyzed using Student's t-test or Mann-Whitney U test based on their distribution. This comprehensive analysis provided insights into the clinical characteristics, prognostic factors, and treatment outcomes across different PanNETs subgroups, particularly emphasizing the distinctions between G3 and NEC categories.
Results
According to the updated WHO 2017 classification, PanNETs could be classified into four groups, including Grade 1 (G1), Grade 2 (G2), Grade 3 (G3) and NEC. Table 1indicates that patients in the G3 (61.4 years) and NEC (64.1 years) groups were generally older than those in the G1 (57.8 years) and G2 (52.1 years) groups. These higher-grade groups (G3 and NEC) also exhibited more frequent symptoms, particularly abdominal pain (G3: 64.0%, NEC: 62.5%), jaundice (G3: 8.0%, NEC: 12.5%), and poor appetite (G3: 12.0%, NEC: 25.0%). In contrast, approximately one-third of G1 (33.3%) and G2 (29.8%) patients were asymptomatic and diagnosed incidentally. The most common symptoms in PanNETs are abdominal pain and hypo/hyperglycemia (Table 1). A greater proportion of G1 patients underwent surgery following the incidental discovery of PanNETs. Patients in the NEC group displayed the highest rates of distant metastases, lymph node involvement, and elevated Ki-67 indices. Additionally, this group had the largest proportion of patients receiving systemic therapies such as chemotherapy.
Subgroup of The WHO 2017 classification of the neuroendocrine neoplasm of the pancreases
| Well differentiated | Poor differentiated | |||
|---|---|---|---|---|
| Subgroup | G1(N=78) | G2(N=57) | G3(N=25) | NEC(N=16) |
| Characteristic | ||||
| Age | 57.8(16-84) | 52.1(16-73) | 61.4(24-85) | 64.1(45-81) |
| Male | 47.4 % | 57.8 % | 60.0 % | 68.8 % |
| Female | 52.6 % | 42.2 % | 40.0 % | 31.3 % |
| Symptoms | ||||
| Abdominal pain | 37.2 % | 47.4 % | 64.0 % | 62.5 % |
| Body weight loss | 3.8 % | 5.3 % | 20.0 % | 12.5 % |
| Diahhrea | 3.8 % | 5.3 % | 0.0 % | 12.5 % |
| Gastrointestinal bleeding | 5.1 % | 3.5 % | 4.0 % | 0.0 % |
| Hypo/Hyperglycemia | 14.1 % | 17.5 % | 0.0 % | 0.0 % |
| jaundice | 0.0 % | 0.0 % | 8.0 % | 12.5 % |
| Poor appetite | 3.8 % | 5.3 % | 12.0 % | 25.0 % |
| No symptoms | 33.3 % | 29.8 % | 16.0 % | 0.0 % |
| AJCC | ||||
| Stage1 | 50.0 % | 17.5 % | 0.0 % | 0.0 % |
| Stage2 | 26.9 % | 26.3 % | 4.0 % | 0.0 % |
| Stage3 | 6.4 % | 7.0 % | 12.0 % | 12.5 % |
| Stage4 | 16.7 % | 49.1 % | 84.0 % | 87.5 % |
| Treatment | ||||
| Surgery | 82.1 % | 63.2 % | 32.0 % | 18.8 % |
| Somatostatin | 16.7 % | 14.0 % | 28.0 % | 12.5 % |
| Chemotherapy | 1.3 % | 21.1 % | 68.0 % | 87.5 % |
| Targeted therapy | 11.5 % | 50.9 % | 16.0 % | 12.5 % |
NEC: Neuroendocrine carcinoma.
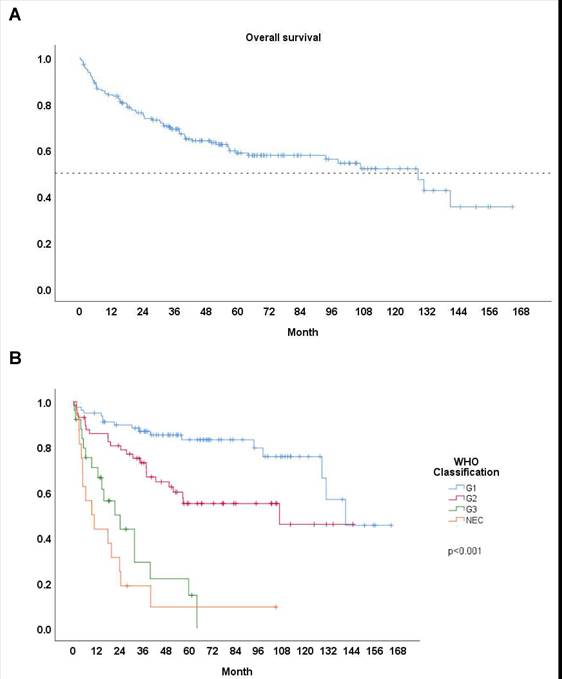
We documented the median survival time and 5-year survival rates to compare different groups of patients with PanNETs. Among the entire cohort of 176 patients, the 5-year survival rate was 58.7%, with a median survival time of 107.6 months (Fig. 1A). Patients with G1 tumors showed the highest survival rates, with a 5-year survival of 83.1% (n = 78) and a median survival time of 141.0 months (Table 2). G2 patients had a 5-year survival rate of 55.0% (n = 57) and a median survival time of 105.2 months. In contrast, G3 patients had poorer outcomes, with a 5-year survival rate of 14.6% (n = 25) and a median survival time of 21.5 months. Patients with NEC exhibited the worst survival outcomes, with a 5-year survival rate of 9.4% (n = 16) and a median survival time of 19.6 months. As highlighted by the updated WHO 2017 classification, the G3 group shows better survival outcomes compared to NEC group, but poorer outcomes compared than G2 group, as confirmed by our findings (Fig. 1B).
Median survival time and 5-year survival
| MST | 5-year survival | |
|---|---|---|
| G1 | 141.0 | 83.1% |
| G2 | 105.2 | 55.0% |
| G3 | 21.5 | 14.6% |
| NEC | 19.6 | 9.4% |
| ALL | 107.6 | 58.7% |
P value <0.001 Note. MST: Median survival time (month). NEC: Neuroendocrine carcinoma.
To investigate the prognostic factors, we conducted both univariate and multivariate analyses (Table 3). The univariate analysis identified several significant prognostic factors, including age over 60 years, absence of symptoms at diagnosis, tumor size > 2cm, lymph node involvement, distant metastasis, Ki-67 index > 55%, and NEC classification under WHO 2017 classification (all p < 0.05). In multivariate analysis, age over 60 years (HR 1.70, p=0.013), tumor size > 2cm (HR 1.893, p = 0.042), lymph node involvement (HR 1.801, p = 0.042), distant metastasis (HR 3.042, p = 0.001), and NEC classification (HR 2.382, p = 0.016) remained independently significant.
The comparison between NET G3 (N = 25) and NEC (N = 16) reveals some remarkable differences in their characteristics and treatment patterns. While tumor size was comparable (5.51 vs 5.07, p = 0.645), NEC showed significantly higher lymph node involvement (81% vs 48%, p = 0.026) and Ki-67 proliferation index (69 vs 43.8, p < 0.001) (Table 4). Table 5 further underscores the distinction in Ki-67 distribution, with NET G3 cases predominantly exhibiting Ki-67 < 55% (18 vs. 2 in NEC) and NEC cases showing Ki-67 > 55% (14 vs. 7 in NET G3), yielding a robust statistical association (p < 0.001). Treatment approaches differed markedly, with NEC patients receiving more chemotherapy overall (88% vs 68%), particularly Etoposide + Cisplatin (69% vs 32%). Notably, NET G3 patients had access to more diverse treatment options, including targeted therapy with Sunitinib (8%) and somatostatin analog therapy (12%), which were not utilized in NEC patients. Notably, NEC patients had a higher objective response rate (29% vs. 19%, p = 0.547), although disease control rate and progression-free survival did not differ significantly.
A Survival curve: All. B Survival curve: Based on WHO 2017 classification.
In our study, Chi-square analysis indicated that a high KI-67 group (cut-off point: KI-67 ≥ 55%) is more prevalent in NEC, with a significance level of P < 0.001 (Table 5). In the subgroup analysis of high-grade neuroendocrine neoplasms, no significant differences were noted in tumor size, lymph node involvement, distant metastasis, or response rate. However, the PFS was significantly longer in patients receiving chemotherapy as first-line systemic therapy, with a PFS of 4.87 months compared to 3.40 months (p = 0.044). The overall survival was also noteworthy, with values of 33.83 months for the low KI-67 group compared to 10.83 months for the high KI-67 group (p = 0.001).
Discussion
Our analysis demonstrated clear prognostic stratification across these groups, with 5-year survival rates ranging from 83.1% in G1 tumors to just 9.4% in NEC cases. The validity of the WHO 2017 classification was particularly evident in distinguishing between G3 and NEC categories, which showed distinct clinical behaviors despite both being high-grade tumors. Our analysis revealed biological and clinical differences between these high-grade categories: NET G3 and NEC demonstrated distinct Ki-67 distribution patterns and significantly different lymph node involvement. Notably, treatment responses and options also differed markedly between these groups, with NET G3 patients having access to more diverse therapeutic options including targeted therapy and somatostatin analogs, while NEC patients primarily received chemotherapy regimens. These characterization of high-grade PanNETs provides evidence supporting the clinical utility of the WHO 2017 classification and suggests that these categories may benefit from distinct therapeutic approaches.
The univariate analysis & multivariate analysis based on WHO 2017 and 2010 criteria of PNET
| Univariate Analysis HR(CI), P value | Multivariate Analysis HR(CI), P value | |
|---|---|---|
| Age, year | ||
| < 60 | ||
| > 60 | 1.666(1.044-2.658), 0.032 | 1.70(1.006-2.890), 0.013 |
| Sex | ||
| Female | ||
| Male | 1.161(0.728-1.851), 0.530 | |
| Carcinoid symptoms | ||
| No | ||
| Yes | 1.301(0.519-3.259), 0.588 | |
| Hypo/hyperglycemia | ||
| No | ||
| Yes | 0.814(0.259-2.593), 0.720 | |
| No symptom | ||
| No | ||
| Yes | 0.499(0.268-0.928), 0.018 | 0.759(0.404-1.427), 0.392 |
| CgA > 10 X upper limit | ||
| No | ||
| Yes | 1.944(1.039-3.637), 0.053 | |
| SSTR positive | ||
| No | ||
| Yes | 0.805(0.399-1.623), 0.534 | |
| Size > 2cm | ||
| No | ||
| Yes | 3.233(1.800-5.806), <0.001 | 1.893(1.024-3.500), 0.042 |
| Lymph node involvement | ||
| No | ||
| Yes | 4.324(2.693-6.491), <0.001 | 1.801 (1.021-3.177), 0.042 |
| Distant metastasis | ||
| No | ||
| Yes | 5.316(3.140-8.999), <0.001 | 3.042(1.618-5.718), 0.001 |
| Ki-67 >55 | ||
| No | ||
| Yes | 2.060(1.250-3.396), 0.007 | |
| WHO2017 | ||
| NET G1/G2/G3 | ||
| NEC | 5.298(2.892-9.703), <0.001 | 2.382(1.178-4.817), 0.016 |
NET: Neuroendocrine tumor; NEC: Neuroendocrine carcinoma.
Subtypes of PanNETs show distinct biological behaviors, ranging from indolent tumors with slow growth and minimal symptoms to aggressive high-grade variants like NEC [14]. Our study reinforces these differences, highlighting the importance of effective classification in clinical practice. Prior research has shown that the updated WHO 2017 classification is more accurate than the WHO 2010 system [15]. Despite a relatively small sample size, our findings support the efficacy of WHO 2017 classification in categorizing PanNETs.
Previous studies have established different molecular mechanisms and genetic backgrounds between G3 and NEC [16]. Based on prior data, the most prevalent mutations in pancreatic neuroendocrine tumors include those in MEN1 (44%), DAXX/ATRX (43%) [17, 18], and genes related to the mTOR pathway. In contrast, the most commonly mutated genes in NEC are TP53 and RB, with NECs exhibiting significantly higher mutation burden [19, 20]. Notably, G3 patients demonstrated a significantly different Ki-67 distribution pattern compared to NEC (p<0.001), with the majority of G3 cases (18/25, 72%) showing Ki-67 indices below 55%, while most NEC cases (14/16, 87.5%) exhibited Ki-67 indices above 55%. These findings underscore the higher proliferative activity in NECs, consistent with their aggressive clinical behavior. Regarding prognosis, our findings suggest that the G3 group may display a more favorable prognosis than previously anticipated, particularly when compared to the NEC group. A deeper understanding of these clinical and pathological factors is essential for optimizing treatment strategies and enhancing patient management in this population.
The comparison between NET G3 and NEC
| NET G3 (N=25) | NEC (N=16) | P value | |
|---|---|---|---|
| Characteristics | |||
| Tumor size | 5.51 | 5.07 | 0.645 |
| Lymph node involvement | 48% | 81% | 0.026 |
| Distant metastasis | 84% | 88% | 0.760 |
| Mitotic index | 12.20 | 15.60 | 0.428 |
| KI-67 | 43.83 | 69.06 | <0.000 |
| 1L systemic treatment | |||
| Chemotherapy | 68% | 88% | |
| Etoposide + Cisplatin | 32% | 69% | |
| Etoposide + Carboplatin | 4% | 6% | |
| Dacarbazine only | 24% | 0% | |
| Dacarbazine + 5FU | 4% | 0% | |
| Gemcitabine + TS-1 | 0% | 6% | |
| Gemcitabine + Nab-Paclitaxel | 0% | 6% | |
| Targeted therapy | 8% | 0% | |
| Sunitinib | 8% | 0% | |
| Somatostatin analog combined | 12% | 0% | |
| No systemic treatment | 20% | 13% | |
| After 1L chemotherapy treatment (image follow up in 3 months) | |||
| Objective response rate | 19% | 29% | 0.547 |
| Disease control rate | 44% | 43% | 0.962 |
| Progression-free survival(month) | 5.36 | 3.40 | 0.743 |
Systemic treatment for PanNETs includes various modalities such as cytotoxic chemotherapy, somatostatin analogs, and targeted therapies [21]. However, it is important to note that there is currently no established standard chemotherapy specifically for this disease, and the response to chemotherapy can vary depending on the tumor grade. The first-line therapy typically consists of a regimen comprising platinum-based agents in combination with etoposide in NEC. Clinical studies have reported response rates for this treatment combination ranging from 31% to 67 [22]. Well-differentiated G3 tumors typically exhibit slow proliferation and tend to be resistant to most chemotherapeutic agents [23]. They have a limited response to platinum-based chemotherapy, so other treatments may be considered, such as temozolomide-based chemotherapy or peptide receptor radiotherapy. In Table 4, we attempted to compare the impact of chemotherapy between G3 and NEC groups. No statistically significant differences were noted in response rate and PFS between the NEC and G3 groups receiving first line chemotherapy. Also, previous data revealed, as KI-67 elevation, response rate increased and survival outcome decreased [24]. We conducted a further investigation into the role of KI-67 in high-grade PanNETs. The low KI-67 group exhibited better OS and PFS. Despite achieving a higher objective response rate (ORR) to initial chemotherapy, the high Ki-67 group is characterized by more aggressive tumor behavior, including a higher prevalence of NEC and distant metastasis. These findings underscore Ki-67 as a critical prognostic factor, with high expression indicating poor long-term despite initial treatment efficacy.
Characteristics of high-grade PanNENs stratified by Ki-67 (<55% vs ≥55%)
| High KI group (N = 15) | Low KI group (N = 16) | P | |
|---|---|---|---|
| High-grade Neuroendocrine neoplasm | N (%) | N (%) | < 0.001 |
| NET G3 | 3 (20%) | 14 (88%) | |
| NEC | 12 (80%) | 2 (13%) | |
| Characteristics | |||
| Tumor size | 5.79 | 0.655 | 0.655 |
| Lymph node involvement | 73% | 56% | 0.335 |
| Distant metastasis | 80% | 88% | 0.588 |
| 1L systemic treatment | |||
| Etoposide + Cisplatin | 80% | 43.7% | |
| Etoposide + Carboplatin | 6.7% | 6.3% | |
| Dacarbazine only | 0% | 43.7% | |
| Dacarbazine + 5FU | 0% | 6.3% | |
| Gemcitabine + TS-1 | 6.7% | 0% | |
| Gemcitabine + Nab-Paclitaxel | 6.7% | 0% | |
| After 1L chemotherapy treatment (image follow up in 3 month) | |||
| Objective response rate | 27% | 19% | 0.614 |
| Disease control rate | 33% | 50% | 0.363 |
| Progression-free survival(month) | 3.40 | 4.87 | 0.044* |
| Overall survival(month) | 10.83 | 33.83 | 0.001* |
NET: Neuroendocrine tumor; NEC: Neuroendocrine carcinoma.
This study has several limitations that should be considered. First, its retrospective design involved analyzing data and recruiting patients over an extended period. Second, the use of Kaplan-Meier methods to estimate cumulative overall survival may be affected by cases with limited follow-up. Additionally, the analysis was confined to a single medical center in Taiwan, potentially limiting statistical power for assessing various factors and survival outcomes. Therefore, a prospectively designed multicenter study with longer follow-up is essential to validate these findings.
Conclusion
Our retrospective analysis highlights the complex interplay between prognostic factors and overall survival in patients with PanNETs, revealing key indicators that significantly influence outcomes and enabling more tailored clinical management. Additionally, the updated WHO 2017 classification has proven pivotal in distinguishing G3 PanNETs from NEC, enhancing patient stratification based on tumor biology and guiding the selection of appropriate treatments. Integrating this refined classification and these key indicators into clinical practice holds substantial potential for improving prognostic assessments and optimizing treatment strategies for high-grade PanNETs.
Acknowledgements
The authors would like to express their gratitude to all colleagues and research staff who contributed to data collection and analysis. We also appreciate the assistance of the pathology department for reviewing the reports and providing critical insights.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethical approval
This study was approved by the Ethics Committee of the Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan (IRB 202500811B0 and conducted in accordance with the Declaration of Helsinki. Informed consent was waived due to the retrospective design of the study.
Author contributions
Wei-Pang Ho: Conceptualization, Investigation, Data Curation, Software, Methodology, Formal Analysis, Writing - Original Draft.
Wen-Kuan Huang: Conceptualization, Supervision, Visualization, Project administration, Resources, Writing - Review & Editing.
Jen-Shi Chen: Conceptualization, Resources, Funding acquisition, Supervision
Ren-Chin Wu: Validation, Methodology.
Wen-Chi Chou, Ming-Mo Hou, Wen-Chi Shen, Pei-Wei Huang, Chia-Hsun Hsieh, Chiao-En Wu, Chih-Chung Hsu, Wen-Cheng Chang, Yung-Chia Kuo, Hung-Chih Hsu, Ching-Fu Chang, Po-Jung Su: Data Curation.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15:409-27
2. Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589-97
3. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y. et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-42
4. Ramachandran A, Madhusudhan KS. Advances in the imaging of gastroenteropancreatic neuroendocrine neoplasms. World J Gastroenterol. 2022;28:3008-26
5. Chang JS, Chen LT, Shan YS, Chu PY, Tsai CR, Tsai HJ. An updated analysis of the epidemiologic trends of neuroendocrine tumors in Taiwan. Sci Rep. 2021;11:7881
6. Fuentes ME, Lu X, Flores NM, Hausmann S, Mazur PK. Combined deletion of MEN1, ATRX and PTEN triggers development of high-grade pancreatic neuroendocrine tumors in mice. Sci Rep. 2024;14:8510
7. Magi L, Marasco M, Rinzivillo M, Faggiano A, Panzuto F. Management of Functional Pancreatic Neuroendocrine Neoplasms. Curr Treat Options Oncol. 2023;24:725-41
8. Perry RR, Feliberti EC, Hughes MS. Management of Pancreatic Neuroendocrine Tumors: Surgical Strategies and Controversies. Endocr Pract. 2024;30:908-16
9. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P. et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-8
10. Inzani F, Petrone G, Rindi G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol Metab Clin North Am. 2018;47:463-70
11. Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang SZ, Zou YP. et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol. 2020;26:2305-22
12. R. L. WHO classification of Tumours of endocrine Organs. fourth ed. IARC. 2017
13. Fujimori N, Miki M, Lee L, Matsumoto K, Takamatsu Y, Takaoka T. et al. Natural history and clinical outcomes of pancreatic neuroendocrine neoplasms based on the WHO 2017 classification; a single-center experience of 30 years. Pancreatology. 2020;20:709-15
14. Tanaka M, Heckler M, Mihaljevic AL, Probst P, Klaiber U, Heger U. et al. Systematic Review and Metaanalysis of Lymph Node Metastases of Resected Pancreatic Neuroendocrine Tumors. Ann Surg Oncol. 2021;28:1614-24
15. Yang M, Zeng L, Ke N-w, Tan C-l, Tian B-l, Liu X-b. et al. World Health Organization grading classification for pancreatic neuroendocrine neoplasms: a comprehensive analysis from a large Chinese institution. BMC Cancer. 2020;20:906
16. Shi M, Fan Z, Xu J, Yang J, Li Y, Gao C. et al. Gastroenteropancreatic neuroendocrine neoplasms G3: Novel insights and unmet needs. Biochim Biophys Acta Rev Cancer. 2021;1876:188637
17. Maxwell JE, Sherman SK, Howe JR. Translational Diagnostics and Therapeutics in Pancreatic Neuroendocrine Tumors. Clin Cancer Res. 2016;22:5022-9
18. Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A. et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199-203
19. Hijioka S, Hosoda W, Matsuo K, Ueno M, Furukawa M, Yoshitomi H. et al. Rb Loss and KRAS Mutation Are Predictors of the Response to Platinum-Based Chemotherapy in Pancreatic Neuroendocrine Neoplasm with Grade 3: A Japanese Multicenter Pancreatic NEN-G3 Study. Clin Cancer Res. 2017;23:4625-32
20. Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A. et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod Pathol. 2017;30:587-98
21. Akirov A, Larouche V, Alshehri S, Asa SL, Ezzat S. Treatment Options for Pancreatic Neuroendocrine Tumors. Cancers (Basel). 2019 11
22. Sorbye H, Baudin E, Borbath I, Caplin M, Chen J, Cwikla JB. et al. Unmet Needs in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms (WHO G3). Neuroendocrinology. 2019;108:54-62
23. Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P. et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-60
24. Childs A, Kirkwood A, Edeline J, Luong TV, Watkins J, Lamarca A. et al. Ki-67 index and response to chemotherapy in patients with neuroendocrine tumours. Endocr Relat Cancer. 2016;23:563-70
Author contact
![]() Corresponding authors: Wen-Kuan Huang, Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan, Tel: +886-03-3281200; E-mail address: medfoxtaiwancom. Jen-Shi Chen, Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan, Tel: +886-03-3281200; E-mail address: js1101org.tw.
Corresponding authors: Wen-Kuan Huang, Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan, Tel: +886-03-3281200; E-mail address: medfoxtaiwancom. Jen-Shi Chen, Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan, Tel: +886-03-3281200; E-mail address: js1101org.tw.

 Global reach, higher impact
Global reach, higher impact