Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(14):4155-4171. doi:10.7150/jca.118694 This issue Cite
Review
Regulating arachidonic acid metabolism: a novel strategy to prevent colorectal inflammatory cancer transformation
1. Institute of Digestive Diseases, Longhua Hospital, China-Canada Center of Research for Digestive Diseases (ccCRDD), Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, China.
2. State Key Laboratory of Integration and Innovation of Classic Formula and Modern Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, China.
3. Shanghai Frontier Research Center of Disease and Syndrome Biology of Inflammatory Cancer Transformation, Shanghai, 200032, China.
*Equal contribution.
Received 2025-6-2; Accepted 2025-9-13; Published 2025-10-1
Abstract
Colorectal cancer (CRC) ranks among the leading causes of cancer-related morbidity and mortality worldwide, with colitis-associated colorectal cancer (CAC) driven by inflammatory cancer transformation. Arachidonic acid (AA), a key ω-6 polyunsaturated fatty acid, and its metabolites, including prostaglandins (PGs) and leukotrienes (LTs), play pivotal roles in this process by modulating inflammation, immune responses, and the intestinal microenvironment. Notably, a multi-enzyme co-expression nanoplatform integrating lipoxygenase (LOX) and phospholipase A2 (PLA2) has been developed, synergistically inducing immunogenic ferroptosis and upregulating AA expression to enhance CD8+ T cell-mediated anti-tumor immunity. Additionally, dual COX-2/soluble epoxide hydrolase (sEH) inhibitors, such as PTUPB, demonstrate enhanced anti-tumor activity and reduced toxicity when combined with cisplatin, offering a promising approach to mitigate gastrointestinal side effects of nonsteroidal anti-inflammatory drugs (NSAIDs). Furthermore, natural products like ginsenoside Rk3 and berberine have been identified to regulate AA metabolism and gut microbiota, alleviating CAC by modulating lipid peroxidation and inflammatory pathways. This review synthesizes these innovative findings, highlighting the role of AA metabolism in maintaining intestinal homeostasis, promoting inflammatory cancer transformation, and serving as a therapeutic target to inhibit CAC progression, thus providing new insights into its prevention and treatment.
Keywords: colorectal cancer, arachidonic acid, gut microbiota, inflammatory cancer transformation, cancer therapy
1. Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors of the digestive system [1]. According to the latest statistics from the National Cancer Center (NCC), CRC has the second highest incidence rate and the fourth highest mortality rate among all malignant tumors, and the incidence rate continues to increase [2]. Colitis-associated colorectal cancer (CAC) refers to CRC arising from chronic intestinal inflammation, primarily in ulcerative colitis (UC) and Crohn's disease (CD) patients. Clinically, CAC is characterized by earlier disease onset, multifocal lesions, and a higher likelihood of aggressive tumor behavior compared to sporadic CRC. The inevitable consequence of CAC is the progression from chronic inflammation to dysplasia and malignancy, driven by persistent epithelial injury, immune dysregulation, and microbial dysbiosis, with potential outcomes including increased metastasis and reduced survival [3].
Polyunsaturated fatty acids (PUFAs) are essential fatty acids that may play a potential role in regulating inflammation, particularly in the pro-cancer inflammatory milieu of the colon. The two main types of PUFAs are omega-3 (ω-3) fatty acids and omega-6 (ω-6) fatty acids. A systematic review found that a high dietary intake of ω-3 fatty acids reduced the risk of CRC, and that the risk was higher with a high dietary ω-6/ω-3 ratio [4]. Furthermore, statistical analyses found that elevated hereditary PUFAs were strongly associated with CRC and emphasized the high expression of ω-6 as a potential mediator [5].
Arachidonic acid (AA) is one of the ω-6 fatty acids and one of the most abundant and widely distributed PUFAs in mammals. AA can be converted to various metabolites in the body, most of which have potent physiological effects and a wide range of actions and are important for cellular regulation. AA and its metabolites regulate inflammatory responses critical to CAC onset and progression [6, 7]. Given its wide range and importance, the functional study of AA metabolic pathways and metabolites has been highly valued by the life science and medical communities, and the present review will systematically elucidate the mechanism of AA and its metabolism in the inflammatory cancer transformation of CAC.
2. Metabolic pathways of arachidonic acid
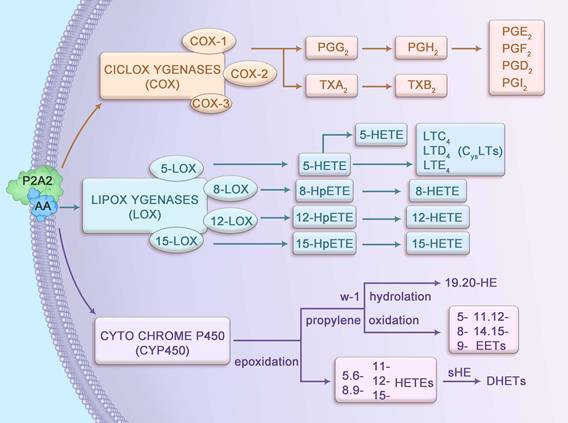
AA, a 20-carbon ω-6 polyunsaturated fatty acid (20:4n-6), possesses four cis-double bonds at positions 5, 8, 11, and 14, conferring high flexibility and reactivity that facilitate its role as a substrate for enzymatic metabolism. Stored primarily as an esterified component of membrane phospholipids, AA is released by cytosolic phospholipase A2 (cPLA2), which is activated by calcium-dependent translocation to the membrane in response to inflammatory stimuli. The liberated AA undergoes metabolism via three primary pathways: cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450), each catalyzed by enzymes with distinct kinetic properties (Figure 1). For instance, COX-2 exhibits a higher affinity for AA compared to COX-1, enabling rapid production of prostaglandin H2 (PGH2) under inflammatory conditions. Similarly, 5-LOX, activated by 5-lipoxygenase-activating protein (FLAP), converts AA into 5-hydroperoxyeicosatetraenoic acid (5-HPETE) with high specificity, subsequently forming leukotriene B4 (LTB4). These metabolites interact with G-protein-coupled receptors (such as EP1-4 for PGE2, BLT1 for LTB4), triggering downstream signaling cascades such as cAMP/PKA and NF-κB, which are critical in CAC pathogenesis [8, 9].
The COX pathway comprises three isoforms: COX-1, COX-2, and COX-3, each with distinct expression patterns and functions. COX-1, constitutively expressed across most tissues, supports physiological processes such as promoting intestinal epithelial cell (IEC) proliferation and enhancing digestive juice secretion [10]. PGs produced by COX-1 maintain gastrointestinal and tissue homeostasis [11] and synergize with enzymes to regulate biological processes, including apoptosis and cell cycle progression [12]. In contrast, COX-2 is inducible, primarily expressed in response to inflammatory stimuli, and is rarely present in resting cells [13]. COX-3 is predominantly found in the cerebral cortex and heart, with its role less clearly defined. In the presence of COX enzymes, AA is converted into PGG2 and PGH2, and occasionally thromboxane A2 (TXA2), which has a short half-life and is rapidly converted to stable TXB2. PGG2 and PGH2 are further transformed by isomerases into prostaglandins such as PGD2, PGF2, PGE2, and PGI2, which mediate inflammatory and homeostatic responses.
The LOX pathway involves four key enzymes—5-LOX, 8-LOX, 12-LOX, and 15-LOX—that metabolize AA into bioactive lipid mediators. The 5-LOX enzyme, activated by FLAP, is the primary producer of leukotrienes (LTs), which regulate both normal homeostasis and inflammatory responses [14]. LTs are categorized into LTB4, a chemokine, and cysteinyl leukotrienes (LTC4, LTD4 and LTE4). LTB4 drives neutrophil recruitment, vascular leakage, and epithelial barrier function, while LTC4 and LTD4 modulate IEC proliferation and survival through effects on vascular permeability. LTE4 serves as a clinical biomarker for asthma triggers [15, 16]. Additionally, 8-LOX, 12-LOX, and 15-LOX convert AA into 8-HPETE, 12-HPETE, and 15-HPETE, respectively, which are subsequently dehydrated to form 8-HETE, 12-HETE, and 15-HETE, contributing to inflammatory signaling.
In the CYP450 pathway, AA undergoes epoxidation to produce 5,6-, 8,9-, 11,12-, and 14,15-epoxyeicosatrienoic acids (EETs), which are hydrolyzed by soluble epoxide hydrolase (sEH) into biologically inactive dihydroxyeicosatrienoic acids (DHETs). Additionally, AA is metabolized via propylene oxidation to yield 5-, 8-, 9-, 11-, 12-, and 15-hydroxyeicosatetraenoic acids (HETEs) and via ω-1 hydroxylation to produce 19- and 20-HETEs. These metabolites regulate vascular tone, inflammation, and cellular signaling, with emerging roles in the inflammatory microenvironment of CAC.
3. Arachidonic acid metabolism is involved in intestinal inflammation and tumorigenesis
The etiology of inflammatory bowel disease (IBD), encompassing UC and CD, remains multifactorial, involving genetic, environmental, and microbial factors. Central to IBD pathogenesis is chronic intestinal inflammation, characterized by epithelial damage and leukocyte infiltration, which is closely linked to the activation of AA metabolic pathways. Elevated AA levels have been observed in the inflamed mucosa of UC patients, with concentrations correlating strongly with the severity of inflammation [17]. Preclinical studies demonstrate that oral AA administration exacerbates inflammation in IBD mouse models, upregulating COX-2 and LTB4 expression, while exerting no significant effect in healthy controls [18]. AA-derived metabolites, such as eicosanoids, activate transient receptor potential vanilloid 4, a calcium channel, leading to increased intracellular calcium and chemokine release, thereby amplifying IBD-associated inflammation [19]. Notably, PGE2, a downstream AA metabolite, promotes Th17 cell-mediated inflammatory responses, further driving disease progression [20]. Clinical studies in adolescent IBD patients reveal significantly elevated levels of TXB2, LTB4, and 9S-HODE during active disease phases compared to remission, with 15S-HETE levels being markedly higher in CD than in UC [21, 22]. Additionally, lipoxygenases ALOX5 and ALOX15 exert proinflammatory effects, and their genetic inactivation confers protection in dextran sulfate sodium (DSS)-induced colitis models [23]. These findings underscore the pivotal role of AA metabolism in sustaining the inflammatory milieu of IBD, a key precursor to CAC.
The role of AA in CRC, particularly CAC, remains controversial, with evidence supporting both anti-tumorigenic and pro-tumorigenic activities. Some studies suggest that AA exerts anti-tumor effects by inhibiting cancer cell proliferation and promoting apoptosis. For instance, AA has been shown to activate neutral sphingomyelinase, increase β2-microglobulin exposure on cell surface membranes for antibody binding, and hydrolyze sphingomyelin to ceramide, a potent inhibitor of proliferation and inducer of apoptosis across various tumor cell lines [24, 25]. Furthermore, AA suppresses CRC cell proliferation by disrupting DNA replication and endogenous fatty acid synthesis, primarily through interference with the G1/S cell cycle transition and DNA repair processes, independent of reactive oxygen species production or caspase-3/7 activation [26, 27]. In contrast, other studies report that AA induces oxidative damage to DNA and proteins, activates caspase-3/7, and promotes apoptosis, thereby inhibiting CRC cell proliferation [28].
Metabolic pathways of arachidonic acid.
Conversely, substantial evidence supports a pro-tumorigenic role for AA and its metabolites. A Mendelian randomization study by Larsson et al. demonstrated a positive correlation between plasma phospholipid AA concentrations and increased risks of colorectal, lung, and esophageal cancers [29]. High dietary AA intake leads to the accumulation of prostaglandins, particularly PGE2, which fosters a pro-inflammatory microenvironment conducive to cancer development [30]. PGE2 enhances CRC cell proliferation, migration, and invasion in an autocrine manner and inhibits inflammasome complex formation (ASC/Caspase-1/NLRP3) in THP-1 cells, promoting a shift from pro-inflammatory M1 to pro-tumorigenic M2 macrophages in the presence of AA [31]. TXA2, another AA metabolite, drives cell growth, migration, and angiogenesis, with elevated levels associated with poor prognosis, reduced survival, and metastatic disease in multiple cancers [32]. Overexpression of 5-LOX in the lipoxygenase pathway correlates strongly with risk factors for malignant transformation of adenomatous polyps [33]. Additionally, 12S-HETE, secreted by CRC cells, enhances cancer-associated fibroblast growth and angiogenesis, further promoting CRC invasiveness [34].
Chronic inflammation in IBD leads to repeated mucosal injury and repair, increasing the risk of dysplastic transformation and CAC development. Given the dual roles of AA in modulating inflammation and tumorigenesis, elucidating the intrinsic mechanisms of AA metabolism in the inflammatory-to-cancerous transition in CAC is critical. This review synthesizes global and domestic research to clarify the complex interplay of AA and its metabolites in driving CAC, providing a foundation for targeted therapeutic strategies to mitigate disease progression and improve clinical outcomes.
4. Mechanisms of Arachidonic acid involvement in inflammatory cancer transformation
CAC, primarily arising from UC and CD, represents a distinct paradigm of inflammatory cancer transformation driven by chronic intestinal inflammation. AA metabolism underpins this process through tightly regulated molecular mechanisms. cPLA2, activated via phosphorylation at Ser505 by inflammatory cytokines (such as IL-1β, TNF-α), selectively hydrolyzes membrane phospholipids to release AA, a process amplified in UC and CD mucosa [35]. The liberated AA is metabolized by COX-2, induced by NF-κB, to produce PGE2, which binds EP2/EP4 receptors to activate cAMP/PKA and PI3K/AKT pathways, promoting epithelial dysplasia [36]. Similarly, 5-LOX, stabilized by FLAP, generates LTB4, which engages BLT1 receptors to enhance NF-κB and STAT3 signaling, driving immune suppression and tumor progression [37]. Novel interventions, such as CRISPR/Cas9-mediated silencing of PLA2G4A, reduce AA availability, attenuating these oncogenic cascades in CAC models [38]. These molecular mechanisms link AA metabolism to the subsequent immune, epithelial, microbial, genetic, and epigenetic alterations driving CAC, as detailed below (Figure 2).
4.1 Tumor immune microenvironment
The tumor immune microenvironment (TIME) comprises immune cells, fibroblasts, blood vessels, signaling molecules, and the extracellular matrix, all of which shape tumor initiation, progression, and metastasis in CAC. AA metabolism influences the TIME by generating pro-inflammatory and immunosuppressive metabolites, such as PGE2 and LTB4, which foster a tumor-permissive environment [39]. Cytosolic PLA2 promotes AA-derived PGE2 production, driving lymphocyte infiltration and M1-to-M2 macrophage polarization, which protects the colon from excessive inflammation but promotes tumor tolerance [40]. Endogenous lipid mediators, formed via COX-2 and prostaglandin D synthase, reduce neutrophil and M2 macrophage polarization, facilitating IBD remission [41]. In trinitrobenzene sulfonic acid (TNBS)-induced colitis mouse models supplemented with AA, T cells increase interferon-gamma (IFN-γ) production in a COX-2-dependent manner, enhancing lymph node cell activation [42]. AA also mediates SLC3A2-dependent reprogramming of macrophage phenotypes, promoting M2 differentiation in both in vitro and in vivo settings [43]. Furthermore, the PLA2G4A/AA axis drives CD39+γδ T-regulatory cells (Tregs) polarization, exacerbating tumor progression and metastasis [44].
To counteract these effects, innovative approaches like a multi-enzyme co-expression nanoplatform integrating LOX and PLA2 have been developed. This platform ind uces immunogenic ferroptosis—a form of programmed cell death—while upregulating AA expression to enhance ACSL4-mediated tumor cell death, synergizing with CD8+ T cell-derived IFN-γ to boost anti-tumor immunity [45]. This strategy highlights the potential of targeting AA metabolism to reverse immunosuppression in CAC, offering a bridge to therapeutic interventions. In the ApcMin/+ model of familial adenomatous polyposis, the amount of CD25+ Treg increased with elevated COX-2 activity [46]. microsomal PGE2 synthase 1 (mPGES-1), a terminal synthase that induces the formation of PGE2, whose absence in tumors reduces collagen deposition and T-cell exhaustion and regulates the TIME [47].
Mechanisms of AA involvement in inflammatory cancer transformation. The blue section in the upper left corner is the intestinal microbiota, the purple section in the upper right corner is the tumor immune microenvironment, the yellow section in the lower left corner is the intestinal barrier, and the pink section in the lower right corner is the Genetic, Epigenetic, and drug. IBD: Inflammatory bowel disease; PGE2: prostaglandins E2; AA: Arachidonic acid; COX-2: cyclooxygenase-2; NF-ҡB: Nuclear Factor Kappa-light-chain-enhancer of Activated B cells; LTB4: Leukotriene B4; IL10: Interleukin-10; IL6: Interleukin-6; TNF-a: Tumor Necrosis Factor-alpha; PGD2: prostaglandins D2; DCs: dendritic cells; ILC2: type 2 innate lymphoid cell; LTC4: Leukotriene C4; LTD4: Leukotriene D4; IL8: Interleukin-8; ER: Endoplasmic reticulum; TJs: Tight Junctions.
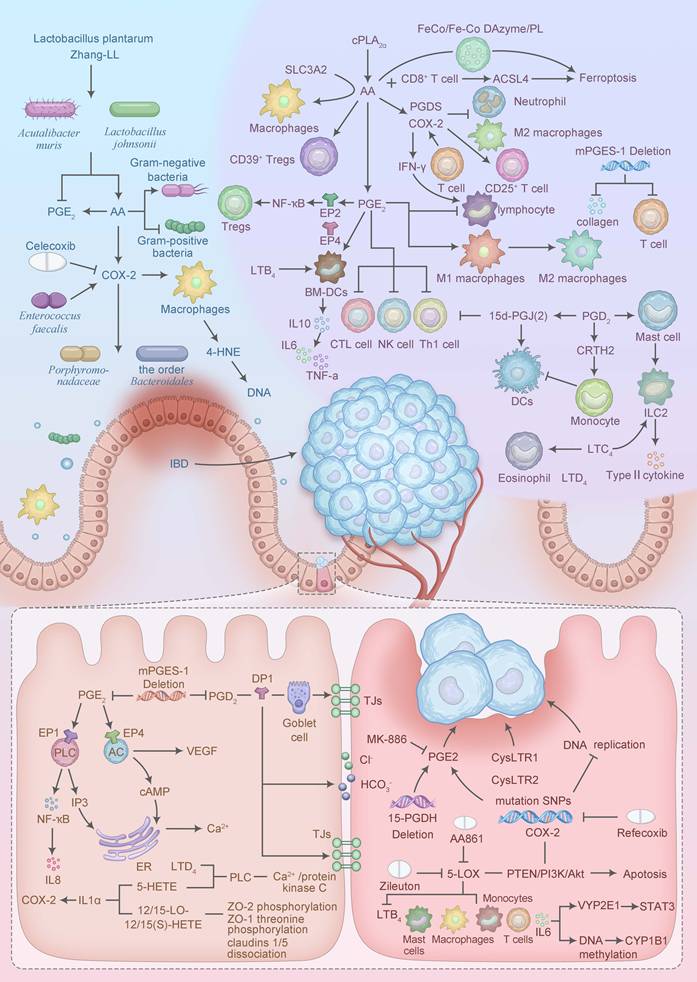
In general, PGE2 promotes acute localized inflammatory responses and phagocyte-mediated immunity in response to the presence of pathogens. PGE2-EP2/EP4 signaling has been reported to induce NF-ҡB gene expression to promote inflammation and cause immunosuppression through recruitment and activation of Tregs [48]. Selenoproteins in macrophages alleviate inflammation and protect DSS-induced IBD mice by enhancing 15-PGDH-dependent oxidation of PGE2 [49]. And in the presence of PGE2, it promotes IL-10 production by bone marrow-derived DCs (BM-DCs), which in turn down-regulates self-produced IL-6, TNF-a, and promotes immune homeostasis [50]. However, high expression of PGE2 in tumor tissues suppresses cytotoxic immune responses in CTL, Th1, and NK cells, leading to immunosuppression [51]. The PGE2 biosynthesis pathway correlates with CD68+ macrophage infiltration and CRC tumor progression [52]. CAR-T therapy is a novel precision-targeted therapy for the treatment of tumors. PGE2 is negatively correlated with memory T cells, and dual blockade of EP2 and EP4 receptors effectively reverses PGE2-mediated inhibition of CAR T cells when it is applied to tumor tissues [53].
PGD2 promotes type 2 immunity by activating the group 2 innate lymphoid cell (ILC2) to produce type 2 cytokines by affecting the supernatant of mast cells [54]. Meanwhile, PGD2 inhibits the migration of monocyte-derived DCs through activation of CRTH2 and, together with the metabolite 15-deoxy-Delta(12,14)-PGJ(2) inhibits TH1 cell chemotaxis and reduces IL-12 secreted by TH1 cells [55].
LTs, as an important inflammatory mediator, play a key role in immune responses. The addition of exogenous LTB4 promoted the proliferation of BM-DCs in in vitro experiments [56]. LTD4 not only enhances the accumulation and proliferation of ILC2 and promotes the release of IL-5 and IL-13, but also induces increases of eosinophil [57]. In addition, LTC4 also induces an increase in ILC2 inducing inflammation [58]. 5-LOX affects tumor immunity during CRC development and has a pro-tumorigenic role in the immune microenvironment [59]. The immunosuppressive TIME shaped by AA metabolites not only promotes tumor growth but also compromises intestinal epithelial integrity, setting the stage for barrier dysfunction.
4.2 Intestinal barrier
The maintenance of intestinal epithelial barrier (IEB) function is critical for intestinal homeostasis, and AA metabolites regulate intestinal electrolytes, epithelial cell proliferation, secretion, and tight junction (TJ) integrity. The COX pathway inhibits Cl-/HCO3- exchange in chromaffin cells, decreasing affinity for Cl- and causing NaCl malabsorption, leading to the development of diarrhea in IBD [60]. The secretion of HCO3- by the intestinal mucosa is also crucial for preventing acidic digestive damage. Studies have found that PGE2 can stimulate the secretion of Cl- and HCO3- in the intestines, which has a protective effect on IEB [61]. PGD2 is able to induce Cl- secretion from the human colonic mucosa by DP1 receptor-mediated means, causing an elevation of cAMP in epithelial cells [62].
In the intestinal mucosal epithelium of IBD patients, increased phospholipid content of AA contributes to the disruption of the intestinal barrier [63]. In animal experiments, the expression of AA and its metabolites (19H-PGF1α and 20H-PGF2α) progressively decreases with the decrease of inflammation, suggesting that mucosal healing is regulated by endogenous lipids [64]. COX-1 mainly produces endogenous PGs engaged in mucosal protection, while COX-2 mainly produces endogenous PGs engaged in ulcer and intestinal lesion healing. It has been shown that COX-2 expression is significantly elevated in the early stages of CRC development, which further affects epithelial cells by influencing the stromal microenvironment of the tumor [65].
PGs play an important role in maintaining intestinal mucosal integrity, especially PGE2. PGE2 is involved in stimulating mucus secretion and down-regulating the immune response through EP4 receptors and is protective against ischemic enteritis and DSS-induced colitis. And activation of EP4 receptors promotes healing of intestinal lesions and is associated with up-regulation of VEGF expression and stimulation of angiogenesis [66]. In addition, EP4 receptors are involved in colorectal homeostasis and cancer development [67]. However, it has also been suggested that PGE2 contributes to the redistribution of intracellular calcium concentration and TJ proteins through multiple signaling pathways, including the PLC-IP 3-Ca2+ and cAMP-PKA pathways, that induces disruption of IEB function [68]. Ptgs2-expressing fibroblasts around intestinal crypts exert paracrine control of tumor-inducing stem cells through the PGE2-Ptger4-Yap signaling axis, which helps drive tumorigenesis [69].
Prostaglandin homeostasis in the intestine is critical for maintaining intestinal homeostasis and influencing tumorigenesis. It was found that in DSS-induced mPGES-1-/- mice, this leads to a decrease in PGE2 and PGD2, resulting in more extensive acute injury affecting recovery. And in DSS-induced ApcMin/+: mPGES-1-/- mice, the number of intestinal polyps was reduced [70]. Pharmacological studies have found that PGD2, through the DP1 receptor, is able to stimulate mucus secretion from goblet cells to reduce intestinal permeability and achieve protection of the IEB [71]. Moreover, PGD2 promotes the regression of inflammation in the gastrointestinal mucosa [72].
PGE2, LTB4, and 5-, 12-, and 15-HETE can protect IECs by inducing proliferation and DNA synthesis in IECs [73]. BLT2, a receptor for LTB4, is expressed only in IECs and epidermal keratinocytes. When BLT2 receptor is overexpressed in IECs it enhances epithelial drug resistance, suggesting that the LTB4-BLT2 axis has a barrier function [74]. DSS-induced colitis in mice is exacerbated in the absence of BLT2 receptor, which may be correlated with the reduced intestinal barrier function [75]. LTD4 and 5-HETE alter the proliferation and DNA synthesis of IECs by activating the phospholipase C/Ca2+/protein kinase C pathway activation alters paracellular permeability and is involved in IEB disruption, a process that is not dependent on protein kinase A activation by cAMP [76]. In addition, LTD4 is able to induce proliferation of Caco-2 cells by binding to the cysteinyl leukotriene receptor (CysLTR), which is dependent on PGE2 [77].
Tight junctions (TJs) are multiprotein complexes composed of transmembrane proteins with cytoskeletal enclosing rings of actin and myosin, which are important components of the intestinal barrier [78]. It was found that 15-HETE regulates IEB permeability and homeostasis through inhibition of adenosine monophosphate-activated protein kinase and increased zonula occludens-1 (ZO-1) expression [79]. 12/15-LO-12/15(S)-HETE axis not only stimulates the phosphorylation of ZO-2, but also stimulates the phosphorylation of ZO-1 threonine and the dissociation of claudins 1/5, which mediates the disruption of endothelial TJs and disrupts the barrier function [80]. In addition, the COX pathway interacts with the LOX pathway; 5(S)-, 12(R)- and 15(S)-HETEs alone have little effect on COX-2 expression, but they synergize with IL-1α to cause increased COX-2 expression in human colonic myofibroblasts [81].
EETs exhibit anti-inflammatory effects and are elevated in UC patients, with reduced sEH expression in the intestinal mucosa [82]. sEH correlates with villin expression, a marker of intestinal cell differentiation [83]. Cyp4a14, a cytochrome P450 family member, promotes oxidative stress and exacerbates DSS-induced colitis, while its knockdown protects the colonic mucosa [84]. IEB disruption by AA metabolism facilitates microbial dysbiosis, amplifying inflammation and CAC risk.
4.3 Intestinal microbiota
The intestinal microbiota maintains immune homeostasis and protects against pathogen invasion, but chronic inflammation disrupts microbial balance, increasing CAC susceptibility [85]. AA metabolism interacts bidirectionally with the microbiota. For example, AA supplementation enhances lipid peroxidation by adherent-invasive Escherichia coli, exacerbating inflammation in CD patients [86]. In another study, AA was found to kill S. aureus through a lipid peroxidation mechanism, in which AA is oxidized to reactive electrophiles, which alters S. aureus macromolecules and produces toxicity [87].
Clinical studies have found that metabolites such as AA are increased in CRC patients, and the abundance of Bacteroides fragilis and Prevotella in the bacterial flora is elevated while the abundance of Blautia and Lachnospiracaea is reduced [88]. ApoE-/- mice not only have disturbed intestinal flora compared to wild-type mice (Lachnospiraceae_FCS020, Ruminococcaceae_UCG-009, Acetatifactor, Lachnoclostridium, and Lactobacillus_gasseri pathogenic bacteria were significantly increased), their metabolism was also significantly altered (AA metabolic pathways of 20-HETE, PGF2α and LTB4 levels were significantly elevated) [89]. These results all indicate suggest a close link between gut flora imbalance and AA metabolism.
Lactobacillus plantarum Zhang-LL regulates the activity of Acutalibacter muris and Lactobacillus johnsonii flora, significantly reduces the expression of PGE2, and promotes AA catabolism, which slows down the process of CRC [90]. Further studies have found that feeding AA significantly increases the number of Gram-negative bacteria such as Escherichia coli and Enterobacter faecalis, and decreases the the number of Gram-positive bacteria Fusarium nucleatum. The rich microecological environment of Gram-negative bacteria accelerated the conversion of AA to PGE2 and promoted tumor growth in AOM/DSS and gut-specific APC-/- model mice. Notably, the pro-carcinogenic effect of AA was unaffected by the removal of Gram-positive bacteria, whereas the pro-carcinogenic effect of AA completely disappeared after the removal of Gram-negative bacteria. This evidence suggests that AA-regulated intestinal flora promote the development of CRC [91].
COX-2 is also closely related to the regulation of intestinal flora. Enterococcus faecalis, a human intestinal commensal, triggers the production of trans-4-hydroxy-2-nonenal (4-HNE) by macrophages via COX-2, which synergistically reinforces the damage of COX-2 to the DNA of the target cells through the bystander effect, leading to the development of CRC [92]. COX-2 inhibitors, such as celecoxib, alter intestinal bacteria, such as Porphyromonadaceae family and the order Bacteroidales, whose metabolites inhibit the development of intestinal polyps in mice [93]. Gut microorganisms are also enriched in CYP450, and the solubility of bacterial CYPs, in contrast to the membrane-bound properties of mammalian CYPs, suggests that intestinal bacteria have a great potential to metabolize xenobiotic compounds.
4.4 Genetics
Genetic polymorphisms are strongly associated with CAC, and clinical studies have found that the COX-2 -765G > C polymorphism is associated with a reduced risk of CD in the Netherlands and an elevated risk of CRC in Asians, whereas the COX2 8473 T > C polymorphism interacts with NASID and is able to reduce the risk of CRC [94-97]. In order to explore the relationship between ALOX5, FLAP, ALOX12 and ALOX15 polymorphisms and CRC risk, a U.S. cohort analysis found that genetic variants in ALOXs may affect the risk of colorectal tumor development and alter the protective effect of NSAID use on CRC [98]. Clinical analyses in northeastern China showed that 12-LOX 261Arg > Gln polymorphisms are closely associated with the risk of CRC development and may serve as a potential marker of CRC prognosis [99].
Mutation or deletion of genes is one of the key factors affecting the number and size of tumors. It is generally accepted that COX-2 is overexpressed in tumors and polyps of CRC patients and CRC mouse models and is thought to promote tumor progression. Nevertheless, single nucleotide polymorphisms (SNPs) in the COX-2 gene may alter the function of the enzyme, thereby altering an individual's risk of developing CRC. Based on clinical cohorts, it has been found that carrying the COX-2 Val511Ala SNP is not associated with a risk of CRC, and that the use of NASID in combination can help reduce the risk of CRC in African Americans [100]. Animal studies have revealed that mice with mutations in the COX-2 gene significantly reduce the number and size of intestinal polyps [101]. The APC gene, a tumor suppressor, is mutated in >80% of sporadic CRC. sporadic CRCs with mutations. When rofecoxib, a COX-2 inhibitor, was used to treat APC mutant mice, the DNA replication rate of their polyps was significantly reduced and was effective in reducing the number and size of intestinal and colonic polyps [102]. In the familial adenomatous polyposis (MMR-proficient CRC) ApcMin+ mice and the Apc∆716 mice, COX-2 gene deletion resulted in reduced intestinal tumor formation [103]. In addition, in vivo and in vitro experiments have shown that knockdown of the COX-2 gene inhibits the proliferation and invasion of CRC cells [104]. In mouse models of ApcMin+ and AOM, the elevation of endogenous PGE2 caused by deletion of the 15-PGDH gene promotes the growth of colonic tumors [105]. Interestingly, knockdown of Ptgs-1 and Ptgs-2 (encoding the COX-1 and COX-2 genes, respectively) greatly reduced the number and size of intestinal polyps in APCmin+ mice [106].
mPGES-1 and mPGES-2 have been associated with poor prognosis in patients with CRC stages I-III [107]. Genetic deletion of mPGES-1 reduces tumor diversity and tumor load in the distal colon, and is significantly protective against carcinogen-induced CRC [108]. Compared with mutant APC, tumors with wild-type APC show higher expression of mPGES-1 [109]. Moreover, mPGES-1 deficiency enhances susceptibility to acute mucosal injury [110]. Genetic variants of LOX were found to be one of the risk factors affecting CRC based on a clinical control trial in the U.S., especially the ALOX15 allele variant [111]. In sporadic adenomas, genetic variants in the COX1, COX2, and ALOX12/15 genes were found to have a significant impact on CRC in recurrent adenomas [112]. Individuals with the ALOX5 VNTR variant genotype are linked to a reduced risk of CRC [98]. Additionally, the heterozygous mutant of ALOX12 is only associated with male CRC patients, revealing a gender bias in functional polymorphisms of ALOX12 in relation to CRC patients [113].
The CysLTR (containing CysLTR1 and CysLTR2) is a G-protein eurameric receptor that mediates the action of CysLT. patients with high expression of CysLTR1 and low expression of CysLTR2 have a poorer prognosis [114]. Animal experiments showed that AOM/DSS model mice had low-grade atypical hyperplasia of colon polyps and reduced inflammation levels in the Cysltr1-/- group compared with the wild-type group, supporting the important role of CysLTR1 in colon tumorigenesis [115]. In addition, based on the biosignature analysis CysLTR2 was positively correlated with immune cell infiltration and immune checkpoints, which could serve as a potential immune target for determining the CRC prognosis as a potential immune target [98].
CYP450 is overexpressed in CRC tissues and cells. Up-regulation of the CYP450 enzyme pathway in CRC plays a crucial role in its pathogenesis and may serve as a new direction for exploring preventive/therapeutic targets in colon cancer. When using pharmacological inhibitors or gene silencing of CYP450 enzymes, AOM/ DSS-induced CRC development can be inhibited [116]. Cytochrome P450 1A1 (CYP1A1) enzyme is one of the most important metabolic enzymes responsible for the metabolism of a wide range of xenobiotics [117]. Meta-analysis based on the exploration of the relationship between genetic variants and CRC risk revealed that CYP1A1 rs1048943 A > G may increase susceptibility to CRC compared to rs4646903 T > C [118]. Overexpression of the CYP24A1 gene in a variety of cancers, including CRC, correlates with tumor invasion, lymph node metastasis, and decreased overall survival [119]. Therefore, investigating overexpression or silencing of a single target, or combining it with immunotherapy, may be a useful tool for chemoprevention of CRC proliferation, invasion, and metastasis as a viable option.
4.5 Epigenetic
Epigenetic changes, including DNA methylation, histone modifications, chromatin remodeling, and noncoding RNA, are significantly associated with colitis-associated cancer development and progression. The CpG-island methylation pathway (CIMP) is associated with KRAS/BRAF mutations, rewiring of cellular metabolism by two oncogenes, prognosis, and resistance to classical chemotherapy. Patients with high CIMP in CRC have activation of the AA metabolic pathway and exhibit hypermetabolism [120]. COX2 methylation in sporadic primary CRC is also closely related to the CpG island methylation phenotype [121]. Transcriptional silencing of 15-LOX-1 promotes CRC, and DNA methylation of the 15-LOX-1 promoter is independently of its transcriptional regulation [122]. However, the current studies on the association of ALOX15 and CRC epigenetic studies are scarce, and the underlying mechanisms can be further explored subsequently.
Clinical studies have revealed that CysLTR methylation and gene expression profiles are associated with progression, prognosis, and metastasis in patients with CRC [123]. Overexpression of IL6 in CRC induces CYP1B1 and CYP2E1 gene expression and alters the metabolic capacity of epithelial cells, with regulation of CYP2E1 expression occurring through a transcriptional mechanism involving STAT3. For CYP1B1 regulation, IL6 downregulates CYP1B1 targeting the microRNA miR27b through a mechanism involving DNA methylation [124]. Streptococcus gallolyticus induces CYP1A enzyme activity in an AhR-dependent manner to regulate expression of epithelial cell biotransformation pathways [125].
5. Arachidonic acid pathway as a target for drugs that inhibit inflammatory cancer transformation
The AA metabolism enzymes COXs and LOXs and their metabolites (such as, PGs and LTs) have been considered as novel targets for cancer prevention and treatment. Currently, many clinical trials and experimental studies have shown that some Nonsteroidal Anti-inflammatory Drugs (NSAIDs), inhibitors and natural products, etc. inhibit the occurrence and development of CRC by regulating AA metabolism.
5.1 Nonsteroidal anti-inflammatory drugs
NSAIDs are common anti-inflammatory drugs with antipyretic, analgesic, and anti-inflammatory effects, and are widely used in cardiovascular and cerebrovascular diseases as well as various types of cancers. There are two main types of NSAIDs, one type is non-selective inhibition of the COX pathway, which includes aspirin, naproxen, ibuprofen, and so on. naproxen, ibuprofen, etc. The other type is a selective COX-2 inhibitor, including celecoxib, refecoxib, etc. Acetylsalicylic acid (aspirin) was the first NSAIDs developed for commercial use in 1897 and was widely used for its anti-inflammatory effects. Epidemiology has found that aspirin reduces mortality and risk of distant metastasis in CRC [126]. Experimental studies have shown that aspirin induces apoptosis in enriched Cancer stem-like cells (CSCs), inhibits tumor progression, and enhances the antitumor effects of chemotherapeutic agents. In addition, aspirin directly interacts with p300 in the nucleus, promotes H3K9 acetylation, activates FasL expression, and induces apoptosis in colorectal CSCs [127].
Celecoxib competitively inhibit COX-2, reducing AA conversion to PGH2 and subsequent PGE2 synthesis, thereby attenuating EP2/EP4-mediated tumor proliferation [128]. Indomethacin, a commonly used potent NSAID, inhibits COX enzymes by reducing AA uptake, thereby inhibiting the malignant development of CRC [129]. Parecoxib, the only non-enteric administered COX-2 inhibitor among NSAIDs, is able to inhibit epithelial-mesenchymal transition and metastasis of human CRC cells by down-regulation of β-conjugated proteins, and inhibit CRC metastasis in combination with chemotherapeutic agents [130].
The selectivity of NSAIDs for COX-1 and COX-2 actions plays different pharmacological roles depending on their structures, and the effective therapeutic effects of NSAIDs on inflammation stem from the selective inhibition of COX-2 [131]. Studies have shown that the greater the selectivity of a drug for COX-2 inhibition, the fewer the gastrointestinal side effects it induces, with a good linear relationship. Currently, 1,3-diaryl pyrazole derivatives were found to have significant inhibitory power and sensitivity to COX-2 enzyme and significant anti-inflammatory activity against COX-1 compared to celecoxib and indomethacin, and have dual anti-inflammatory and anti-cancer activity for the treatment of CRC [132].
Clinical trials, epidemiologic and experimental studies have shown that NSAIDs reduce the risk of CRC and mortality and prevent the progression of colitis to CRC. However, the major adverse effects of treatment with NSAIDs lead to gastrointestinal damage (including UC, bleeding, and even perforation) and cardiovascular side effects. Thus, the search for more effective improvements or combinations is an ongoing problem.
5.2 Single-target inhibitors
Currently, there are many inhibitors targeting metabolic enzymes or metabolites in the AA pathway, and the inhibitory effects of these inhibitors on CRC are mostly at the stage of experimental studies in animals. mPGES-1 enzyme, a COX downstream enzyme, is a membrane-associated protein with low expression in most tissues, and it can be induced to be produced by proinflammatory cytokines or tumorigenic conditions [133]. MK-886, target mPGES-1, a downstream enzyme in the COX pathway, reducing PGE2 formation without affecting COX-1-mediated mucosal protection [134].
In addition to COX pathway inhibitors, there are also LOX pathway inhibitors such as Zileuton (5-LOX inhibitor) and PD146176 (15-LOX-1 inhibitor). Zileuton is used to treat asthma patients by inhibiting 5-LOX, blocking the production of LTB4 and the BLT1-driven inflammatory cascade reaction. Elias Gounaris et al. found that APCΔ468/+ mice consuming food containing Zileuton for 12 consecutive weeks showed a decrease in serum LTB4 concentration, as well as a significant reduction in tumor-infiltrating mast cells, macrophages, mature monocytes, and pro-inflammatory T-regs at the site of the polyp, which was effective in decreasing the tumors and polyp formation [135]. PD146176, a selective 15-LOX-1 inhibitor, significantly inhibited 13-HODE production to promote tumor growth in human CRC HCA-7 cells, while inhibiting 12-HETE production to inhibit tumor growth in mouse CRC MC38 cells [136].
Within the LOX pathway, LTs also have the potential to prevent CRC. A prospective study showed that montelukast targeting the leukotriene pathway by cysteinyl leukotriene receptor antagonist (LTRA) inhibited the formation of ACFs and cell proliferation in IECs, suggesting that LTRA has the potential to prevent CRC [137]. In addition, COX-2 inhibitor (NS-398) or 5-LOX inhibitor (AA861) inhibits CRC tumor invasion and proliferation by promoting apoptosis through modulation of the PTEN/PI3K/Akt pathway [138]. GSK2256294, an sEH inhibitor, reduces the production of IL2, IL12p70, IL10, and TNFα in IBD patients. Interestingly, GSK2256294 has different potential effects on UC and CD, reducing IL4 and IFNγ levels in the former and IL1β levels in the latter, respectively [139].
5.3 Dual Inhibitors
Frequent inhibition of either the COX or LOX pathway results in the conversion of AA metabolism from one to the other, which can lead to serious consequences. COX/LOX inhibitors inhibit both the COX pathway and the LOX pathway, inhibiting the production of their downstream products, improving therapeutic efficiency and reducing adverse effects associated with a single inhibitor. Meanwhile, dual COX/LOX inhibitors provide a safe and effective theoretical basis for the study of new anti-inflammatory drugs. Mukhopadhyay N et al. summarized plant-based natural products with dual inhibition of COX/LOX bioactivity in different species, including Tannins, Steroids, Flavonoids, Alkaloids, etc. emphasizing the importance of natural product derivatives [140]. Meshram MA et al. conducted a review of synthetic bis-COX-2/5-LOX inhibitors covering Thiazoles, 2,3,4-Trisubstituted thiophenes, Pyrazoloquinazolines and others. The design of these novel scaffolds retains the basic structural features of COX-2 and LOX-5 activity while synergizing or enhancing the activity of bis-COX-2/5-LOX, contributing to the discovery of molecules with superior anti-inflammatory activity [141].
sEH is the major epoxide hydrolase involved in the metabolism of EET and is encoded by the EPHX-2 gene on chromosome 8. sEH has been shown to be overexpressed in colitis and CRC [142]. Inhibition of sEH on the one hand increases EET to enhance the bioavailability of EET, which has significant anti-inflammatory effects and protective effects on the lungs, heart, gastrointestinal tract, and blood-brain barrier; and on the other hand, it reduces the product DHET, which is involved in monocyte chemoattractant protein-1 (MCP-1)-mediated monocyte chemotaxis [143].
When sEH inhibitors are co-administered with NASID, they are effective in treating cancer and reduce the side effects caused by NASID, and the underlying mechanisms may be related to decreasing monocyte recruitment and inflammation, blocking the endoplasmic reticulum (ER)/mitochondrial stress induced with NASID to reduce epithelial vascular barrier damage, or increase tissue repair and angiogenesis related [144]. 4-(5-phenyl-3-{3-[3-(4-trifluoromethyl-phenyl)-ureido]-propyl}-pyrazol-1-yl)-benzenesulfonamide (PTUPB) is a dual COX-2/sEH inhibitor with antitumor activity and organ-protective effects. PTUPB, when used in combination with cisplatin, enhances antitumor properties without increasing toxicity [145]. The combination of sEH inhibitors with other drugs is an effective strategy in the transformation of inflammatory cancers, which can be further investigated in clinical trials.
3,3'-Diindolymethane (DIM) is a novel COX1/2 and ERK1/2 inhibitor derived from the derived from indole-3-carbinol found in broccoli and cabbage. In an in vivo mouse model, oral administration of DIM inhibits the growth of xenograft colon tumors and can be used in the chemotherapy of CRC [146]. Therefore, the design of simultaneous multi-target blockade can effectively overcome the side effects of the drug and suggest new ideas for the development of effective and safe new drugs.
5.4 Natural products
Most of the natural products used in the treatment of cancer are derived from plant extracts, and their derived drugs have the advantage of fewer residues and lower side effects (Table 1).
Natural products that play a role in inflammatory cancer transformation in colorectal cancer
| Ingredients | Origins | Experimental model | Cell lines/animals | Mechanisms | Anticancer/anticarcinogenic effects | References |
|---|---|---|---|---|---|---|
| Ginseng and Sinensis | In vivo | DSS-induced mice model | Regulation of metabolic pathways such as arachidonic acid metabolismBeneficial bacteria (such as Muribaculaceae_norank, Lachnospiraceae and Akkermansia)↑Harmful bacteria (such as Bacteroides, Parabacteroides and Desulfovibrio)↓ | Improvement of colitis | 147 | |
| Ginsenoside Rk3 | Ginseng | In vivo | High-fat diet-induced mice model | PGE2, PGD2, TXB2, HETE, and HODE↓EET and diHOME↑ | Improve obesity-induced intestinal inflammation | 148 |
| Protopanaxatriol saponin | Ginseng | In vivo | DSS-induced mice model | TNF-α, IL-6, and IL-1↓MPO and NO↓ | Inhibit metabolic dysfunctionReversing abnormal metabolite changesAmelioration of pathological damage | 149 |
| Clinopodium chinense Kuntze | In vitroIn vivo | Mouse macrophage RAW264.7 cellDSS-induced mice model | LPS-TLR4-NF-κB-iNOS/COX-2 signaling pathwayNO, PGE2, IL-6, IL-10 and TNF-ɑ↑ | Reduces systemic inflammationRegulates metabolism | 150 | |
| Jasminum elongatum | In vivo | DSS-induced mice model | IκB/p65/COX-2/arachidonic acid pathway | Improvement of UC mice | 151 | |
| Chrysanthemum polysaccharides | In vivo | TNBS/ethanol induced rat model | P-p65, TLR4, P-STAT3 and P-JAK2 | Improvement of colitis rats | 152 | |
| Pistacia lentiscus oil | In vivo | TNBS-induced rat model | Vesiculitis and cryptoinflammation↓ | Protects against intestinal inflammation | 153 | |
| Acacia saligna butanol extract and its nanoformulation | In vivo | Acetic acid-induced mice model | COX-2, PGE2 and IL1β↓ | Improvement of intestinal mucosal lesions and inflammatory infiltrates | 154 | |
| 6-Gingerol | Zingiber officinale Roscoe | In vitroIn vivo | Human CRC cell lines Caco2DSS-induced mice model | Iron load and MDA↓GSSG↓ SOD, GSH↑ | Anti-inflammatory, antioxidant | 155 |
| In vitroIn vivo | Human CRC cell lines HCT116 cellsXenograft mouse model | LTA4 hydrolase↓Proliferation↓ | Inhibition of CRC progression | 156 | ||
| Berberine | Coptis chinensis and many other plants | DSS-induced mice modelBBR-induced fecal microbiota transplantation model | AA metabolism pathway↓ | Regulates the intestinal microbiomeImproves serum metabolic balance | 158 | |
| In vitroIn vivo | Human CRC cell lines SW620 and LoVo cellsXenograft mouse model | COX-2/PGE2- JAK2/STAT3 signaling pathway↓ | Inhibited CRC invasion and metastasis | 159 | ||
| In vitro | Human CRC cell lines SW480 cell | Arrested SW480 cell cycle at G2/M phaseMitochondriamediated intrinsic apoptosis↑Angiogenesis and inflammation markers↓ | Chemopreventive effect on CRC | 160 | ||
| Emodin | Rheum officinale | In vitro | Human CRC cell lines SW620 and HCT116 cellsAOM/DSS-induced mice model | Inflammatory cell, cytokine and pro-inflammatory enzymes↓CD3+ T lymphocytes↑ | Inhibits cancer-associated intestinal inflammation and prevents CRC progression | 161 |
| Inositol hexapphosphate | In vitro | Human CRC cell lines Caco2 cells | COX-2, 5-LOX, PGE2 and LTB4↓ | Prevention of CRC | 162 | |
| Celastrol | Tripterygium wilfordii Hook F | In vitro | Human CRC cell lines HCT116 and SW620 | Cell apoptosis↑Cell cycle arrestNF-κB/COX-2 pathway↓ | Effective treatment of CRC | 163 |
| Ellagic acid | Ellagitannin | In vivo | 1,2-dimethylhydrazine-induced mice model | NF-κB, COX-2, iNOS, TNF-α and IL-6↓5'-ND, gamma-GT, CEA, AFP, CD, ALP, LDH↓ | Chemopreventive effect on CRC | 165 |
| Lycopene | Lycopersicum esculentum | In vitroIn vivo | Human CRC cell lines HT29 cellsXenograft mouse model | p21(CIP1/WAF1) and p27(Kip1)↑Proliferating cell nuclear antigen, β-catenin, cyclin D1 and c-Myc proteins↓MMP-7, MMP-9, COX-2 and PGE2↓ | Synergistic fish oil inhibits CRC growth and progression | 166 |
| Lizhong Decoction (LZD) | Zingiberis Rhizoma, Radix Ginseng, Rhizoma Atractylodis Macrocephalae and Radix Glycyrrhizae | In vivo | DSS-induced mice | Improvement of metabolites in plasma and urine | Ameliorate of DSS-induced colitis mice | 167 |
| Zhilining Formula (ZLN) | Andrographis herba, Sophorae flavescentis radix and Aucklandia radix | In vivo | DSS-induced mice | MPO, IL1β, TNF-α, IL18↓AHR↑, NF-κBp65 axis↓COX-2↓ | Repairing the intestinal mucosal barrierReduce persistent inflammation | 168 |
| Yinhua Miyanling tablets | Lonicerae Japonicae Flos, Scu tellariae Barbatae Herba, Pol ygoni Avicularis Herba, Pyrrosiae Folium, Clematis Armandii Caulis, Lophatheri Herba, Plantaginis Semen, Dianthi Herba and Junci Medulla | In vitroIn vivo | Human CRC cell lines Caco2 cellDSS-induced mice model | TNF-α, IL-6, iNOS↓MPO, MDA, SOD↓ | Improvement of colonic mucosal damage | 169 |
| Huang-lian-Jie-du decoction (HLJDD) | Copptidis Rhizoma, Scutellaria Radix, Phelodendri Chinensis Cortex and Gardenia Fructus | In vivo | DSS-induced mice model | COX-2, PLA2 and 5-LOX↓ | Reversing metabolite abnormalitiesAlleviates UC mice | 170 |
| Sanwu Baisan Decoction | Badoushuang, Zhebeimu and Jiegeng | In vitroIn vivo | Mouse CRC cell line CT26Xenograft mouse model | TLR4/COX-2/PGE2↓Induces apoptosis | Inhibition of CRC progression | 171 |
The gut microbiota-metabolite axis may be one of the important mechanisms for the treatment of IBD. The combination of ginseng and Sinensis effectively increases the abundance of beneficial bacteria and decreases the abundance of harmful bacteria through metabolic pathways such as AA metabolism [147]. ginsenoside Rk3, a natural anti-inflammatory active ingredient extracted from ginseng, can improve obesity-induced intestinal inflammation by regulating lipid metabolism [148]. Protopanaxatriol saponin is also a major active ingredient of ginseng, which can ameliorate pathological damage and reverse abnormal metabolite changes in UC mice through metabolic pathways such as AA [149]. Traditional Chinese medicine Clinopodium chinense Kuntze (CC) has anti-inflammatory, antidiarrheal, and hemostatic activities, and it was found that CC can reduce inflammation through the LPS-TLR4-NF-κB-iNOS/COX-2 signaling pathway, and regulate endogenous metabolites such as AA to alleviate UC [150]. Jasminum elongatum alleviates UC physiological and pathological symptoms and reverses DSS-induced UC mice via the IκB/p65/COX-2/AA pathway [151]. Also, Chrysanthemum polysaccharides ameliorate 2,4,6-trinitrobenzenesulfonic acid (TNBS)/ethanol-induced colitis in rats by adjusting multiple metabolites including AA [152]. Animal experiments in which the Pistacia lentiscus oil was administered first, and in which TNBS was given to induce UC, significantly reduced vesiculation and crypt inflammation [153]. Acacia saligna butanol extract and its nanoformulation can reduce COX-2, PGE2 and IL1β levels, normalize metabolite levels, and ameliorate intestinal mucosal lesions and inflammatory infiltration in UC mice [154].
Some natural products have dual anti-inflammatory and cancer inhibiting activities for both IBD and CRC. natural phenolics, 6-Gingerol (6-G), one of the constituents of Zingiber officinale Roscoe, is able to inhibit ferrometabolism through AA metabolism, exerting anti-inflammatory and antioxidant effects to ameliorate UC [155]. 6-G also inhibits the growth of CRC by inhibiting LTA4 hydrolase [156]. Berberine, an isoquinoline alkaloid, is found in Coptis chinensis and many other plants [157]. Berberine has been found to be able to improve serum metabolic homeostasis by inhibiting the AA metabolic pathway and modulating the intestinal microbiome, thereby treating UC [158]. In CRC, berberine prevents the growth, migration and invasion of CRC cells in vitro and in vivo by targeting the COX-2/PGE2-JAK2 and STAT3-MMP-2/MMP-9 signaling pathways [159]. Moreover, berberine is also able by targeting various pathways, such as the NF-κB/COX-2 pathway, to result in the cell cycle arrest, induction of apoptosis, and inhibition of inflammatory response in CRC cells [160]. Emodin, a plant root extract, reduces intestinal inflammation associated with carcinogenesis [161].
Inositol hexakisphosphate (IP6) is a natural phytochemical. Małgorzata Kapral et al. found that IP6 prevents CRC by limiting inflammatory events in the colon epithelium by regulating the expression of COX-2 and 5-LOX proteins, as well as by affecting the synthesis and secretion of PGE2 and LTB4 [162]. A natural product, Celastrol, isolated from Tripterygium wilfordii Hook F, can regulate the NF-κB/COX-2 pathway to block the cell cycle and induce apoptosis, and is a potent antitumor inhibitor [163]. Products present in some fruits, nuts and vegetables also have anticancer activity, such as ellagic acid, a hydrolyzed metabolite of ellagitannins [164]. Umesalma and sudhandiran found that ellagic acid prevented the development of CRC in rats induced by the chemical carcinogen 1,2dimethylhydrazine by targeting the NF-κB/COX-2 pathway [165]. lycopene is isolated from tomatoes. Using a mouse xenograft colon cancer model and in vitro experiments, Tang et al. found that lycopene and fish oil synergistically inhibited COX-2 and PGE2, thereby inhibiting CRC development [166].
Chinese herbal formula is considered as one of the common protocols for effective treatment of CAC. Lizhong Decoction (LZD) improves UC by modulating endogenous metabolites such as AA [167]. Zhilining Formula (ZLN) repairs the intestinal mucosal barrier and attenuates persistent inflammation in UC mice by modulating AA metabolism [168]. Yinhua Miyanling tablets also has a favorable therapeutic effect on UC by ameliorating colonic mucosal damage through multiple endogenous metabolites and AA metabolic pathways, among others [169]. Also, Huang lian Jie du decoction (HLJDD) inhibited colonic pathological injury by regulating AA metabolism and alleviated UC in mice [170]. Sanwu Baisan Decoction exerts anti-CRC effects by inhibiting the TLR-4/COX-2/PGE-2 pathway, inhibiting the secretion of anti-tumor-promoting immune cytokines, inducing apoptosis of tumor cells, and maintaining intestinal flora [171].
6. Conclusions
AA metabolism drives the inflammatory cancer transformation in CAC through eicosanoid-mediated pathways. In CAC, novel insights highlight AA's role in epigenetic regulation, where COX-2 methylation correlates with CpG island methylation phenotypes, promoting KRAS/BRAF-driven oncogenesis. Additionally, 12S-HETE enhances cancer-associated fibroblast activity, fostering tumor invasiveness via stromal remodeling. A pioneering approach involves CRISPR-based ALOX5/15 gene editing, which suppresses LTB4 production and inhibits tumor growth in preclinical CAC models, offering a targeted strategy to disrupt pro-tumorigenic inflammation. Furthermore, AA's interaction with the gut microbiota, particularly Gram-negative bacteria, amplifies PGE2 production, accelerating CAC progression, while microbiota-modulating agents like berberine counteract this effect by reducing lipid peroxidation. Clinically, serum LTB4 and urinary PGE-M levels serve as non-invasive biomarkers for CAC risk stratification. Dual COX/LOX inhibitors, such as licofelone, mitigate compensatory pathway shunting, enhancing therapeutic efficacy with reduced gastrointestinal toxicity compared to NSAIDs. Future research should leverage AI-driven profiling of AA metabolite signatures to guide personalized therapies and explore integration with immune checkpoint inhibitors to boost anti-tumor immunity in CAC. These advancements position AA metabolism as a transformative target for preventing and treating inflammation-driven colorectal cancer.
Acknowledgements
This work was supported by National Nature Science Foundation of China, No. 82320108022, 82322076, 82405506; Shanghai "Science and Technology Innovation Action Plan" medical innovation research special project of major difficult disease diagnosis and treatment strategy clinical research (22Y31920100); National funding program for postdoctoral researchers (GZC20231707).
Author contributions
Sisi Ren: Investigation, Data curation, Writing -Original draft preparation, Visualization; Lu Lu: Writing -Original draft preparation, Reviewing and Editing, Funding acquisition, Visualization; Hang Su: Data curation, Visualization; Zongping Li: Data curation; Sumei Li: Data curation; Jiashu Pan: Data curation, Visualization; Yujing Liu: Data curation, Visualization; Guang Ji: Writing - Reviewing and Editing, Supervision, Funding acquisition; Hanchen Xu: Formal analysis, Investigation, Data curation, Writing -Reviewing and Editing, Supervision, Funding acquisition.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sinha R. Colorectal cancer. Clinical radiology. 2021;76:870
2. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A. et al. Cancer treatment and survivorship statistics, 2022. CA: a cancer journal for clinicians. 2022;72:409-436
3. Praveen TK, Gangadharappa HV, Abu Lila AS, Moin A, Mehmood K, Krishna KL. et al. Inflammation targeted nanomedicines: Patents and applications in cancer therapy. Seminars in cancer biology. 2022;86:645-663
4. Lu Y, Li D, Wang L, Zhang H, Jiang F, Zhang R. et al. Comprehensive Investigation on Associations between Dietary Intake and Blood Levels of Fatty Acids and Colorectal Cancer Risk. Nutrients. 2023;15:730
5. Haycock PC, Borges MC, Burrows K, Lemaitre RN, Burgess S, Khankari NK. et al. The association between genetically elevated polyunsaturated fatty acids and risk of cancer. EBioMedicine. 2023;91:104510
6. Ortiz-Placín C, Castillejo-Rufo A, Estarás M, González A. Membrane Lipid Derivatives: Roles of Arachidonic Acid and Its Metabolites in Pancreatic Physiology and Pathophysiology. Molecules (Basel, Switzerland). 2023;28:4316
7. McCarty MF, DiNicolantonio JJ. Minimizing Membrane Arachidonic Acid Content as a Strategy for Controlling Cancer: A Review. Nutrition and cancer. 2018;70:840-850
8. Li YW, Guo Q, Peng QQ, Shen Q, Nie ZK, Ye C. et al. Recent Development of Advanced Biotechnology in the Oleaginous Fungi for Arachidonic Acid Production. ACS synthetic biology. 2022;11:3163-3173
9. Chandrasekharan JA, Marginean A, Sharma-Walia N. An insight into the role of arachidonic acid derived lipid mediators in virus associated pathogenesis and malignancies. Prostaglandins & other lipid mediators. 2016;126:46-54
10. Pannunzio A, Coluccia M. Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature. Pharmaceuticals (Basel, Switzerland). 2018;11:101
11. Riehl TE, Alvarado D, Ee X, Zuckerman A, Foster L, Kapoor V. et al. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut. 2019;68:1003-1013
12. Sohail R, Mathew M, Patel KK, Reddy SA, Haider Z, Naria M. et al. Effects of Non-steroidal Anti-inflammatory Drugs (NSAIDs) and Gastroprotective NSAIDs on the Gastrointestinal Tract: A Narrative Review. Cureus. 2023;15:e37080
13. Chen Y. Design and construction of COX-2 specific fluorescent probes. Molecular and cellular probes. 2019;48:101472
14. Zhang DX, Gauthier KM, Chawengsub Y, Holmes BB, Campbell WB. Cyclooxygenase- and lipoxygenase-dependent relaxation to arachidonic acid in rabbit small mesenteric arteries. American journal of physiology Heart and circulatory physiology. 2005;288:H302-309
15. Merchant N, Bhaskar L, Momin S, Sujatha P, Reddy ABM, Nagaraju GP. 5-Lipoxygenase: Its involvement in gastrointestinal malignancies. Critical reviews in oncology/hematology. 2018;127:50-55
16. Aparoy P, Reddy KK, Reddanna P. Structure and ligand based drug design strategies in the development of novel 5- LOX inhibitors. Current medicinal chemistry. 2012;19:3763-3778
17. Pearl DS, Masoodi M, Eiden M, Brümmer J, Gullick D, McKeever TM. et al. Altered colonic mucosal availability of n-3 and n-6 polyunsaturated fatty acids in ulcerative colitis and the relationship to disease activity. Journal of Crohn's & colitis. 2014;8:70-79
18. Naito Y, Ji X, Tachibana S, Aoki S, Furuya M, Tazura Y. et al. Effects of arachidonic acid intake on inflammatory reactions in dextran sodium sulphate-induced colitis in rats. The British journal of nutrition. 2015;114:734-745
19. D'Aldebert E, Cenac N, Rousset P, Martin L, Rolland C, Chapman K. et al. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:275-285
20. Monk JM, Turk HF, Fan YY, Callaway E, Weeks B, Yang P. et al. Antagonizing arachidonic acid-derived eicosanoids reduces inflammatory Th17 and Th1 cell-mediated inflammation and colitis severity. Mediators of inflammation. 2014;2014:917149
21. Kikut J, Mokrzycka M, Drozd A, Grzybowska-Chlebowczyk U, Ziętek M, Szczuko M. Involvement of Proinflammatory Arachidonic Acid (ARA) Derivatives in Crohn's Disease (CD) and Ulcerative Colitis (UC). Journal of clinical medicine. 2022;11:1861
22. Heydeck D, Kakularam KR, Labuz D, Machelska H, Rohwer N, Weylandt K. et al. Transgenic mice overexpressing human ALOX15 under the control of the aP2 promoter are partly protected in the complete Freund's adjuvant-induced paw inflammation model. Inflammation research. 2023;72:1649-1664
23. Marbach-Breitrück E, Rohwer N, Infante-Duarte C, Romero-Suarez S, Labuz D, Machelska H. et al. Knock-In Mice Expressing a 15-Lipoxygenating Alox5 Mutant Respond Differently to Experimental Inflammation Than Reported Alox5(-/-) Mice. Metabolites. 2021;11:698
24. Tallima H, El Ridi R. Mechanisms of Arachidonic Acid In Vitro Tumoricidal Impact. Molecules (Basel, Switzerland). 2023;28:1727
25. Piazzesi A, Afsar SY, van Echten-Deckert G. Sphingolipid metabolism in the development and progression of cancer: one cancer's help is another's hindrance. Molecular oncology. 2021;15:3256-3279
26. Tallima H, Azzazy HME, El Ridi R. Cell surface sphingomyelin: key role in cancer initiation, progression, and immune evasion. Lipids in health and disease. 2021;20:150
27. González-Fernández MJ, Fabrikov D, Ramos-Bueno RP, Guil-Guerrero JL, Ortea I. SWATH Differential Abundance Proteomics and Cellular Assays Show In Vitro Anticancer Activity of Arachidonic Acid- and Docosahexaenoic Acid-Based Monoacylglycerols in HT-29 Colorectal Cancer Cells. Nutrients. 2019;11:2984
28. Ortea I, González-Fernández MJ, Ramos-Bueno RP, Guil-Guerrero JL. Proteomics Study Reveals That Docosahexaenoic and Arachidonic Acids Exert Different In Vitro Anticancer Activities in Colorectal Cancer Cells. Journal of agricultural and food chemistry. 2018;66:6003-6012
29. Larsson SC, Carter P, Vithayathil M, Mason AM, Michaëlsson K, Baron JA. et al. Genetically predicted plasma phospholipid arachidonic acid concentrations and 10 site-specific cancers in UK biobank and genetic consortia participants: A mendelian randomization study. Clinical nutrition (Edinburgh, Scotland). 2021;40:3332-3337
30. Cilenti F, Barbiera G, Caronni N, Iodice D, Montaldo E, Barresi S. et al. A PGE(2)-MEF2A axis enables context-dependent control of inflammatory gene expression. Immunity. 2021;54:1665-1682.e1614
31. Park HJ, Kim J, Saima FT, Rhee KJ, Hwang S, Kim MY. et al. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochemical and biophysical research communications. 2018;498:988-995
32. Ashton AW, Zhang Y, Cazzolli R, Honn KV. The Role and Regulation of Thromboxane A(2) Signaling in Cancer-Trojan Horses and Misdirection. Molecules (Basel, Switzerland). 2022;27:6234
33. Wasilewicz MP, Kołodziej B, Bojułko T, Kaczmarczyk M, Sulzyc-Bielicka V, Bielicki D. et al. Overexpression of 5-lipoxygenase in sporadic colonic adenomas and a possible new aspect of colon carcinogenesis. International journal of colorectal disease. 2010;25:1079-1085
34. Stadler S, Nguyen CH, Schachner H, Milovanovic D, Holzner S, Brenner S. et al. Colon cancer cell-derived 12(S)-HETE induces the retraction of cancer-associated fibroblast via MLC2, RHO/ROCK and Ca(2+) signalling. Cellular and molecular life sciences: CMLS. 2017;74:1907-1921
35. Zhao X, Liu R, Chen Y, Hettinghouse A, Liu C. Cytosolic Phospholipase A2 Is Required for Fexofenadine's Therapeutic Effects against Inflammatory Bowel Disease in Mice. International journal of molecular sciences. 2021;22:11155
36. Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115-122
37. Tang C, Wang A, Zhao Y, Mou W, Jiang J, Kuang J. et al. Leukotriene B4 receptor knockdown affects PI3K/AKT/mTOR signaling and apoptotic responses in colorectal cancer. Biomolecules & biomedicine. 2024;24:968-981
38. Khorshid Sokhangouy S, Alizadeh F, Lotfi M, Sharif S, Ashouri A, Yoosefi Y. et al. Recent advances in CRISPR-Cas systems for colorectal cancer research and therapeutics. Expert review of molecular diagnostics. 2024;24:677-702
39. Tian J, Zhang L, La X, Fan X, Li A, Wu C. et al. Tumor-secreted GRP78 induces M2 polarization of macrophages by promoting lipid catabolism. Cellular signalling. 2023;108:110719
40. Murase R, Sato H, Yamamoto K, Ushida A, Nishito Y, Ikeda K. et al. Group X Secreted Phospholipase A2 Releases ω3 Polyunsaturated Fatty Acids, Suppresses Colitis, and Promotes Sperm Fertility. The Journal of biological chemistry. 2016;291:6895-6911
41. Kim W, Jang JH, Zhong X, Seo H, Surh YJ. 15-Deoxy-△(12,14)-Prostaglandin J(2) Promotes Resolution of Experimentally Induced Colitis. Frontiers in immunology. 2021;12:615803
42. Acedo SC, Gotardo EM, Lacerda JM, de Oliveira CC, de Oliveira Carvalho P, Gambero A. Perinodal adipose tissue and mesenteric lymph node activation during reactivated TNBS-colitis in rats. Digestive diseases and sciences. 2011;56:2545-2552
43. Li Z, Chen S, He X, Gong S, Sun L, Weng L. SLC3A2 promotes tumor-associated macrophage polarization through metabolic reprogramming in lung cancer. Cancer science. 2023;114:2306-2317
44. Zhan Y, Zheng L, Liu J, Hu D, Wang J, Liu K. et al. PLA2G4A promotes right-sided colorectal cancer progression by inducing CD39+γδ Treg polarization. JCI insight. 2021;6:e148028
45. Liu Y, Niu R, Deng R, Song S, Wang Y, Zhang H. Multi-enzyme Co-expressed Dual-Atom Nanozymes Induce Cascade Immunogenic Ferroptosis via Activating Interferon-γ and Targeting Arachidonic Acid Metabolism. Journal of the American Chemical Society. 2023;145:8965-8978
46. Faluyi OO, Fitch P, Howie SEM. An increased CD25-positive intestinal regulatory T lymphocyte population is dependent upon Cox-2 activity in the Apc(min/+) model. Clinical and experimental immunology. 2018;191:32-41
47. Fukuda Y, Kim SH, Bustos MA, Cho SN, Roszik J, Burks JK. et al. Inhibition of Microsomal Prostaglandin E2 Synthase Reduces Collagen Deposition in Melanoma Tumors and May Improve Immunotherapy Efficacy by Reducing T-cell Exhaustion. Cancer research communications. 2023;3:1397-1408
48. Thumkeo D, Punyawatthananukool S, Prasongtanakij S, Matsuura R, Arima K, Nie H. et al. PGE(2)-EP2/EP4 signaling elicits immunosuppression by driving the mregDC-Treg axis in inflammatory tumor microenvironment. Cell reports. 2022;39:110914
49. Kaushal N, Kudva AK, Patterson AD, Chiaro C, Kennett MJ, Desai D. et al. Crucial role of macrophage selenoproteins in experimental colitis. Journal of immunology (Baltimore, Md: 1950). 2014;193:3683-3692
50. Harizi H, Norbert G. Inhibition of IL-6, TNF-alpha, and cyclooxygenase-2 protein expression by prostaglandin E2-induced IL-10 in bone marrow-derived dendritic cells. Cellular immunology. 2004;228:99-109
51. Finetti F, Travelli C, Ercoli J, Colombo G, Buoso E, Trabalzini L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology. 2020;9:434
52. Zhang C, Hu Z, Pan Z, Ji Z, Cao X, Yu H. et al. The arachidonic acid metabolome reveals elevation of prostaglandin E2 biosynthesis in colorectal cancer. The Analyst. 2024;149:1907-1920
53. Akbari B, Soltantoyeh T, Shahosseini Z, Jadidi-Niaragh F, Hadjati J, Brown CE. et al. PGE2-EP2/EP4 signaling elicits mesoCAR T cell immunosuppression in pancreatic cancer. Frontiers in immunology. 2023;14:1209572
54. Xue L, Salimi M, Panse I, Mjösberg JM, McKenzie AN, Spits H. et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. The Journal of allergy and clinical immunology. 2014;133:1184-1194
55. Gosset P, Bureau F, Angeli V, Pichavant M, Faveeuw C, Tonnel AB. et al. Prostaglandin D2 affects the maturation of human monocyte-derived dendritic cells: consequence on the polarization of naive Th cells. Journal of immunology (Baltimore, Md: 1950). 2003;170:4943-4952
56. Harizi H, Gualde N. Dendritic cells produce eicosanoids, which modulate generation and functions of antigen-presenting cells. Prostaglandins, leukotrienes, and essential fatty acids. 2002;66:459-466
57. Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. The Journal of allergy and clinical immunology. 2013;132:205-213
58. Lund SJ, Portillo A, Cavagnero K, Baum RE, Naji LH, Badrani JH. et al. Leukotriene C4 Potentiates IL-33-Induced Group 2 Innate Lymphoid Cell Activation and Lung Inflammation. Journal of immunology (Baltimore, Md: 1950). 2017;199:1096-1104
59. Cheon EC, Khazaie K, Khan MW, Strouch MJ, Krantz SB, Phillips J. et al. Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APCDelta468 mice. Cancer research. 2011;71:1627-1636
60. Rahman MM, Borthakur A, Afroz S, Arthur S, Sundaram U. Unique Regulation of Intestinal Villus Epithelial Cl(-)/HCO(3)(-) Exchange by Cyclooxygenase Pathway Metabolites of Arachidonic Acid in a Mouse Model of Spontaneous Ileitis. International journal of molecular sciences. 2021;22:4171
61. Sellers ZM, Illek B, Figueira MF, Hari G, Joo NS, Sibley E. et al. Impaired PGE2-stimulated Cl- and HCO3- secretion contributes to cystic fibrosis airway disease. PloS one. 2017;12:e0189894
62. Najar M, Alsabri SG, Guedi GG, Merimi M, Lavoie F, Grabs D. et al. Role of epigenetics and the transcription factor Sp1 in the expression of the D prostanoid receptor 1 in human cartilage. Frontiers in cell and developmental biology. 2023;11:1256998
63. Maimó-Barceló A, Martín-Saiz L, Barceló-Nicolau M, Salivo S, Pérez-Romero K, Rodriguez RM. et al. Lipid signature associated with chronic colon inflammation reveals a dysregulation in colonocyte differentiation process. Biochimica et biophysica acta Molecular and cell biology of lipids. 2024;1869:159528
64. Lee Y, Choo J, Kim SJ, Heo G, Pothoulakis C, Kim YH. et al. Analysis of endogenous lipids during intestinal wound healing. PloS one. 2017;12:e0183028
65. Ayiomamitis GD, Notas G, Vasilakaki T, Tsavari A, Vederaki S, Theodosopoulos T. et al. Understanding the Interplay between COX-2 and hTERT in Colorectal Cancer Using a Multi-Omics Analysis. Cancers. 2019;11:1536
66. Takeuchi K, Amagase K. Roles of Cyclooxygenase, Prostaglandin E2 and EP Receptors in Mucosal Protection and Ulcer Healing in the Gastrointestinal Tract. Current pharmaceutical design. 2018;24:2002-2011
67. Endo S, Suganami A, Fukushima K, Senoo K, Araki Y, Regan JW. et al. 15-Keto-PGE(2) acts as a biased/partial agonist to terminate PGE(2)-evoked signaling. The Journal of biological chemistry. 2020;295:13338-13352
68. Rodríguez-Lagunas MJ, Martín-Venegas R, Moreno JJ, Ferrer R. PGE2 promotes Ca2+-mediated epithelial barrier disruption through EP1 and EP4 receptors in Caco-2 cell monolayers. American journal of physiology Cell physiology. 2010;299:C324-334
69. Roulis M, Kaklamanos A, Schernthanner M, Bielecki P, Zhao J, Kaffe E. et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature. 2020;580:524-529
70. Montrose DC, Nakanishi M, Murphy RC, Zarini S, McAleer JP, Vella AT. et al. The role of PGE2 in intestinal inflammation and tumorigenesis. Prostaglandins & other lipid mediators. 2015;116-117:26-36
71. Hayashi A, Sakamoto N, Kobayashi K, Murata T. Enhancement of prostaglandin D(2)-D prostanoid 1 signaling reduces intestinal permeability by stimulating mucus secretion. Frontiers in immunology. 2023;14:1276852
72. Medani M, Collins D, Mohan HM, Walsh E, Winter DC, Baird AW. Prostaglandin D2 regulates human colonic ion transport via the DP1 receptor. Life sciences. 2015;122:87-91
73. Cabral M, Martín-Venegas R, Moreno JJ. Role of arachidonic acid metabolites on the control of non-differentiated intestinal epithelial cell growth. The international journal of biochemistry & cell biology. 2013;45:1620-1628
74. Nakamura M, Shimizu T. Therapeutic target of leukotriene B(4) receptors, BLT1 and BLT2: Insights from basic research. Biochimie. 2023;215:60-68
75. Yokomizo T. Two distinct leukotriene B4 receptors, BLT1 and BLT2. Journal of biochemistry. 2015;157:65-71
76. Rodríguez-Lagunas MJ, Storniolo CE, Ferrer R, Moreno JJ. 5-Hydroxyeicosatetraenoic acid and leukotriene D4 increase intestinal epithelial paracellular permeability. The international journal of biochemistry & cell biology. 2013;45:1318-1326
77. Cabral M, Martín-Venegas R, Moreno JJ. Leukotriene D4-induced Caco-2 cell proliferation is mediated by prostaglandin E2 synthesis. Physiological reports. 2015;3:e12417
78. Ferrer R, Moreno JJ. Role of eicosanoids on intestinal epithelial homeostasis. Biochemical pharmacology. 2010;80:431-438
79. Pochard C, Coquenlorge S, Jaulin J, Cenac N, Vergnolle N, Meurette G. et al. Defects in 15-HETE Production and Control of Epithelial Permeability by Human Enteric Glial Cells From Patients With Crohn's Disease. Gastroenterology. 2016;150:168-180
80. Chattopadhyay R, Dyukova E, Singh NK, Ohba M, Mobley JA, Rao GN. Vascular endothelial tight junctions and barrier function are disrupted by 15(S)-hydroxyeicosatetraenoic acid partly via protein kinase C ε-mediated zona occludens-1 phosphorylation at threonine 770/772. The Journal of biological chemistry. 2014;289:3148-3163
81. Di Mari JF, Saada JI, Mifflin RC, Valentich JD, Powell DW. HETEs enhance IL-1-mediated COX-2 expression via augmentation of message stability in human colonic myofibroblasts. American journal of physiology Gastrointestinal and liver physiology. 2007;293:G719-728
82. Qiu YE, Qin J, Luo Y, Qin SL, Mu YF, Cun R. et al. Increased epoxyeicosatrienoic acids may be part of a protective mechanism in human ulcerative colitis, with increased CYP2J2 and reduced soluble epoxide hydrolase expression. Prostaglandins & other lipid mediators. 2018;136:9-14
83. Cizkova K, Koubova K, Foltynkova T, Jiravova J, Tauber Z. Soluble Epoxide Hydrolase as an Important Player in Intestinal Cell Differentiation. Cells, tissues, organs. 2020;209:177-188
84. Xuan Q, Zhou Y, Tan B, Xiao Z, Dong S, Dai F. et al. Mice Deficient in Cyp4a14 Have An Increased Number of Goblet Cells and Attenuated Dextran Sulfate Sodium-Induced Colitis. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2018;50:2272-2282
85. Fu Q, Ma X, Li S, Shi M, Song T, Cui J. New insights into the interactions between the gut microbiota and the inflammatory response to ulcerative colitis in a mouse model of dextran sodium sulfate and possible mechanisms of action for treatment with PE&AFWE. Animal models and experimental medicine. 2024;7:83-97
86. Wen W, Xu Y, Qian W, Huang L, Gong J, Li Y. et al. PUFAs add fuel to Crohn's disease-associated AIEC-induced enteritis by exacerbating intestinal epithelial lipid peroxidation. Gut microbes. 2023;15:2265578
87. Beavers WN, Monteith AJ, Amarnath V, Mernaugh RL, Roberts LJ 2nd, Chazin WJ. et al. Arachidonic Acid Kills Staphylococcus aureus through a Lipid Peroxidation Mechanism. mBio. 2019 10
88. Du X, Li Q, Tang Z, Yan L, Zhang L, Zheng Q. et al. Alterations of the Gut Microbiome and Fecal Metabolome in Colorectal Cancer: Implication of Intestinal Metabolism for Tumorigenesis. Frontiers in physiology. 2022;13:854545
89. Sun Y, Wu D, Zeng W, Chen Y, Guo M, Lu B. et al. The Role of Intestinal Dysbacteriosis Induced Arachidonic Acid Metabolism Disorder in Inflammaging in Atherosclerosis. Frontiers in cellular and infection microbiology. 2021;11:618265
90. Zhu J, Liu W, Bian Z, Ma Y, Kang Z, Jin J. et al. Lactobacillus plantarum Zhang-LL Inhibits Colitis-Related Tumorigenesis by Regulating Arachidonic Acid Metabolism and CD22-Mediated B-Cell Receptor Regulation. Nutrients. 2023;15:4512
91. Xu C, Gu L, Hu L, Jiang C, Li Q, Sun L. et al. FADS1-arachidonic acid axis enhances arachidonic acid metabolism by altering intestinal microecology in colorectal cancer. Nature communications. 2023;14:2042
92. Wang X, Allen TD, Yang Y, Moore DR, Huycke MM. Cyclooxygenase-2 generates the endogenous mutagen trans-4-hydroxy-2-nonenal in Enterococcus faecalis-infected macrophages. Cancer prevention research (Philadelphia, Pa). 2013;6:206-216
93. Ferrara CR, Bai JDK, McNally EM, Putzel GG, Zhou XK, Wang H. et al. Microbes Contribute to Chemopreventive Efficacy, Intestinal Tumorigenesis, and the Metabolome. Cancer prevention research (Philadelphia, Pa). 2022;15:803-814
94. de Vries HS, te Morsche RH, van Oijen MG, Nagtegaal ID, Peters WH, de Jong DJ. The functional -765G→C polymorphism of the COX-2 gene may reduce the risk of developing crohn's disease. PloS one. 2010;5:e15011
95. Zhu W, Wei BB, Shan X, Liu P. -765G>C and 8473T>C polymorphisms of COX-2 and cancer risk: a meta-analysis based on 33 case-control studies. Molecular biology reports. 2010;37:277-288
96. Peng Q, Yang S, Lao X, Tang W, Chen Z, Lai H. et al. Meta-analysis of the association between COX-2 polymorphisms and risk of colorectal cancer based on case-control studies. PloS one. 2014;9:e94790
97. Gong Z, Bostick RM, Xie D, Hurley TG, Deng Z, Dixon DA. et al. Genetic polymorphisms in the cyclooxygenase-1 and cyclooxygenase-2 genes and risk of colorectal adenoma. International journal of colorectal disease. 2009;24:647-654
98. Kleinstein SE, Heath L, Makar KW, Poole EM, Seufert BL, Slattery ML. et al. Genetic variation in the lipoxygenase pathway and risk of colorectal neoplasia. Genes, chromosomes & cancer. 2013;52:437-449
99. Li S, Zhao X, Wu Z, Li Y, Zhu L, Cui B. et al. Polymorphisms in arachidonic acid metabolism-related genes and the risk and prognosis of colorectal cancer. Familial cancer. 2013;12:755-765
100. Sansbury LB, Millikan RC, Schroeder JC, North KE, Moorman PG, Keku TO. et al. COX-2 polymorphism, use of nonsteroidal anti-inflammatory drugs, and risk of colon cancer in African Americans (United States). Cancer causes & control: CCC. 2006;17:257-266
101. Fournier DB, Gordon GB. COX-2 and colon cancer: potential targets for chemoprevention. Journal of cellular biochemistry Supplement. 2000;34:97-102
102. Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E. et al. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer research. 2001;61:1733-1740
103. Roser C, Tóth C, Renner M, Herpel E, Schirmacher P. Expression of apoptosis repressor with caspase recruitment domain (ARC) in familial adenomatous polyposis (FAP) adenomas and its correlation with DNA mismatch repair proteins, p53, Bcl-2, COX-2 and beta-catenin. Cell communication and signaling: CCS. 2021;19:15
104. Li ZG, Wang XY, Chang JL, Xie WB, Liu TF, Zhang QL. et al. The establishment of supramolecular immunobead real-time PCR and the identification of Cox-2 as a metastasis-related marker in colorectal carcinoma. Oncology reports. 2012;28:977-984
105. Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang AV, Kraft P. et al. Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science (New York, NY). 2021;371:eabc8059
106. Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C. et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer research. 2000;60:4705-4708
107. Yarla NS, Pathuri G, Gali H, Terzyan S, Panneerselvam J, Chandrakesan P. et al. Discovery and Development of a Novel mPGES-1/5-LOX Dual Inhibitor LFA-9 for Prevention and Treatment of Chronic Inflammatory Diseases. Journal of inflammation research. 2020;13:1261-1278
108. Nakanishi M, Menoret A, Tanaka T, Miyamoto S, Montrose DC, Vella AT. et al. Selective PGE(2) suppression inhibits colon carcinogenesis and modifies local mucosal immunity. Cancer prevention research (Philadelphia, Pa). 2011;4:1198-1208
109. Elander N, Zhou J, Ungerbäck J, Dimberg J, Söderkvist P. Association between adenomatosis polyposis coli functional status and microsomal prostaglandin E synthase-1 expression in colorectal cancer. Molecular carcinogenesis. 2009;48:401-407
110. Nakanishi M, Perret C, Meuillet EJ, Rosenberg DW. Non-cell autonomous effects of targeting inducible PGE2 synthesis during inflammation-associated colon carcinogenesis. Carcinogenesis. 2015;36:478-486
111. Wen H, Li F, Bukhari I, Mi Y, Guo C, Liu B. et al. Comprehensive Analysis of Colorectal Cancer Immunity and Identification of Immune-Related Prognostic Targets. Disease markers. 2022;2022:7932655
112. Kraus S, Hummler S, Toriola AT, Poole EM, Scherer D, Kotzmann J. et al. Impact of genetic polymorphisms on adenoma recurrence and toxicity in a COX2 inhibitor (celecoxib) trial: results from a pilot study. Pharmacogenetics and genomics. 2013;23:428-437
113. Prasad VV, Padma K. Non-synonymous polymorphism (Gln261Arg) of 12-lipoxygenase in colorectal and thyroid cancers. Familial cancer. 2012;11:615-621
114. Bengtsson AM, Jönsson G, Magnusson C, Salim T, Axelsson C, Sjölander A. The cysteinyl leukotriene 2 receptor contributes to all-trans retinoic acid-induced differentiation of colon cancer cells. BMC cancer. 2013;13:336
115. Osman J, Savari S, Chandrashekar NK, Bellamkonda K, Douglas D, Sjölander A. Cysteinyl leukotriene receptor 1 facilitates tumorigenesis in a mouse model of colitis-associated colon cancer. Oncotarget. 2017;8:34773-34786
116. Wang W, Yang J, Edin ML, Wang Y, Luo Y, Wan D. et al. Targeted Metabolomics Identifies the Cytochrome P450 Monooxygenase Eicosanoid Pathway as a Novel Therapeutic Target of Colon Tumorigenesis. Cancer research. 2019;79:1822-1830
117. Kyoreva M, Li Y, Hoosenally M, Hardman-Smart J, Morrison K, Tosi I. et al. CYP1A1 Enzymatic Activity Influences Skin Inflammation Via Regulation of the AHR Pathway. The Journal of investigative dermatology. 2021;141:1553-1563.e1553
118. Zhu X, Wang Z, He J, Wang W, Xue W, Wang Y. et al. Associations between CYP1A1 rs1048943 A > G and rs4646903 T > C genetic variations and colorectal cancer risk: Proof from 26 case-control studies. Oncotarget. 2016;7:51365-51374
119. Sadeghi H, Nazemalhosseini-Mojarad E, Yaghoob-Taleghani M, Amin-Beidokhti M, Yassaee VR, Aghdaei HA. et al. miR-30a promoter variation contributes to the increased risk of colorectal cancer in an Iranian population. Journal of cellular biochemistry. 2019;120:7734-7740
120. Saraggi D, Fassan M, Mescoli C, Scarpa M, Valeri N, Michielan A. et al. The molecular landscape of colitis-associated carcinogenesis. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2017;49:326-330
121. Toyota M, Shen L, Ohe-Toyota M, Hamilton SR, Sinicrope FA, Issa JP. Aberrant methylation of the Cyclooxygenase 2 CpG island in colorectal tumors. Cancer research. 2000;60:4044-4048
122. Zuo X, Shen L, Issa JP, Moy O, Morris JS, Lippman SM. et al. 15-Lipoxygenase-1 transcriptional silencing by DNA methyltransferase-1 independently of DNA methylation. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:1981-1992
123. Ghatak S, Satapathy SR, Sjölander A. DNA Methylation and Gene Expression of the Cysteinyl Leukotriene Receptors as a Prognostic and Metastatic Factor for Colorectal Cancer Patients. International journal of molecular sciences. 2023 24
124. Patel SA, Bhambra U, Charalambous MP, David RM, Edwards RJ, Lightfoot T. et al. Interleukin-6 mediated upregulation of CYP1B1 and CYP2E1 in colorectal cancer involves DNA methylation, miR27b and STAT3. British journal of cancer. 2014;111:2287-2296
125. Taddese R, Roelofs R, Draper D, Wu X, Wu S, Swinkels DW. et al. Streptococcus gallolyticus Increases Expression and Activity of Aryl Hydrocarbon Receptor-Dependent CYP1 Biotransformation Capacity in Colorectal Epithelial Cells. Frontiers in cellular and infection microbiology. 2021;11:740704
126. Guo CG, Ma W, Drew DA, Cao Y, Nguyen LH, Joshi AD. et al. Aspirin Use and Risk of Colorectal Cancer Among Older Adults. JAMA oncology. 2021;7:428-435
127. Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best practice & research Clinical gastroenterology. 2010;24:121-132
128. Xu XT, Hu WT, Zhou JY, Tu Y. Celecoxib enhances the radiosensitivity of HCT116 cells in a COX-2 independent manner by up-regulating BCCIP. American journal of translational research. 2017;9:1088-1100
129. Orido T, Fujino H, Kawashima T, Murayama T. Decrease in uptake of arachidonic acid by indomethacin in LS174T human colon cancer cells; a novel cyclooxygenase-2-inhibition-independent effect. Archives of biochemistry and biophysics. 2010;494:78-85
130. Wong CH, Chang WL, Lu FJ, Liu YW, Peng JY, Chen CH. Parecoxib expresses anti-metastasis effect through inhibition of epithelial-mesenchymal transition and the Wnt/β-catenin signaling pathway in human colon cancer DLD-1 cell line. Environmental toxicology. 2022;37:2718-2727
131. Leathers TA, Rogers CD. Nonsteroidal anti-inflammatory drugs and implications for the cyclooxygenase pathway in embryonic development. American journal of physiology Cell physiology. 2023;324:C532-c539
132. Shaker AMM, Shahin MI, AboulMagd AM, Abdel Aleem SA, Abdel-Rahman HM, Abou El Ella DA. Novel 1,3-diaryl pyrazole derivatives bearing methylsulfonyl moiety: Design, synthesis, molecular docking and dynamics, with dual activities as anti-inflammatory and anticancer agents through selectively targeting COX-2. Bioorganic chemistry. 2022;129:106143
133. Jakobsson PJ, Thorén S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7220-7225
134. Kamei D, Murakami M, Nakatani Y, Ishikawa Y, Ishii T, Kudo I. Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis. The Journal of biological chemistry. 2003;278:19396-19405
135. Gounaris E, Heiferman MJ, Heiferman JR, Shrivastav M, Vitello D, Blatner NR. et al. Zileuton, 5-lipoxygenase inhibitor, acts as a chemopreventive agent in intestinal polyposis, by modulating polyp and systemic inflammation. PloS one. 2015;10:e0121402
136. Chang J, Jiang L, Wang Y, Yao B, Yang S, Zhang B. et al. 12/15 Lipoxygenase regulation of colorectal tumorigenesis is determined by the relative tumor levels of its metabolite 12-HETE and 13-HODE in animal models. Oncotarget. 2015;6:2879-2888
137. Higurashi T, Ashikari K, Tamura S, Saigusa Y, Takatsu T, Misawa N. et al. Leukotriene Receptor Antagonist Therapy for the Chemoprevention of Human Rectal Aberrant Crypt Foci: Nonrandomized, Open-Label, Controlled Trial. Cancer prevention research (Philadelphia, Pa). 2022;15:661-668
138. Chang J, Tang N, Fang Q, Zhu K, Liu L, Xiong X. et al. Inhibition of COX-2 and 5-LOX regulates the progression of colorectal cancer by promoting PTEN and suppressing PI3K/AKT pathway. Biochemical and biophysical research communications. 2019;517:1-7
139. Reisdorf WC, Xie Q, Zeng X, Xie W, Rajpal N, Hoang B. et al. Preclinical evaluation of EPHX2 inhibition as a novel treatment for inflammatory bowel disease. PloS one. 2019;14:e0215033
140. Mukhopadhyay N, Shukla A, Makhal PN, Kaki VR. Natural product-driven dual COX-LOX inhibitors: Overview of recent studies on the development of novel anti-inflammatory agents. Heliyon. 2023;9:e14569
141. Meshram MA, Bhise UO, Makhal PN, Kaki VR. Synthetically-tailored and nature-derived dual COX-2/5-LOX inhibitors: Structural aspects and SAR. European journal of medicinal chemistry. 2021;225:113804
142. Zhang W, Li H, Dong H, Liao J, Hammock BD, Yang GY. Soluble epoxide hydrolase deficiency inhibits dextran sulfate sodium-induced colitis and carcinogenesis in mice. Anticancer research. 2013;33:5261-5271
143. Norwood S, Liao J, Hammock BD, Yang GY. Epoxyeicosatrienoic acids and soluble epoxide hydrolase: potential therapeutic targets for inflammation and its induced carcinogenesis. American journal of translational research. 2010;2:447-457
144. Inceoglu B, Bettaieb A, Haj FG, Gomes AV, Hammock BD. Modulation of mitochondrial dysfunction and endoplasmic reticulum stress are key mechanisms for the wide-ranging actions of epoxy fatty acids and soluble epoxide hydrolase inhibitors. Prostaglandins & other lipid mediators. 2017;133:68-78
145. Wang F, Zhang H, Ma AH, Yu W, Zimmermann M, Yang J. et al. COX-2/sEH Dual Inhibitor PTUPB Potentiates the Antitumor Efficacy of Cisplatin. Molecular cancer therapeutics. 2018;17:474-483
146. Tian X, Liu K, Zu X, Ma F, Li Z, Lee M. et al. 3,3'-Diindolylmethane inhibits patient-derived xenograft colon tumor growth by targeting COX1/2 and ERK1/2. Cancer letters. 2019;448:20-30
147. Wan Y, Yang L, Li H, Ren H, Zhu K, Dong Z. et al. Zingiber officinale and Panax ginseng ameliorate ulcerative colitis in mice via modulating gut microbiota and its metabolites. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2022;1203:123313
148. Wang W, Chen H, Zhang W, Fan D, Deng J, Yang H. Ginsenoside Rk3 Ameliorates Obesity-Induced Colitis by Modulating Lipid Metabolism in C57BL/6 Mice. Journal of agricultural and food chemistry. 2024;72:2997-3007
149. Wu F, Lai S, Feng H, Liu J, Fu D, Wang C. et al. Protective Effects of Protopanaxatriol Saponins on Ulcerative Colitis in Mouse Based on UPLC-Q/TOF-MS Serum and Colon Metabolomics. Molecules (Basel, Switzerland). 2022 27
150. Wang Y, Shao Z, Song C, Zhou H, Zhao J, Zong K. et al. Clinopodium chinense Kuntze ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by reducing systematic inflammation and regulating metabolism. Journal of ethnopharmacology. 2023;309:116330
151. Qiu J, Xiao G, Yang M, Huang X, Cai D, Xie C. et al. Integrated network pharmacology and metabolomics reveal the mechanisms of Jasminum elongatum in anti-ulcerative colitis. Scientific reports. 2023;13:22449
152. Tao JH, Duan JA, Zhang W, Jiang S, Guo JM, Wei DD. Polysaccharides From Chrysanthemum morifolium Ramat Ameliorate Colitis Rats via Regulation of the Metabolic Profiling and NF-κ B/TLR4 and IL-6/JAK2/STAT3 Signaling Pathways. Frontiers in pharmacology. 2018;9:746
153. Naouar MS, Mekki LZ, Charfi L, Boubaker J, Filali A. Preventive and curative effect of Pistacia lentiscus oil in experimental colitis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2016;83:577-583
154. Abdallah HMI, Ammar NM, Abdelhameed MF, Gendy A, Ragab TIM, Abd-ElGawad AM. et al. Protective Mechanism of Acacia saligna Butanol Extract and Its Nano-Formulations against Ulcerative Colitis in Rats as Revealed via Biochemical and Metabolomic Assays. Biology. 2020;9:195
155. Li W, Zhang Y, Wang Q, Wang Y, Fan Y, Shang E. et al. 6-Gingerol ameliorates ulcerative colitis by inhibiting ferroptosis based on the integrative analysis of plasma metabolomics and network pharmacology. Food & function. 2024;15:6054-6067
156. Jeong CH, Bode AM, Pugliese A, Cho YY, Kim HG, Shim JH. et al. [6]-Gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer research. 2009;69:5584-5591
157. Bishayee A, Block K. A broad-spectrum integrative design for cancer prevention and therapy: The challenge ahead. Seminars in cancer biology. 2015;35(Suppl):S1-s4
158. Yang T, Qin N, Liu F, Zhao Y, Liu W, Fan D. Berberine regulates intestinal microbiome and metabolism homeostasis to treat ulcerative colitis. Life sciences. 2024;338:122385
159. Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H. et al. Berberine Inhibits Invasion and Metastasis of Colorectal Cancer Cells via COX-2/PGE2 Mediated JAK2/STAT3 Signaling Pathway. PloS one. 2015;10:e0123478
160. Chidambara Murthy KN, Jayaprakasha GK, Patil BS. The natural alkaloid berberine targets multiple pathways to induce cell death in cultured human colon cancer cells. European journal of pharmacology. 2012;688:14-21
161. Zhang Y, Pu W, Bousquenaud M, Cattin S, Zaric J, Sun LK. et al. Emodin Inhibits Inflammation, Carcinogenesis, and Cancer Progression in the AOM/DSS Model of Colitis-Associated Intestinal Tumorigenesis. Frontiers in oncology. 2020;10:564674
162. Kapral M, Wawszczyk J, Sośnicki S, Jesse K, Węglarz L. Modulating effect of inositol hexaphosphate on arachidonic acid-dependent pathways in colon cancer cells. Prostaglandins & other lipid mediators. 2017;131:41-48
163. Zhang H, Zhao X, Shang F, Sun H, Zheng X, Zhu J. Celastrol Inhibits the Proliferation and Induces Apoptosis of Colorectal Cancer Cells via Downregulating NF-κB/COX-2 Signaling Pathways. Anti-cancer agents in medicinal chemistry. 2022;22:1921-1932
164. Weber D, Wheat JM, Curri GM. Inflammation and cancer: tumor initiation, progression and metastasis, and Chinese botanical medicines. Zhong xi yi jie he xue bao = Journal of Chinese integrative medicine. 2010;8:1006-1013
165. Umesalma S, Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic & clinical pharmacology & toxicology. 2010;107:650-655
166. Tang FY, Pai MH, Kuo YH, Wang XD. Concomitant consumption of lycopene and fish oil inhibits tumor growth and progression in a mouse xenograft model of colon cancer. Molecular nutrition & food research. 2012;56:1520-1531
167. Wang L, Tao JH, Chen YF, Shen YM, Jiang S. Lizhong Decoction Ameliorates Ulcerative Colitis in Mice via Regulation of Plasma and Urine Metabolic Profiling. Chinese journal of integrative medicine. 2022;28:1015-1022
168. Zhou R, Huang K, Chen S, Wang M, Liu F, Liu F. et al. Zhilining Formula alleviates DSS-induced colitis through suppressing inflammation and gut barrier dysfunction via the AHR/NF-κBp65 axis. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2024;129:155571
169. Wang C, Yu H, Li Z, Wu J, Gao P, He S. et al. Novel applications of Yinhua Miyanling tablets in ulcerative colitis treatment based on metabolomics and network pharmacology. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2024;128:155366
170. Yuan Z, Yang L, Zhang X, Ji P, Hua Y, Wei Y. Mechanism of Huang-lian-Jie-du decoction and its effective fraction in alleviating acute ulcerative colitis in mice: Regulating arachidonic acid metabolism and glycerophospholipid metabolism. Journal of ethnopharmacology. 2020;259:112872
171. Yiqian J, Xibin Z, Wenyuan PU, Chunxiang Z. Sanwu Baisan decoction inhibits colorectal cancer progression in mice by remodeling gut microbiota and tumorigenesis. Journal of traditional Chinese medicine = Chung i tsa chih ying wen pan. 2023;43:466-473
Author contact
![]() Corresponding authors: Guang Ji: jiliversina.com, jgedu.cn. Institute of Digestive Diseases, Longhua Hospital, China-Canada Center of Research for Digestive Diseases (ccCRDD), Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, China. Hanchen Xu: hanson0702com, hcxuedu.cn. Institute of Digestive Diseases, Longhua Hospital, China-Canada Center of Research for Digestive Diseases (ccCRDD), Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, China.
Corresponding authors: Guang Ji: jiliversina.com, jgedu.cn. Institute of Digestive Diseases, Longhua Hospital, China-Canada Center of Research for Digestive Diseases (ccCRDD), Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, China. Hanchen Xu: hanson0702com, hcxuedu.cn. Institute of Digestive Diseases, Longhua Hospital, China-Canada Center of Research for Digestive Diseases (ccCRDD), Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, China.

 Global reach, higher impact
Global reach, higher impact