3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(14):4127-4138. doi:10.7150/jca.116790 This issue Cite
Research Paper
Development and validation of a nomogram to predict prognosis of patients with combined hepatocellular-cholangiocarcinoma after hepatic resection
1. Department of Hepatobiliary Pancreatic Tumor Center, Chongqing University Cancer Hospital, Chongqing, China.
2. Department of Organ Transplantation, The Second Affiliated Hospital of Nanchang University, Nanchang, 330038, Jiangxi, China.
3. Radiation Oncology Center, Chongqing University Cancer Hospital, School of Medicine, Chongqing University, Chongqing, China.
#These authors contributed equally to this work.
Received 2025-5-1; Accepted 2025-9-13; Published 2025-9-29
Abstract
Background: Combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CCA) is a rare primary liver cancer characterized by a low incidence but a poor prognosis. The purpose of the study was to develop a clinical prediction model utilizing non-invasive blood markers to effectively evaluate the prognosis of cHCC-CCA patients following hepatic resection.
Methods: The retrospective analysis was conducted on 125 patients with cHCC-CCA who underwent hepatic resection between April 2013 and October 2022. All cHCC-CCA patients were randomly assigned to the training group (n = 63) and the validation group (n =62). A nomogram based on patient clinical factors was established using cox regression analysis. Receiver operating characteristic curves (ROCs) were used to assess the predictive performance of the model. Calibration and decision curves were employed to evaluate the model's prediction accuracy and goodness of fit.
Results: Multivariate analysis revealed significant associations between lymphatic metastasis, microvascular invasion (MVI), gamma-glutamyl transpeptidase to albumin ratio (GAR), carcinoembryonic antigen (CEA), prothrombin time (PT), alpha-fetoprotein (AFP), hepatitis B virus (HBV), and overall survival. Based on these prognostic factors, a nomogram model was established and validated using the validation set. Calibration curves demonstrated good consistency in the 1-year, 3-year, and 5-year survival rates of patients. Additionally, the ROC analysis indicated the model's strong predictive ability, and the decision curves confirmed its clinical applicability.
Conclusion: This study successfully developed a nomogram model for predicting survival outcomes in patients with cHCC-CCA following hepatectomy.
Keywords: combined hepatocellular carcinoma and cholangiocarcinoma, gamma-glutamyl transpeptidase to albumin ratio, nomogram, prognosis
Introduction
Liver cancer as a significant global health burden, not only ranks as one of the most common malignancies, but also is the third leading cause of cancer-related mortality worldwide[1]. In 2018 alone, there were approximately 841,080 new cases and 781,631 deaths were attributed to liver cancer[2]. Among the primary liver cancer subtypes, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CCA) are the main entities[3]. cHCC-CCA, a composite neoplasm comprising elements of both HCC and CCA, accounts for 0.4% to 14.2% of primary liver cancer cases[4]. This unique subtype predominantly affects middle-aged and older males, with viral hepatitis being a major risk factor in Asian populations, while the etiology in Western populations is multifactorial[5]. cHCC-CCA is characterized by insidious onset, rapid progression, and early metastatic potential. Radical hepatectomy is currently the primary treatment approach for cHCC-CCA[6]. However, despite effective hepatectomy, many patients experience tumor recurrence, leading to a 5-year survival rate of less than 30%[7]. Enhancing the prognosis of cHCC-CCA patients remains a challenging clinical dilemma. Thus, developing a more accurate predictive model for survival outcomes following hepatic resection in cHCC-CCA patients would aid clinicians in formulating optimal treatment strategies to improve overall survival rates.
Clinical prediction models incorporating various prognostic factors have been successfully employed to predict outcomes in numerous malignancies, including liver cancer and colorectal cancer[8-10]. Notably, Tang et al established a prediction model based on radiomics to assess overall survival outcome in cHCC-CCA patients[11]. While Wu et al developed a recurrence prediction model utilizing clinical information[12]. Nevertheless, despite these notable contributions, further investigations are warranted to deepen our understanding of cHCC-CCA and address existing knowledge gaps.
In this study, we collected comprehensive clinical information, including tumor markers, blood parameters, and pathological features, from patients diagnosed with cHCC-CCA. Based on identified prognostic risk factors, we aimed to establish a prognostic model based on non-invasive blood markers to accurately evaluate the survival outcomes of patients with cHCC-CCA following hepatic resection.
Materials and Methods
Patients
A total of 125 patients who were diagnosed with cHCC-CCA and underwent hepatic resection at Chongqing University Cancer Hospital between April 2013 and October 2022 were adopted in the study. Only patients with complete follow-up information were considered. The cHCC-CCA patients were randomly assigned to the training group and the validation group at a 1:1 ratio. The inclusion criteria: (1) confirmation of cHCC-CCA through pathology after hepatectomy, and (2) availability of complete postoperative follow-up data. The exclusion criteria included: (1) incomplete follow-up information; (2) presence of other tumors or extrahepatic metastasis; (3) receipt of preoperative anticancer treatments such as chemotherapy, radiotherapy, or immunotherapy; and (4) presence of other chronic diseases, such as uncontrolled diabetes, chronic kidney disease (stage ≥3), congestive heart failure. Patients with cirrhosis were included in this study. The Ethics Committee of Chongqing University Cancer Hospital approved the study and all patients signed informed consent forms. The study was implemented in accordance with the Declaration of Helsinki.
Data collection and follow-up
All preoperative clinical information, including general patient characteristics, laboratory parameters, and tumor pathological features, was extracted from the hospital's electronic medical records. The collected data included gender, age, survival time, survival status, viral hepatitis infection, liver cirrhosis, ascites, tumor capsule status, blood loss, tumor number, microvascular invasion (MVI), tumor thrombus, satellite lesions, lymphatic metastasis, tumor size, alpha-fetoprotein (AFP) levels, Child-Pugh grade, American Joint Committee on Cancer (AJCC) stage, red blood cell (RBC) count, white blood cell (WBC) count, neutrophil count, lymphocyte count, hemoglobin (Hb) level, platelet count (PLT), macrophage count, total bilirubin (TBil) level, albumin (ALB) level, aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, carbohydrate antigen 19-9 (CA19-9) level, carcinoembryonic antigen (CEA) level, alkaline phosphatase (ALP) level, gamma-glutamyl transpeptidase (GGT) level, prothrombin time (PT), plasma fibrinogen (FIB) level, and gamma-glutamyl transpeptidase to albumin ratio (GAR). GAR was calculated by dividing the GGT count by the ALB count. Follow-up assessments were initiated 3 months after surgery and conducted at least twice a year during the first 2 years. The follow-up period ended in October 2022. Follow-up information included blood biochemical tests, abdominal dynamic enhanced computed tomography, magnetic resonance imaging, or PET-CT scans. Overall survival (OS) was recognized as the time from hepatectomy to death or the last follow-up in patients with cHCC-CCA.
Statistical analysis
All statistical analyses were implemented through R software (version 4.0.2). All cHCC-CCA patients were randomly allocated into the training group and the validation group at a ratio of approximately 1:1. Pearson's correlation coefficient was applied to analyze the correlation between variables, and a heatmap was generated. The Kaplan-Meier methods were employed to construct survival curves. The optimal cut-off values for continuous variables were determined using X-tile software, which identifies the best stratification threshold based on survival outcomes. The independent samples t-test was applied for normally distributed continuous variables, while non-normally distributed continuous data was analyzed by wilcoxon rank-sum test. The chi-square test was used for categorical variables. Cox regression models were utilized to analyze the prognostic risk factors. Variables with p value < 0.05 in the multivariate analysis were incorporated into the nomogram construction. Receiver operating characteristic curves (ROC) were adopted to assess the predictive performance of the model[13]. Finally, decision curves were employed to evaluate the clinical utility of the model[14]. P-value less than 0.05 was considered statistically significant.
Results
The best cut-off value for GAR
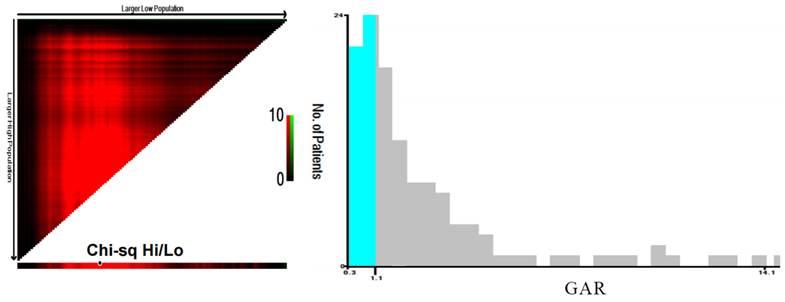
X-Tile software was utilized to analyze the optimal cut-off value for the continuous variable, GAR, and convert it into a binary variable. The optimal cut-off value for GAR was determined to be 1.14 (Figure 1).
Determination of the optimal cut-off value for the GAR using X-tile software. The X-tile analysis identified the optimal cut-off value of GAR that best stratifies patients with combined hepatocellular-cholangiocarcinoma (cHCC-CCA) after hepatic resection based on overall survival. The figure illustrates the best division point along with corresponding chi-square values and survival curves.
Basic patient information
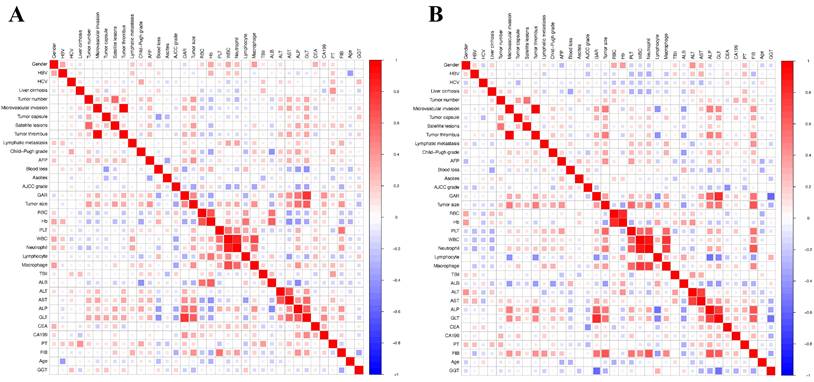
A total of 125 patients with cHCC-CCA who underwent radical hepatectomy and had complete follow-up data were included in the study. Of these patients, 105 patients were male. The majority of patients were over 50 years old, with an average age of 51.34 ± 10.73. Microvascular invasion (MVI) was observed in 42 patients (33.6%), while lymphatic metastasis was present in 16 patients (12.8%). Elevated AFP levels (> 400 ng/mL) were observed in 36 patients (28.8%). Among the patients, 81 patients were infected with HBV, while only 4 patients were infected with HCV. Cirrhosis was found in a significant proportion of patients (68%). Furthermore, 58 patients (46.4%) had two or more tumors. The division was based on a 1:1 ratio, resulting in 63 patients in the training group and 62 patients in the validation group. Baseline clinical features showed no significantly discrepancy between the two groups. The median overall survival was 20.6 months in the training group and 21.4 months in the validation group. Table 1 presented the basic patient characteristics. Additionally, spearman correlation analysis was conducted on the clinical information of patients in the two groups. Positive correlations were indicated by red, while negative correlations were denoted by blue (Figure 2).
Univariate analysis and multivariate cox regression
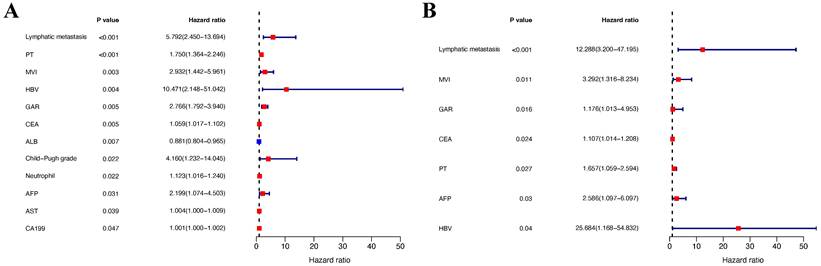
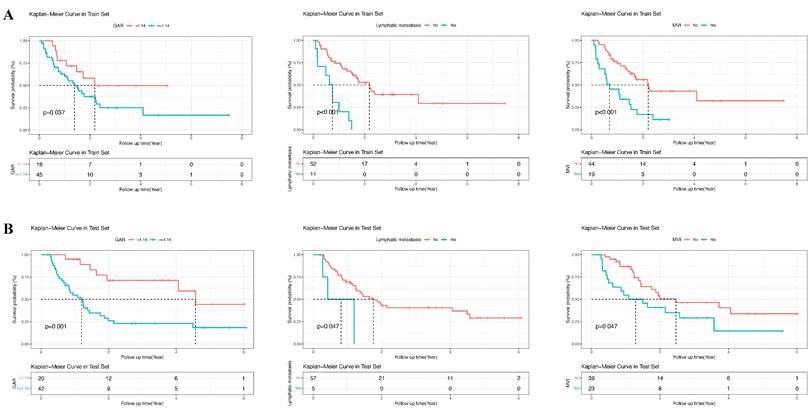
In the training group, univariate analysis was initially performed, followed by multivariate analysis of variables with a P-value < 0.05 to identify the prognostic risk factors. Univariate analysis revealed that lymphatic metastasis, PT, MVI, HBV, GAR, ALB, AST, Child-Pugh grade, neutrophils, CEA, AFP, and CA19-9 were significant risk factors. These variables were then included in the multivariate analysis, which demonstrated that lymphatic metastasis, MVI, GAR, PT, AFP, CEA, and HBV were independent prognostic risk factors for patients with cHCC-CCA (P-value < 0.05) (Table 2). The forest plots were used to depict the results (Figure 3). Additionally, survival curves were described for the binary variables of the independent risk factors, and the results displayed that the P-values for the three binary variables (MVI, lymphatic metastasis, and GAR) were all less than 0.05 (P-value < 0.05) (Figure 4).
Baseline characteristics of patients with cHCC-CCA in the training and validation groups.
| Variables | Total (n = 125) | Training (n = 63) | Validation (n = 62) | P-value |
|---|---|---|---|---|
| Gender, n (%) | 0.838 | |||
| Female | 20 (16) | 11 (17) | 9 (15) | |
| Male | 105 (84) | 52 (83) | 53 (85) | |
| HBV, n (%) | 0.903 | |||
| No | 44 (35) | 23 (37) | 21 (34) | |
| Yes | 81 (65) | 40 (63) | 41 (66) | |
| HCV, n (%) | 1 | |||
| No | 121 (97) | 61 (97) | 60 (97) | |
| Yes | 4 (3) | 2 (3) | 2 (3) | |
| Liver cirrhosis, n (%) | 1 | |||
| No | 40 (32) | 20 (32) | 20 (32) | |
| Yes | 85 (68) | 43 (68) | 42 (68) | |
| Tumor number, n (%) | 0.181 | |||
| Single tumor | 67 (54) | 38 (60) | 29 (47) | |
| Multiple tumors | 58 (46) | 25 (40) | 33 (53) | |
| Microvascular invasion;, n (%) | 0.528 | |||
| No | 83 (66) | 44 (70) | 39 (63) | |
| Yes | 42 (34) | 19 (30) | 23 (37) | |
| Tumor capsule, n (%) | 1 | |||
| No | 43 (34) | 22 (35) | 21 (34) | |
| Yes | 82 (66) | 41 (65) | 41 (66) | |
| Satellite lesions, n (%) | 0.122 | |||
| No | 78 (62) | 44 (70) | 34 (55) | |
| Yes | 47 (38) | 19 (30) | 28 (45) | |
| Lymphatic metastasis, n (%) | 0.192 | |||
| No | 109 (87) | 52 (83) | 57 (92) | |
| Yes | 16 (13) | 11 (17) | 5 (8) | |
| Child-Pugh grade, n (%) | 1 | |||
| A | 118 (94) | 59 (94) | 59 (95) | |
| B | 7 (6) | 4 (6) | 3 (5) | |
| AFP, n (%) | 1 | |||
| <400 | 89 (71) | 45 (71) | 44 (71) | |
| ≥400 | 36 (29) | 18 (29) | 18 (29) | |
| Ascites, n (%) | 0.888 | |||
| No | 89 (71) | 44 (70) | 45 (73) | |
| Yes | 36 (29) | 19 (30) | 17 (27) | |
| AJCC grade, n (%) | 0.73 | |||
| I | 14 (11) | 7 (11) | 7 (11) | |
| II | 21 (17) | 11 (17) | 10 (16) | |
| III | 90 (72) | 45 (71) | 45 (73) | |
| GAR, n (%) | 0.8 | |||
| <1.14 | 38 (30) | 18 (29) | 20 (32) | |
| ≥1.14 | 87 (70) | 45 (71) | 42 (68) | |
| Tumor size, Median (Q1,Q3) | 6 (4, 8) | 6.1 (4, 8.6) | 5.5 (3.82, 7.65) | 0.259 |
| Age, Mean ± SD | 51.34 ± 10.73 | 52.05 ± 10.25 | 50.63 ± 11.23 | 0.462 |
| RBC, Mean ± SD | 4.64 ± 0.47 | 4.67 ± 0.49 | 4.62 ± 0.45 | 0.576 |
| Hb, Mean ± SD | 140.98 ± 15.55 | 139.22 ± 16.61 | 142.76 ± 14.3 | 0.204 |
| PLT, Median (Q1,Q3) | 144 (94, 184) | 144 (99, 194) | 143 (92.5, 177.75) | 1 |
| WBC, Median (Q1,Q3) | 5.75 (4.73, 7.06) | 5.65 (4.74, 7.14) | 5.86 (4.74, 6.98) | 0.537 |
| Neutrophil, Median (Q1,Q3) | 3.5 (2.9, 4.62) | 3.51 (2.91, 4.62) | 3.49 (2.88, 4.61) | 0.838 |
| Lymphocyte, Median (Q1,Q3) | 1.36 (1.1, 1.81) | 1.23 (0.98, 1.81) | 1.42 (1.18, 1.8) | 0.098 |
| Macrophage, Median (Q1,Q3) | 0.41 (0.3, 0.51) | 0.37 (0.3, 0.51) | 0.43 (0.32, 0.5) | 0.305 |
| TBil, Median (Q1,Q3) | 13 (9.7, 17) | 13 (9.55, 16.75) | 12.95 (9.85, 16.85) | 0.634 |
| ALB, Median (Q1,Q3) | 42.3 (38.9, 44.3) | 42.3 (40.25, 44.85) | 42.2 (38.38, 43.9) | 0.392 |
| ALT, Median (Q1,Q3) | 34 (26, 48) | 34 (25.5, 49) | 34 (26.25, 48) | 0.795 |
| AST, Median (Q1,Q3) | 36 (28, 48) | 36 (31, 54) | 36 (27.25, 46.75) | 0.662 |
| GGT, Median (Q1,Q3) | 53 (44, 132) | 60.5 (73.5, 125) | 46 (28, 66.5) | 0.086 |
| ALP, Median (Q1,Q3) | 101 (75, 135) | 102 (77, 129.5) | 99 (72.25, 135.75) | 0.68 |
| CEA, Median (Q1,Q3) | 2.19 (1.52, 3.3) | 2.2 (1.51, 3.56) | 2.19 (1.56, 3.29) | 0.933 |
| CA19-9, Median (Q1,Q3) | 27.3 (13.98, 72.75) | 29.88 (15.29, 87.42) | 21.73 (11.33, 51.6) | 0.174 |
| PT, Median (Q1,Q3) | 11.9 (11.35, 12.8) | 11.95 (11.33, 12.88) | 11.9 (11.4, 12.8) | 0.887 |
| FIB, Median (Q1,Q3) | 3.12 (2.41, 3.9) | 3.2 (2.5, 3.89) | 2.98 (2.14, 4.3) | 0.379 |
| Median OS (months) | 21 | 20.6 | 21.4 | 0.82 |
Abbreviations: RBC, red blood cell; Hb, hemoglobin; PLT, platelets; WBC, white blood cell; TBil, total bilirubin; ALB, albumin; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time; FIB, fibrinogen; CA19-9, carbohydrate antigen 19-9; GAR, gamma-glutamyl transpeptidase to albumin ratio.
Heatmaps showing the correlation between clinical and laboratory variables. (A) Training cohort. (B) Validation cohort. Pearson correlation coefficients are represented using a color gradient, with red indicating a positive correlation and blue indicating a negative correlation. The heatmaps help visualize potential collinearity or associations among variables considered in the Cox regression model.
Forest plots of Cox proportional hazards regression analyses for overall survival in the training cohort. (A) Univariate analysis. (B) Multivariate analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) are shown for each variable. Significant prognostic factors identified in the univariate analysis were further included in the multivariate analysis to identify independent predictors of survival.
Univariate and multivariate cox regression analysis
| Characteristics | Category | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| AFP | <400 | Ref | Ref | Ref | Ref |
| ≥400 | 2.199 ( 1.074 - 4.503 ) | 0.031 | 2.586 ( 1.097 - 6.097 ) | 0.03 | |
| Ascites | No | Ref | Ref | ||
| Yes | 0.462 ( 0.199 - 1.07 ) | 0.072 | |||
| Tumor capsule | No | Ref | Ref | ||
| Yes | 0.992 ( 0.488 - 2.014 ) | 0.982 | |||
| Child-Pugh grade | A | Ref | Ref | Ref | Ref |
| B | 4.16 ( 1.232 - 14.045 ) | 0.022 | 0.12 ( 0.001 - 12.548 ) | 0.372 | |
| Gender | Female | Ref | Ref | ||
| Male | 1.458 ( 0.561 - 3.788 ) | 0.439 | |||
| GAR | <1.14 | Ref | Ref | Ref | Ref |
| ≥1.14 | 2.766 ( 1.792 - 3.94 ) | 0.005 | 1.176 ( 1.013 - 4.953 ) | 0.016 | |
| Blood loss | <500ml | Ref | Ref | ||
| ≥500ml | 1.372 ( 0.59 - 3.192 ) | 0.462 | |||
| HCV | No | Ref | Ref | ||
| Yes | 1.178 ( 0.566 - 2.451 ) | 0.662 | |||
| AJCC Grade | I | Ref | Ref | ||
| II | 1.248 ( 0.52 - 2.994 ) | 0.62 | |||
| III | 0.657 ( 0.259 - 1.662 ) | 0.375 | |||
| HBV | No | Ref | Ref | Ref | Ref |
| Yes | 10.471 ( 2.148 - 51.042 ) | 0.004 | 25.684 ( 1.168 - 54.832 ) | 0.04 | |
| Liver cirrhosis | No | Ref | Ref | ||
| Yes | 1.143 ( 0.511 - 2.556 ) | 0.745 | |||
| Lymphatic metastasis | No | Ref | Ref | Ref | Ref |
| Yes | 5.792 ( 2.45 - 13.694 ) | <0.001 | 12.288 ( 3.2 - 47.195 ) | <0.001 | |
| Microvascular invasion | No | Ref | Ref | Ref | Ref |
| Yes | 2.932 ( 1.442 - 5.961 ) | 0.003 | 3.292 ( 1.316 - 8.234 ) | 0.011 | |
| Satellite lesions | No | Ref | Ref | ||
| Yes | 1.849 ( 0.902 - 3.791 ) | 0.093 | |||
| Tumor thrombus | No | Ref | Ref | ||
| Yes | 1.32 ( 0.607 - 2.871 ) | 0.483 | |||
| Tumor number | Single tumor | Ref | Ref | ||
| Multiple tumors | 1.055 ( 0.515 - 2.161 ) | 0.884 | |||
| Tumor size | 1.047 ( 0.961 - 1.141 ) | 0.293 | |||
| RBC | 1.053 ( 0.476 - 2.33 ) | 0.899 | |||
| Hb | 0.994 ( 0.972 - 1.016 ) | 0.573 | |||
| PLT | 1.004 ( 0.999 - 1.009 ) | 0.155 | |||
| WBC | 1.103 ( 0.993 - 1.226 ) | 0.068 | |||
| Neutrophil | 1.123 ( 1.016 - 1.24 ) | 0.022 | 0.828 ( 0.69 - 0.994 ) | 0.043 | |
| Lymphocyte | 0.834 ( 0.492 - 1.414 ) | 0.501 | |||
| Macrophage | 1.647 ( 0.251 - 10.791 ) | 0.603 | |||
| TBil | 0.988 ( 0.922 - 1.057 ) | 0.72 | |||
| ALB | 0.881 ( 0.804 - 0.965 ) | 0.007 | 0.897 ( 0.771 - 1.043 ) | 0.157 | |
| ALT | 1.002 ( 1 - 1.003 ) | 0.072 | |||
| AST | 1.004 ( 1 - 1.009 ) | 0.039 | 1.005 ( 1 - 1.01 ) | 0.064 | |
| ALP | 1.002 ( 0.997 - 1.007 ) | 0.439 | |||
| GGT | 1.163 ( 0.997 - 1.392 ) | 0.083 | |||
| CEA | 1.059 ( 1.017 - 1.102 ) | 0.005 | 1.107 ( 1.014 - 1.208 ) | 0.024 | |
| CA19-9 | 1.001 ( 1 - 1.002 ) | 0.047 | 1.001 ( 0.997 - 1.003 ) | 0.112 | |
| PT | 1.75 ( 1.364 - 2.246 ) | <0.001 | 1.657 ( 1.059 - 2.594 ) | 0.027 | |
| FIB | 1.248 ( 0.899 - 1.731 ) | 0.185 | |||
Abbreviations: RBC, red blood cell; Hb, hemoglobin; PLT, platelets; WBC, white blood cell; TBil, total bilirubin; ALB, albumin; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time; FIB, fibrinogen; CA19-9, carbohydrate antigen 19-9; GAR, gamma-glutamyl transpeptidase to albumin ratio.
Kaplan-Meier survival curves for overall survival stratified by GAR-based risk groups. (A) Training cohort. (B) Validation cohort. Patients were divided into high-risk and low-risk groups according to the GAR cut-off value. The log-rank test was used to compare survival differences between the two groups, demonstrating the prognostic value of GAR.
Construction and validation of the nomogram
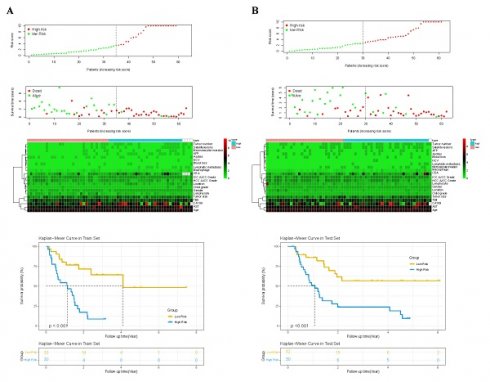
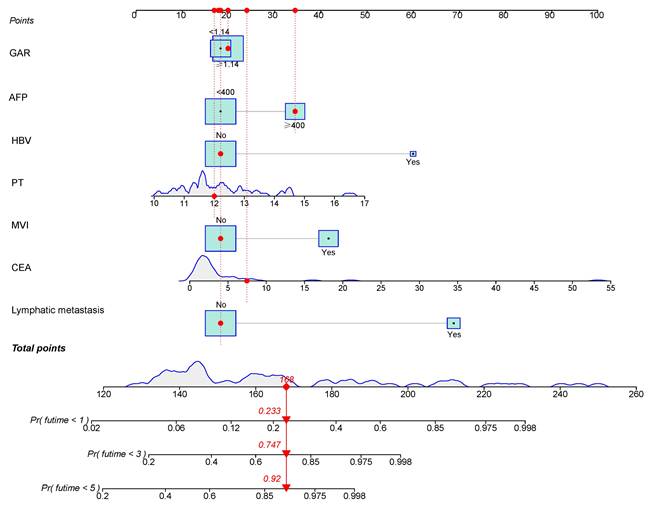
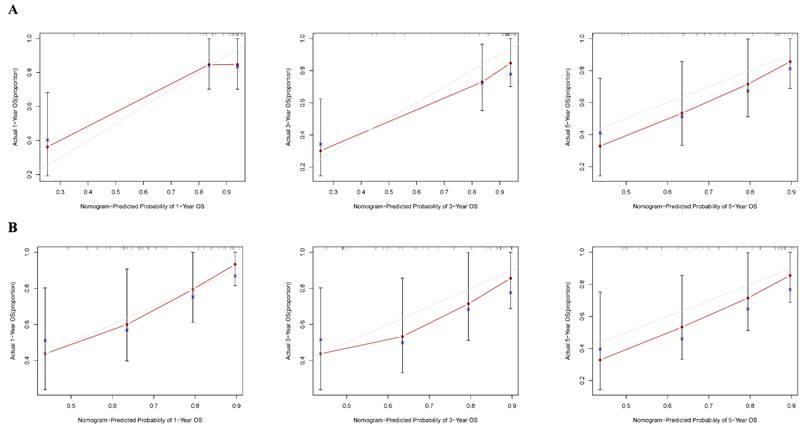
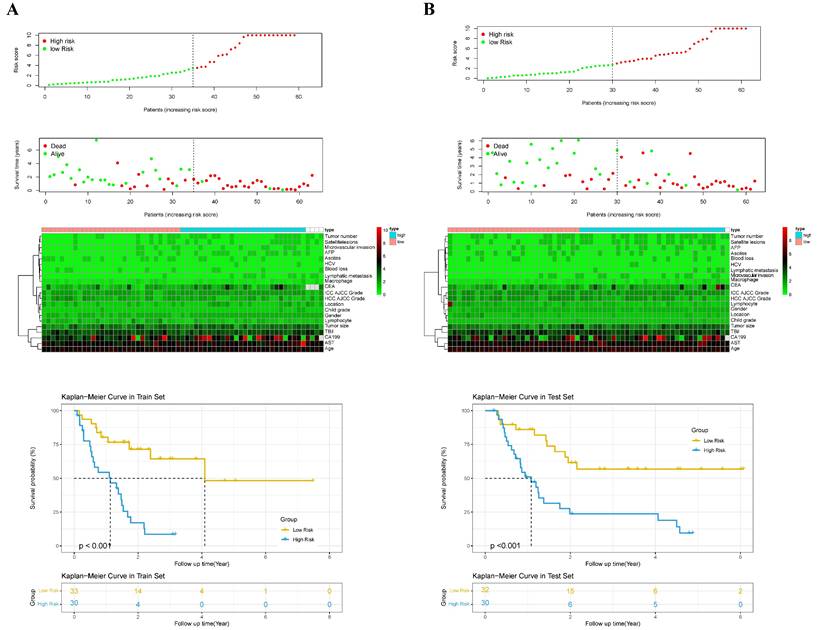
According to the independent prognostic factors, a prognostic model for overall survival (OS) in cHCC-CCA patients was constructed (Figure 5). The corresponding score for each prognostic index can be determined from the column graph. The sum of the eight scores yielded the total score, which corresponded to the predicted OS rates for patients at 1, 3, and 5 years. To further evaluate and validate the nomogram, calibration curves were plotted for 1, 3, and 5-year survival rates in both the training and validation groups. The results demonstrated that predicted survival rates were in good agreement with actual survival rates (Figure 6). Furthermore, prognostic risk maps, risk heat maps, and survival time plots were generated to analyze the prognostic risk of cHCC-CCA patients, revealing that patients in the high-risk group had more unfavorable prognostic outcome (Figure 7).
Predictive power and discrimination of the model
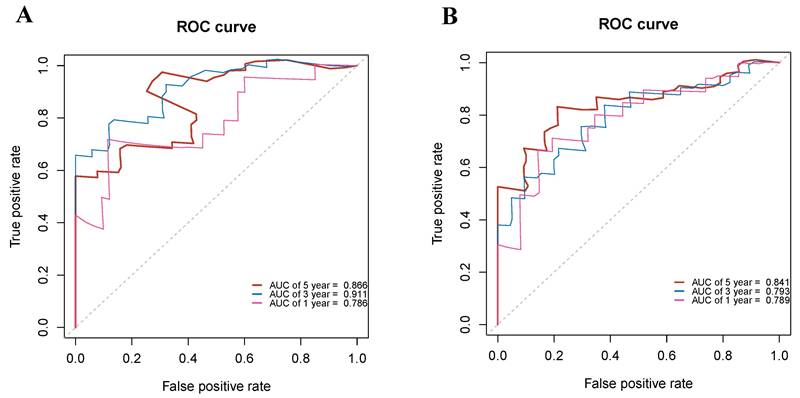
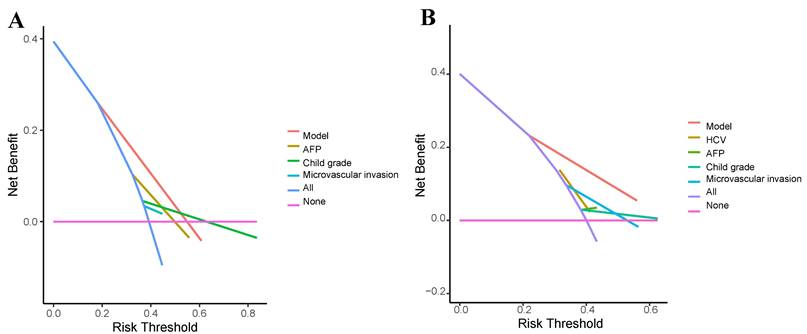
To analyze the accuracy of the prediction model, ROC curves were plotted for different survival times. In the training group, the areas under the ROC curve of 1, 3, and 5-year OS were 0.786, 0.911, and 0.866, respectively (Figure 8A). In the validation group, the p areas under the ROC curve of 1, 3, and 5-year OS were 0.789, 0.793, and 0.841, respectively (Figure 8B). Lastly, decision curves were generated in two groups, demonstrating that the prediction model had higher accuracy compared to individual clinical variables (Figure 9).
Discussion
Liver cancer is a significant global health concern, and cHCC-CCA is a subtype associated with limited research and clinical consensus regarding its clinical characteristics, treatment strategies, and prognosis. The low incidence of cHCC-CCA and varying classification criteria have contributed to the limited knowledge in this area. Previous studies have identified several factors, such as tumor differentiation, tumor size, CA19-9, AFP, and Child-Pugh score, as the prognostic risk factors in cHCC-CCA patients[15, 16]. However, the variables included in these studies were limited. In our study, we built a nomogram incorporating different variables to evaluate the prognostic outcome of patients with cHCC-CCA and assessed its performance in the training group. The calibration curves, ROC curves, and decision curves demonstrated the favorable prognostic predictive capability of the model.
cHCC-CCA is more commonly observed in men, and our study revealed that male patients accounted for 80% of the cohort[2]. In terms of viral etiology, 78% of patients were infected with HBV, while only 0.03% were infected with HCV. Cumulative studies have confirmed the important role of HBV infection in the progression of cHCC-CCA[5]. Regarding tumor markers, elevated AFP levels were observed in 28.8% of patients, which is lower than the previously reported 50%[17]. Furthermore, our study observed a higher proportion of patients with cirrhosis (68%) compared to previous studies, possibly due to the inclusion of predominantly HBV-infected patients[18].
Nomogram for predicting 1-, 3-, and 5-year overall survival in patients with cHCC-CCA after hepatic resection. The nomogram incorporates independent prognostic factors identified in multivariate Cox analysis. Each variable corresponds to a specific score, and the total score predicts individual survival probabilities at 1, 3, and 5 years.
Calibration curves assessing the predictive performance of the nomogram. (A) Training cohort. (B) Validation cohort. The calibration plots compare the predicted survival probabilities from the nomogram with the observed outcomes at 1, 3, and 5 years. The 45-degree dashed line indicates perfect prediction, and close alignment with this line indicates good model calibration.
Kaplan-Meier survival curves based on nomogram-derived risk scores. (A) Training cohort. (B) Validation cohort. Patients were stratified into high- and low-risk groups using the median risk score from the nomogram. The survival curves demonstrate significant survival differences between groups, indicating the discriminatory power of the risk model.
Receiver operating characteristic (ROC) curves evaluating the performance of the nomogram in predicting overall survival. (A) ROC curves for 1-, 3-, and 5-year survival in the training cohort. (B) ROC curves for 1-, 3-, and 5-year survival in the validation cohort. The area under the curve (AUC) values reflects the predictive accuracy of the nomogram at each time point.
Decision curve analysis (DCA) for the clinical utility of the nomogram. (A) Training cohort. (B) Validation cohort. DCA evaluates the net clinical benefit of using the nomogram across a range of threshold probabilities, comparing it to default strategies (treat-all or treat-none). The higher net benefit across relevant thresholds indicates the model's potential utility in clinical decision-making.
Through univariate and multivariate analyses, we identified lymphatic metastasis, PT, MVI, HBV, GAR, ALB, AST, Child-Pugh grade, CEA, AFP, and CA19-9 as independent prognostic risk factors for patients with cHCC-CCA. The findings were consistent with previous researches that have implicated lymph node metastasis, MVI, CEA, and AFP in the prognosis of cHCC-CCA[19, 20].
Radical resection remains the primary treatment modality for cHCC-CCA patients. Previous studies have shown that radical resection is correlated with significantly improved survival outcome compared to palliative resection or non-surgical treatment[21]. In our study, the median survival time after radical resection was 21 months, which surpassed the mean survival time reported in previous research. Due to the similarities of cHCC-CCA with HCC in respect of portal and hepatic vein invasion and similarities with ICC in lymph node metastasis, radical resection, negative tumor margin and lymph node dissection are essential for achieving better outcomes[22].
The prognostic significance of GAR, a composite marker consisting of GGT and ALB, was demonstrated in our study. GAR has been previously used as a stratification tool in chronic hepatitis B patients and has emerged as a prognostic risk factor in HCC, ICC, and pancreatic cancer patients following radical resection[23-25]. High GGT levels are related to increased tumor cell proliferation, invasion, and metastasis due to elevated reactive oxygen species and oxidative stress[26]. Serum GGT levels have also been proposed as prognostic indicators in various cancers[27]. On the other hand, ALB, produced by the liver, reflects nutritional status and liver function. Hypoalbuminemia is associated with immune cell dysfunction and immune evasion, leading to poorer survival outcomes in cancer patients[28]. GAR provides a simple and effective means of evaluating the prognosis of tumor patients, reflecting both malnutrition and inflammation. Lymphocyte-to-CRP ratio is a promising indicator and has been proven to have potential prognostic value in HCC and ICC[29]. Lymphocyte-to-CRP ratio should be considered in future comparative or integrative prognostic models.
Additionally, we identified PT as an independent prognostic risk indicator for cHCC-CCA patients after hepatectomy. Prolonged PT has been associated with adverse overall survival in HCC, increased recurrence risk in colorectal cancer, and poor prognosis in cholangiocarcinoma patients[30, 31].
The study had a few disadvantages. It was a single-center retrospective study, which may introduce bias. Furthermore, the insufficient sample size may limit the generalizability and reliability of the findings. Additionally, our study predominantly included cHCC-CCA patients with HBV-infected, and further validation was needed to assess the applicability of the model for non-HBV-infected cHCC-CCA patients.
Conclusions
We successfully developed a nomogram incorporating clinical risk factors to evaluate the overall survival outcomes of cHCC-CCA patients after hepatic resection. This prognostic nomogram provides a practical tool for stratifying cHCC-CCA patients into different risk categories based on readily available clinical and pathological parameters. In clinical settings, it can assist surgeons and oncologists in preoperative counseling, postoperative surveillance planning, and individualized decision-making for adjuvant therapies. For example, patients identified as high risk by the model may benefit from more intensive follow-up or consideration for early adjuvant treatments.
Abbreviations
cHCC-CCA: combined hepatocellular carcinoma and cholangiocarcinoma; ROC: Receiver operating characteristic curves; MVI: microvascular invasion; GAR: gamma-glutamyl transpeptidase to albumin ratio; CEA: carcinoembryonic antigen; PT: prothrombin time; AFP: alpha-fetoprotein; HBV: hepatitis B virus; AJCC: American Joint Committee on Cancer; RBC: red blood cell; WBC: white blood cell; Hb: hemoglobin; PLT: platelet; TBil: total bilirubin; ALB: albumin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CA19-9: carbohydrate antigen 19-9; ALP: alkaline phosphatase; GGT: gamma-glutamyl transpeptidase; FIB: plasma fibrinogen; OS: overall survival.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82203823), the China Postdoctoral Science Foundation (2023M730436, 2022TQ0393), and the Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0477, CSTB2024NSCQ-QCXMX0051, CSTB2022NSCQ-MSX1174), and Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau) (2024ZDXM008).
Data availability statement
All the data have been included in this study. Further information is available from the corresponding author upon request.
Author contributions
Conception and design: RQL, HL and DWL. Development of methodology: RQL and HL. Analysis and interpretation of data: RQL, YW, XXL and HL. Writing, review, and revision of the manuscript: RQL, SW, YSD and DWL. Administrative, technical, or material support: TW, LX, and WYX. Study supervision: HL and DWL. All authors approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021;71:209-49
2. Schizas D, Mastoraki A, Routsi E, Papapanou M, Tsapralis D, Vassiliu P. et al. Combined hepatocellular-cholangiocarcinoma: An update on epidemiology, classification, diagnosis and management. Hepatobiliary & pancreatic diseases international: HBPD INT. 2020;19:515-23
3. Li DB, Si XY, Wang SJ, Zhou YM. Long-term outcomes of combined hepatocellular-cholangiocarcinoma after hepatectomy or liver transplantation: A systematic review and meta-analysis. Hepatobiliary & pancreatic diseases international: HBPD INT. 2019;18:12-8
4. Wang AQ, Zheng YC, Du J, Zhu CP, Huang HC, Wang SS. et al. Combined hepatocellular cholangiocarcinoma: Controversies to be addressed. World journal of gastroenterology. 2016;22:4459-65
5. Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: An update. Journal of hepatology. 2021;74:1212-24
6. Calderaro J, Ghaffari Laleh N, Zeng Q, Maille P, Favre L, Pujals A. et al. Deep learning-based phenotyping reclassifies combined hepatocellular-cholangiocarcinoma. Nature communications. 2023;14:8290
7. Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H. et al. Mixed hepatocellular cholangiocarcinoma tumors: Cholangiolocellular carcinoma is a distinct molecular entity. Journal of hepatology. 2017;66:952-61
8. Liu Z, Xu Y, Xu G, Baklaushev VP, Chekhonin VP, Peltzer K. et al. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC gastroenterology. 2021;21:103
9. Wang S, Cheng L, Dou L, Kuang Y, Huang Y, Wen T. et al. Geriatric nutritional risk index and body composition dictate the prognosis of elderly patients with intrahepatic cholangiocarcinoma. Frontiers in nutrition. 2025;12:1565317
10. Gu YG, Xue HY, Ma ES, Jiang SR, Li JH, Wang ZX. A novel nomogram to predict the recurrence of hepatocellular carcinoma after liver transplantation using extended selection criteria. Hepatobiliary & pancreatic diseases international: HBPD INT. 2025;24:252-60
11. Tang YY, Zhao YN, Zhang T, Chen ZY, Ma XL. Comprehensive radiomics nomogram for predicting survival of patients with combined hepatocellular carcinoma and cholangiocarcinoma. World journal of gastroenterology. 2021;27:7173-89
12. Wu Y, Liu H, Zeng J, Chen Y, Fang G, Zhang J. et al. Development and validation of nomogram to predict very early recurrence of combined hepatocellular-cholangiocarcinoma after hepatic resection: a multi-institutional study. World journal of surgical oncology. 2022;20:60
13. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. European heart journal. 2014;35:1925-31
14. Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ. et al. Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. European urology. 2018;74:796-804
15. Yamashita YI, Aishima S, Nakao Y, Yoshizumi T, Nagano H, Kuroki T. et al. Clinicopathological characteristics of combined hepatocellular cholangiocarcinoma from the viewpoint of patient prognosis after hepatic resection: High rate of early recurrence and its predictors. Hepatology research: the official journal of the Japan Society of Hepatology. 2020;50:863-70
16. Zhang G, Chen BW, Yang XB, Wang HY, Yang X, Xie FC. et al. Prognostic analysis of patients with combined hepatocellular-cholangiocarcinoma after radical resection: A retrospective multicenter cohort study. World journal of gastroenterology. 2022;28:5968-81
17. Chu KJ, Lu CD, Dong H, Fu XH, Zhang HW, Yao XP. Hepatitis B virus-related combined hepatocellular-cholangiocarcinoma: clinicopathological and prognostic analysis of 390 cases. European journal of gastroenterology & hepatology. 2014;26:192-9
18. Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC. et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. American journal of surgery. 2005;189:120-5
19. Hou GM, Liu HL, Wu H, Zeng Y. Prediction of Prognosis for cHCC-CC Patients After Surgery: Comparison of Tumor Marker Score Based on AFP, CEA, CA19-9, and Other Clinical Stages. Annals of surgical oncology. 2021;28:7647-60
20. Yin X, Zhang BH, Qiu SJ, Ren ZG, Zhou J, Chen XH. et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Annals of surgical oncology. 2012;19:2869-76
21. Kim SH, Park YN, Lim JH, Choi GH, Choi JS, Kim KS. Characteristics of combined hepatocelluar-cholangiocarcinoma and comparison with intrahepatic cholangiocarcinoma. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2014;40:976-81
22. Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. International journal of clinical practice. 2008;62:1271-8
23. Li Q, Lu C, Li W, Huang Y, Chen L. The gamma-glutamyl transpeptidase-to-albumin ratio predicts significant fibrosis and cirrhosis in chronic hepatitis B patients. Journal of viral hepatitis. 2017;24:1143-50
24. Li H, Liu R, Li J, Li J, Wu H, Wang G. et al. Prognostic significance of gamma-glutamyl transpeptidase to albumin ratio in patients with intrahepatic cholangiocarcinoma after hepatectomy. Journal of cellular and molecular medicine. 2022;26:3196-202
25. Li S, Xu H, Wu C, Wang W, Jin W, Gao H. et al. Prognostic value of γ-glutamyltransferase-to-albumin ratio in patients with pancreatic ductal adenocarcinoma following radical surgery. Cancer medicine. 2019;8:572-84
26. Xia J, Song P, Sun Z, Sawakami T, Jia M, Wang Z. Advances of diagnostic and mechanistic studies of γ-glutamyl transpeptidase in hepatocellular carcinoma. Drug discoveries & therapeutics. 2016;10:181-7
27. Kunutsor SK, Apekey TA, Van Hemelrijck M, Calori G, Perseghin G. Gamma glutamyltransferase, alanine aminotransferase and risk of cancer: systematic review and meta-analysis. International journal of cancer. 2015;136:1162-70
28. McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutrition and cancer. 2001;39:210-3
29. Iseda N, Itoh S, Yoshizumi T, Tomiyama T, Morinaga A, Shimagaki T. et al. Lymphocyte-to-C-reactive protein ratio as a prognostic factor for hepatocellular carcinoma. International journal of clinical oncology. 2021;26:1890-900
30. Wang XP, Mao MJ, He ZL, Zhang L, Chi PD, Su JR. et al. A retrospective discussion of the prognostic value of combining prothrombin time(PT) and fibrinogen(Fbg) in patients with Hepatocellular carcinoma. Journal of Cancer. 2017;8:2079-87
31. Wang HS, Ge XX, Li QP, Nie JJ, Miao L. Clinical Significance of Prothrombin Time in Cholangiocarcinoma Patients with Surgeries. Canadian journal of gastroenterology & hepatology. 2019;2019:3413969
Author contact
![]() Corresponding authors: Dewei Li, Email: lidewei406com; Hui Li, Email: lihedu.cn.
Corresponding authors: Dewei Li, Email: lidewei406com; Hui Li, Email: lihedu.cn.

 Global reach, higher impact
Global reach, higher impact