Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(14):4081-4098. doi:10.7150/jca.114837 This issue Cite
Review
From Herb to Hope: A Systematic Exploration of Medicinal Plants' Role in Cancer Therapy
1. King Saud Bin Abdulaziz University for Health Sciences (KSAU-HS), Riyadh, Saudi Arabia.
2. King Abdullah International Medical Research Center (KAIMRC), Riyadh, Saudi Arabia.
3. Ministry of National Guard Health Affairs (MNGHA), Riyadh, Saudi Arabia.
4. Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
5. Microbiology and Immunology Unit, Natural and Health Sciences Research Center, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Received 2025-4-1; Accepted 2025-7-8; Published 2025-9-27
Abstract
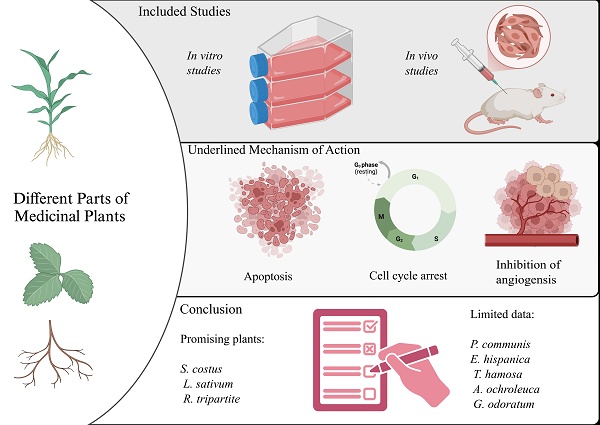
Medicinal plants play a critical role in drug development, serving as a valuable source of bioactive compounds. Cancer, characterized by uncontrolled cell proliferation, presents significant challenges in treatment due to its multifaceted nature. This study aims to evaluate the anticancer potentials of selected medicinal plants specifically focusing on in vitro and in vivo studies that evaluate therapeutic implications for cancer treatment. A systematic review was conducted to assess both in vitro and in vivo studies involving selected medicinal plants: Saussurea costus, Lepidium sativum, Rhus tripartite, Pyrus communis, Chenopodium murale, Erucaria hispanica, Trigonella hamosa, Argemone ochroleuca, and Galium odoratum. The review involved analyzing cancer cell lines, plant parts used, extraction methods, and mechanisms of action reported in the literature. A total of sixty-nine articles were identified that investigated the anticancer properties of the selected plants. Notably, S. costus, L. sativum, and R. tripartite exhibited significant anticancer potential. In contrast, P. communis, C. murale, E. hispanica, T. hamosa, A. ochroleuca, and G.odoratum had limited studies available. The predominant mechanism of action identified for the anticancer activity was the induction of apoptosis. The findings indicate that these medicinal herbs possess promising therapeutic potential as anti-cancer agents. However, further research is warranted for P. communis, C. murale, E. hispanica, T. hamosa, A. ochroleuca, and G. odoratum to enhance understanding of their anticancer activities and explore their full therapeutic capabilities.
Keywords: Saussurea costus, Lepidium sativum, Rhus tripartite, Pyrus communis, Chenopodium murale, Erucaria hispanica, Trigonella hamosa, Argemone ochroleuca, Galium odoratum, anticancer, in vitro, in vivo
1. Introduction
Medicinal plants have played an important role in the discovery of innovative treatments for a variety of diseases. Compounds derived from many herbal plants have demonstrated important therapeutic effects on human pathologies [1]. As a result, medicinal plants are increasingly recognized as a potential source for identifying candidate drugs, particularly in the search for cancer therapies and in efforts to minimize cancer cell resistance to treatment.
Cancer is one of the diseases that has been an obstacle in the scientific and medical fields due to its complex biological nature. The use of plants as potential cancer therapies has been a subject of interest throughout history [2]. Globally, approximately 20 million new cancer cases and 9 million cancer-related deaths were reported in 2022 [3]. The treatment of these diseases is becoming more challenging due to resistance to current treatments. In addition to resistance, the side effects of the cancer therapies impact the patient's quality of life. Thus, it is a global concern, as it can hinder treatment and has an impact on the prognosis of the disease, prompting researchers to pursue novel pharmacological solutions.
In recent years, there has been an ongoing quest for effective drugs as a preventive therapies for cancer, and to overcome the resistance issue [4]. In this context, medicinal plants are being studied as possible anticancer treatments. Recent findings on medicinal plants (Saussurea costus, Lepidium sativum, Rhus tripartite, Pyrus communis, Chenopodium murale, Erucaria hispanica, Trigonella hamosa, Argemone ochroleuca, and Galium odoratum) have increased the scientific interest in their bioactive compounds, particularly anticancer properties [5-13]. Traditionally, these plants have been used for a variety of purposes, including antimicrobial, antioxidant, and anticancer applications. They contain a variety of secondary metabolites, such as polyphenols, flavonoids, steroids, which enhance their ability to induce the cancer cell death via different mechanisms.
The main aim of this systematic review is to assemble all current studies that explore and analyze the anticancer activity of the extracted parts of the selected medicinal plants in both in vitro and in vivo models.
2. Methods
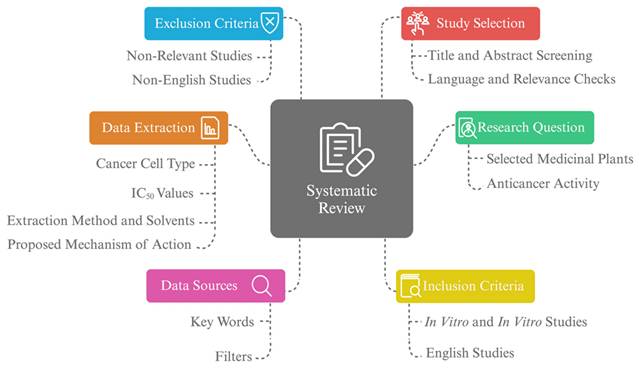
2.1. Research questions
This systematic review focuses on the analysis of the anticancer activity for the extracts or isolated compounds of selected plants (Saussurea costus, Lepidium sativum, Rhus tripartite, Pyrus communis, Chenopodium murale, Erucaria hispanica, Trigonella hamosa, Argemone ochroleuca, and Galium odoratum) and their effects on in vitro and in vivo models, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We included studies published within the past 24 years (2000-2024) to ensure adequate representation of historical and recent research findings regarding the selected medicinal plants (Figure 1).
2.2. Data sources
Google Scholar was utilized as a database to identify the studies that investigate the anticancer properties of the selected medicinal plants. As a search strategy, to conduct a comprehensive search, we utilized certain keywords and specific phrases, it involves “Saussurea costus" AND ("anticancer" OR "cytotoxic") AND ("in vitro" OR "in vivo"), " Lepidium sativum" AND ("anticancer" OR "cytotoxic") AND ("in vitro" OR "in vivo"), and so forth for each plant. The search is limited by the filter to include the articles that are published between 01/01/2000 to 01/08/2024 in English.
2.3. Inclusion criteria
Selected articles were required to investigate the potential anticancer effects for the chosen medicinal plants using in vitro or in vivo models, with plant extracts or isolated compounds tested against cancer cell lines or tumor-induced animal models.
2.4. Exclusion criteria
Articles were excluded if they did not evaluate anticancer activity or did not involve cancer cell lines or tumor models. Additionally, non-English articles, clinical trials, and non-original studies (e.g., systematic reviews or meta-analysis) were excluded.
The systematic review approach and scope. A systematic review focused on the anticancer activity of selected medicinal plants, including original studies such as in vitro and in vivo. Data selection was based on the study's title, language, and relevance objectives, with data sources determined by keywords and filters. Data extracted includes cancer cell type, extraction methods, IC50 values, and proposed mechanism of action.
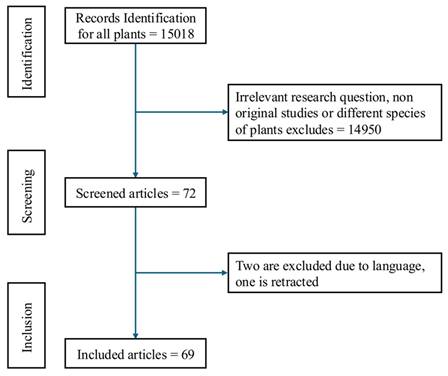
Flow chart illustrating the study selection process: A total of 15,018 records were identified, of which 14,950 were excluded due to irrelevant research questions or different plant species. After screening 72 records, 2 were excluded due to language barriers and one was retracted resulting in 69 studies included in the final analysis.
2.5. Study selection
The screening of the preselected articles, using the described search strategy, was conducted by a single author and initially based on title and abstract review (Figure 2). Duplicates, studies irrelevant to the research question, and those investigating different plant species were excluded. Subsequently, the studies that are written with different languages other than English were eliminated. The final selection of the eligible studies was carried out by a single author.
2.6. Data extraction
Data extraction was carried out by a single author for all included articles. The extracted information encompassed several key aspects: the type of cancer cells treated with the plant extracts, the specific plant name, the part of the plant utilized in the study, the extraction methods and solvents employed by researchers, and the results, including values for IC50. Additionally, any proposed mechanisms of action suggested by the studies were recorded.
3. Results
To assess their anticancer activity and mechanism of action, a comprehensive review of in vitro and in vivo studies on nine plants— Saussurea costus, Lepidium sativum, Rhus tripartite, Pyrus communis, Chenopodium murale, Erucaria hispanica, Trigonella hamosa, Argemone ochroleuca, and Galium odoratum —was conducted. The selected medicinal plants have been investigated for their cytotoxic potential against a variety of cancers, including acute myeloid leukemia, breast cancer, lung cancer, colon cancer, tongue squamous cell carcinoma, colorectal cancer, liver cancer, prostate cancer, melanoma, cervical cancer, neuroblastoma, adenocarcinoma, ovarian cancer, esophageal cancer, gastric cancer, and skin cancer. Following a thorough examination for sixty-one in vitro and in vivo studies, the findings revealed that the S. costus, L. sativum, R. tripartite, C. murale, P. communis, E. hispanica, T. hamosa were investigated for their cytotoxic activity. No studies were found evaluating the anticancer properties of A. ochroleuca and G. odoratums' anticancer properties (Table 1).
Comprehensive overview of anticancer studies on selected medicinal plants.
| Plant Name | Number of Studies Identified | Types of Experimental Approach |
|---|---|---|
| S. costus | Forty studies | In vitro, and in vivo |
| L. sativum | Fifteen studies | In vitro, and in vivo |
| R. tripartite | Four studies | In vitro |
| P. communis | Two studies | In vitro |
| C. murale | One study | In vitro |
| E. hispanica | One study | In vitro |
| T. hamosa | One study | In vitro |
| A. ochroleuca | No study | Not applicable |
| G. odoratum's | No study | Not applicable |
*No study: No reported studies available for these plants.
The proposed mechanism of action is mostly related to generation of reactive oxygen species (ROS) and induction of apoptosis.
3.1. Saussurea costus
S. costus was the most extensively studied plant across diverse types of cancer, including breast cancer (MCF-7, MDA-MB-231, SK-BR-3, MDA-MB-453), colon cancer (HTC116, Caco2, LS174T, HT-15, HT-29), liver cancer (HepG-2, HuH-7, PLC/PRF/5, SMMC-7721), lung cancer (A549, SK-MES-1), gastric cancer (AGS), central nervous system (CNS) cancer (XF498, IMR-32, SH-SY5Y, Rat B103), prostate cancer (PC-3, LNCaP, DU145), leukemia (HL-60, Jurkat E6-1, THP-1), cervical cancer (HeLa), esophageal cancer (Eca109, KYSE150), ovarian cancer (SK-OV-3, OVCAR3), soft tissue sarcoma (SW-872, SW-982, TE-671) [14-33].
In vitro studies utilized different parts of S. costus; however; root was the most utilized part for the most cancer types, while a few studies tested leaves and fruits of S. costus for breast (MCF-7), colon (CaCo-2), liver cancer (HepG2) [22,25,29,34-38]. Other studies investigated isolated compounds, namely costunolide and dehydrocostuslactone [14-16,18,20,21,32,39-41].
Breast cancer was the most studied cancer type, with MCF-7 as the predominant cell line, in addition to MDA-MB-231, SK-BR-3, and MDA-MB-453, demonstrating the versatility of extracts and solvents. In a study conducted by Peng et al. (2013), the roots were extracted using liquid-liquid extraction with methanol and ethyl acetate and tested on MCF-7 cell lines, revealing IC50 values ranging from 1.7 to 6.1 μg/mL[28]. Similar findings were reported by Bhushan et al. (2023), where liquid-liquid extraction was applied to root extracts and tested on the MDA-MB-231 cell line using additional solvents such as hexane, chloroform, ethanol, and butanol[37]. A similar trend was observed, where hexane and chloroform showed IC50 values of 5.3-12.18 μg/mL, whereas ethanol and butanol exhibited higher IC50 values, ranging from 20 to >100 μg/mL. Comparable results were observed for other plant parts, including leaves and fruits, as summarized in Table 2.
Comprehensive analysis of in vitro anticancer studies on Saussurea costus: cell lines, extracts, and mechanisms of action.
| References | Results Description | Proposed Mechanisms | IC50 | Extraction Method | Part Used | Cancer Type |
|---|---|---|---|---|---|---|
| Breast Cancer | ||||||
| [34] | Decrease the cellular proliferation and control the cancer invasiveness | Inhibition of NF-κB and MMP-9 | 10 μg/mL | Ethanol by maceration liquid-liquid extraction | Roots | MCF-7 |
| [44] | Decrease cellular proliferation | Apoptosis regulations | 20 μg/mL | Ethanol | ||
| [43] | Decrease cellular proliferation | Apoptosis by induction of ROS | 80 μg/mL | Methanol by soxhlet extraction + MgO nanoparticles | ||
| [78] | Decrease cellular proliferation | Apoptosis regulations and cell cycle effects | 122.5 μg/mL | Methanol by maceration | ||
| [28] | Decrease cellular proliferation | Not determined | 1.7-6.1 µg/mL | Water, Methanol by sonication, Ethyl Acetate by liquid-liquid extraction | ||
| [30] | Decrease cellular proliferation | Apoptosis regulations and caspase activity | 0.54 - 25.5 µg/mL | Methanol, Hexane, Chloroform, Ethyl Acetate, n-Butanol by liquid-liquid extraction | Leaves | |
| [84] | Decrease cellular proliferation, | Inhibition of NF-κB and MMP-7 | 0.5 mg/mL | Aqueous extracts by maceration | Fruits | |
| [42] | Decrease cellular proliferation, | Not determined | 0.46 μg/mL | Supercritical Carbon Dioxide Extraction | Powder | |
| [39] | Decrease cellular proliferation, | Intrinsic apoptosis and mitophagy activation | 30.16 μM | Single isolated metabolite (costunolide) | Not applicable | |
| [14] | Decrease cellular proliferation, | Not determined | 26.7 - 114.6 µg/mL | Palladium nanoparticle with aqueous extracts | Entire plant | |
| [37] | Decrease cellular proliferation, | Not determined | 5.353 - >100 μg/mL | Ethanol by maceration, Hexane, Chloroform, n-Butanol by liquid-liquid extraction | Roots | MDA-MB-231 |
| [34] | Decrease the cellular proliferation and control the cancer invasiveness | Inhibition of NF-κB and MMP-9 | Ethanol extracts did not inhibit 50% of cells | Ethanol by maceration liquid-liquid extraction | ||
| [47] | Decrease the cellular proliferation and control the cancer invasiveness | TNFα and NF-κB inhibition | 50 μg/mL | Ethanol by sonication | ||
| [26] | Decrease cellular proliferation | Not determined | 0.56 - 0.88 μM/ml | Hexane by soxhlet for 72 hours | ||
| [15] | Decrease cellular proliferation | Not determined | 21.5 µM | Single isolated metabolite (dehydrocostuslactone) | Not applicable | |
| [39] | Decrease cellular proliferation | Intrinsic apoptosis and mitophagy activation | 12.76 μM | Single isolated metabolite (costunolide ) | Not applicable | SK-BR-3 |
| [15] | Decrease cellular proliferation | Not determined | 25.6 µM | Single isolated metabolite (dehydrocostuslactone) | Not applicable | |
| [15] | Decrease cellular proliferation | Not determined | 43.2 µM | Single isolated metabolite (dehydrocostuslactone) | Not applicable | MDA-MB-453 |
| Colon Cancer | ||||||
| [37] | Decrease cellular proliferation | Not determined | 4.717->100 μg/mL | Ethanol by maceration, Hexane, Chloroform, n-Butanol (liquid-liquid extraction) | Roots | HCT-116 |
| [35] | Decrease cellular proliferation | Apoptosis regulation and angiogenesis reduction | 82.64 μg/mL | Ethanol and Hexane by cold percolation | ||
| [21] | The extracts did not affect the cellular viability (reduced cells ~ 20%, however it induced apoptosis | Cell Cycle Arrest, Gene Expression Changes (BCL2., CASP3, TP53, BAX), Mitochondrial Dysfunction | Hexane extracts did not show effect on the viability | Hexane by sonication | ||
| [30] | Decrease cellular proliferation | Apoptosis regulation and caspases activities modulation | 0.4-24.9 µg/mL | Methanol, Hexane, Chloroform, Ethyl acetate, n-Butanol by liquid-liquid extraction | Leaves | |
| [42] | Decrease cellular proliferation | Not determined | 0.44µg/mL | Supercritical Carbon Dioxide Extraction | Powder | |
| [14] | Decrease cellular proliferation, nanoparticles formulation enhances the anticancer activity | Not determined | 7.8 - 82.5 µg/mL | Palladium nanoparticles | Not determined | |
| [84] | Decrease cellular proliferation | Apoptosis | 1 mg/mL | Aqueous extracts by maceration | Fruits | CaCo-2 |
| [46] | Decrease cellular proliferation | Not applicable | 1.16 - 1.55 μM | Methanol | Roots | HCT-15 |
| [21] | Decrease cellular proliferation | Apoptosis regulation, caspase activity | 6.20 - 15.78 µM | Bilosome-based on single isolated metabolites (costunolide) | Not applicable | LS174T |
| [21] | The extracts did not affect the cellular viability (reduced cells ~ 20%, however it induced apoptosis | Cell Cycle Arrest, Gene Expression Changes (BCL2., CASP3, TP53, BAX), Mitochondrial Dysfunction, | The hexane extracts did not show effect on the viability | Hexane by sonication | Roots | HT-29 |
| Liver Cancer | ||||||
| [36] | Decrease cellular proliferation | Apoptosis regulation, caspase activity | 56.76 µg/mL | Chloroform, n-Butanol, Ethyl Acetate by soxhlet apparatus | Roots | HepG2 |
| [35] | Decrease cellular proliferation | Apoptosis and reduction of the angiogenesis | 154.30 μg/mL | Ethanol, Hexane by cold percolation | ||
| [85] | Decrease cellular proliferation | Apoptosis regulation | 1.10 - 3.5 mg/mL | Aqueous, Ethanol, Hydro-ethanol | ||
| [86] | Decrease cellular proliferation | Apoptosis regulation and reactive oxygen species induction | 20 mM | Methanol, Water, Petroleum Ether, n-Butanol, Acetone, Ethyl Acetate | ||
| [36] | Decrease cellular proliferation | Apoptosis regulation, caspase activity | 56.76 µg/mL | Chloroform, n-Butanol, Ethyl Acetate by soxhlet apparatus | ||
| [87] | Decrease cellular proliferation | Autophagy inhibition | 5 - 20 μg/mL | Hexane, Ethyl Acetate, n-Butanol, Water | ||
| [86] | Decrease cellular proliferation | Apoptosis regulation and reactive oxygen species induction | 20 mM | Methanol, Water, Petroleum Ether, n-Butanol, Acetone, Ethyl Acetate | ||
| [30] | Decrease cellular proliferation | Apoptosis regulation, caspase activity | 0.5-33.2 µg/mL | Methanol, Hexane, Chloroform, Ethyl Acetate, n-Butanol by liquid-liquid extraction | Leaves | |
| [42] | Decrease cellular proliferation | Not determined | 0.74 μg/mL | Supercritical Carbon Dioxide | Powder | |
| [18] | Decrease cellular proliferation | Intrinsic apoptosis, ER stress induction, MAPK activation, Phosphorylation signaling | 16.7 µM | Single isolated metabolite (dehydrocostuslactone) | Not applicable | |
| [14] | Decrease cellular proliferation, nanoparticles formulation enhances the anticancer activity | Not determined | 11.8 - 91.5 µg/mL | Palladium nanoparticles | Not applicable | |
| [18] | Decrease cellular proliferation | Intrinsic apoptosis, ER stress induction, MAPK activation, Phosphorylation signaling; Apoptosis | 18.8 µM | Single isolated metabolite (dehydrocostuslactone) | Not applicable | PLC/PRF/5 |
| [86] | Decrease cellular proliferation | Apoptosis regulation and reactive oxygen species induction | 20 µM | Methanol, Water, Petroleum Ether, n-Butanol, Acetone, Ethyl Acetate | Roots | SMMC-7721 |
| Lung Cancer | ||||||
| [37] | Decrease cellular proliferation | Not determined | 11.875->100 μg/mL | Ethanol by maceration, Hexane, Chloroform, n-Butanol by liquid-liquid extraction | Roots | A-549 |
| [23] | Decrease cellular proliferation | Not determined | 38.5->100 µg/m | Ethanol, Chloroform, Ethyl Acetate, n-Butanol | ||
| [46] | Decrease cellular proliferation | Not determined | 1.64-2.97 μM | Methanol | ||
| [17] | Decrease cellular proliferation | Not determined | 37.90 µg/ml | Chloroform, Ethanol by soxhlet apparatus | ||
| [26] | Decrease cellular proliferation | Not determined | 3.9 - 7.4 μM/ml | Hexane by soxhlet apparatus for 72 hours | ||
| [40] | Decrease cellular proliferation | Gene expression activity (BCL2, P53, BAX), suppression of the TNFα and NF-κB | 6.1 -13.4 µM | Single isolated metabolites (costunolide) | Not applicable | |
| [20] | Decrease cellular proliferation | Cell cycle affects, Apoptosis regulation, Protein expression changes (p53, p27, p21) | ~50-60 µM | Single isolated metabolites (costunolide) | Not applicable | SK-MES-1 |
| Neuroblastoma | ||||||
| [26] | Decrease cellular proliferation | Not determined | 4.1 - 4.2 μM/mL; | Hexane by soxhlet apparatus for 72 hours | Roots | IMR-32 |
| [32] | Decrease cellular proliferation and control the cancer invasiveness | Apoptosis regulation and reduction the invasion of the cell | 1.26-6.52 μM | Two isolated metabolites (dehydrocostus lactone and costunolide) | Not applicable | |
| [46] | Decrease cellular proliferation | Not determined | 0.43 - 1.70 μM; | Methanol; | Roots; | XF498 |
| [29] | Decrease cellular proliferation | Apoptosis regulation, caspase activity, gene expression activity (BCL2, P53, BAX), changes in protein expression (AKT and GSK-3β activity) | 15 μg/mL | Ethanol | Roots | SH-SY5Y |
| [29] | Decrease cellular proliferation | Apoptosis regulation, caspase activity, gene expression activity (BCL2, P53, BAX), changes in protein expression (AKT and GSK-3β activity) | 20 μg/mL | Ethanol | Roots | B103 |
| [32] | Decrease cellular proliferation and control the cancer invasiveness | Apoptosis regulation and reduction the invasion of the cell | 1.26-6.52 μM | Two isolated metabolites (dehydrocostus lactone and costunolide) | Not applicable | LA-N-1 |
| [32] | Decrease cellular proliferation and control the cancer invasiveness | Apoptosis regulation and reduction the invasion of the cell | 1.26-6.52 μM | Two isolated metabolites (dehydrocostus lactone and costunolide) | Not applicable | SK-N-SH |
| Prostate Cancer | ||||||
| [26] | Decrease cellular proliferation | Not determined | 0.64 - 3.4 μM/ml | Hexane by soxhlet apparatus for 72 hours | Roots | DU-145 |
| [21] | Decrease cellular proliferation | Apoptosis regulation, caspase activity, gene expression activity (BCL2, P53, BAX) | Hexane extracts did not affect the viability | Hexane by sonication | ||
| [38] | The extracts did not affect the cellular viability (reduced cells ~ 20%, however it induced apoptosis and inhibits the cell migration | Changes in TIMP, MMP-9 expression, inhibition of cell migration | Hexane did not affect the viability of the cell | |||
| [33] | Decrease cellular proliferation | Apoptosis regulation, gene expression activity (BCL2, P53, BAX), effects on androgen signaling, decrease cell migration, effects on the autophagy activity | 50 µg/ml | Ethanol | Roots | LNCaP |
| [21] | Decrease cellular proliferation | Apoptosis regulation, caspase activity, gene expression activity (BCL2, P53, BAX) | Hexane extracts did not affect the viability | Hexane by sonication | ||
| [38] | Decrease cellular proliferation | Not determined | 3.37 - >100 μg/mL | Ethanol by maceration, Hexane, Chloroform, n-Butanol (liquid-liquid extraction) | Roots | PC-3 |
| [38] | The extracts did not affect the cellular viability (reduced cells ~ 20%, however it induced apoptosis and inhibits the cell migration | Changes in TIMP, MMP-9 expression, inhibition of cell migration | Hexane did not affect the viability of the cell | Hexane by sonication | Roots | TRAMP-C |
| Gastric Cancer | ||||||
| [45] | Decrease cellular proliferation | Apoptosis regulation, caspase activity, gene expression activity (BCL2, P53, BAX), cell cycle effects | 100 μg/mL | Ethanolic extracts by sonication followed by freeze-drying | Roots | AGS |
| [31] | Decrease cellular proliferation | Apoptosis regulation, caspase activity, gene expression activity (BCL2, P53, BAX), cell cycle effects | 79 μg/mL | Ethanolic extracts by sonication followed by freeze-drying | ||
| [41] | Decrease cellular proliferation | Not determined | 4.5 μM | Single isolated metabolites (costunolide) | Not applicable | |
| Leukemia | ||||||
| [22] | Decrease cellular proliferation | Apoptosis regulation, cell cycle effects, and decrease the proteins expression of the multidrug resistance | Not determined | Methanol; Petroleum Ether, Methanol by soxhlet apparatus | Roots | CCRF-CEM |
| [25] | Decrease cellular proliferation | Apoptosis and NF-κB inhibition | 5 mM | Partitioning into Ethyl Acetate, Water, n-Butanol | Roots | HL-60 |
| [46] | Decrease cellular proliferation | Apoptosis and Cell cycle effects | Not determined | Methanol | Roots | U937 |
| Cervical Cancer | ||||||
| [26] | Decrease cellular proliferation | Not determined | 1.1 - 2.4 μM/mL | Hexane by soxhlet apparatus for 72 hours | Roots | SIHA |
| [87] | Decrease cellular proliferation | Apoptosis regulation and reactive oxygen species induction | 20 µM | Methanol, Water, Petroleum Ether, n-Butanol, Acetone, Ethyl Acetate | Roots | HeLa |
| Esophageal Cancer | ||||||
| [27] | Decrease cellular proliferation and inhibit cell migration | Migration Inhibition, Prefoliation Prevention, Apoptosis Induction; Inhibition of the cell migration and expression of MMp-2 and MPP-9, modulation of the autophagy process, Induction of ROS | 10.55 - 43.75 µM | Ethanol by maceration, Petroleum Ether by liquid-liquid extraction | Roots | Eca109 |
| [27] | Decrease cellular proliferation and inhibit cell migration | Migration Inhibition, Prefoliation Prevention, Apoptosis Induction; Inhibition of the cell migration and expression of MMp-2 and MPP-9, modulation of the autophagy process, Induction of ROS | 6.97 - 24.29 μg/mL | Hydro-distillation using a Clevenger-type device | ||
| [27] | Decrease cellular proliferation and inhibit cell migration | Migration Inhibition, Prefoliation Prevention, Apoptosis Induction; Inhibition of the cell migration and expression of MMp-2 and MPP-9, modulation of the autophagy process, Induction of ROS | 8.35 - 40.78 µM | Ethanol by maceration, Petroleum Ether by liquid-liquid extraction | Roots | KYSE150 |
| Ovarian Cancer | ||||||
| [46] | Decrease cellular proliferation | Not determined | 1.65 - 1.83 μM | Methanol | Roots | SK-OV-3 |
| [15] | Decrease cellular proliferation | Not determined | 10.8 μM | Single isolated metabolite (dehydrocostus lactone) | Not applicable | |
| [15] | Decrease cellular proliferation | Not determined | 13.9 μM | Single isolated metabolite (dehydrocostus lactone) | Not applicable | OVCAR3 |
| Colorectal Cancer | ||||||
| [16] | Decrease cellular proliferation | Apoptosis regulation, caspase activity, suppression of the TNFα and NF-κB, suppression of the Suppression of Nuclear Translocation | 5nM | Two isolated metabolites (dehydrocostus lactone and costunolide) | Not applicable | SW-480 |
| Oral Cancer | ||||||
| [24] | Decrease cellular proliferation | Apoptosis regulation, caspase activity | 30 µg/mL | Methanol | Roots | KB |
| Skin Cancer | ||||||
| [46] | Decrease cellular proliferation | Not determined | 0.55- 0.59 μM | Methanol | Roots | SK-MEL-2 |
| Soft Tissue Sarcoma | ||||||
| [22] | Decrease cellular proliferation | Apoptosis regulation, caspase activity, gene expression activity (BCL2, P53, BAX) | 7.41 - 9.71 μg/mL | Methanol; Petroleum Ether, Methanol by soxhlet apparatus | Roots | SW-872 |
| [22] | Decrease cellular proliferation | Apoptosis regulation, caspase activity, gene expression activity (BCL2, P53, BAX) | 6.17 - 9.61 μg/mL | Methanol; Petroleum Ether, Methanol by soxhlet apparatus | Roots | SW-982 |
| [22] | Decrease cellular proliferation | Apoptosis regulation, caspase activity, gene expression activity (BCL2, P53, BAX) | 8.33 - 9.75 μg/mL | Methanol; Petroleum Ether, Methanol by soxhlet apparatus | Roots | TE-671 |
| Pancreatic Cancer | ||||||
| [26] | Decrease cellular proliferation | Not Determined | 0.26 - 1.2 μM/mL | Hexane | Roots | PANC1 |
*Not applicable: The studies did not extract the compound, they tested the isolated compounds, which are mainly (dehydrocostus lactone or costunolide).
*Not determined: The studies did not investigate the mechanism of action of anticancer activity.
Additionally, other studies have explored alternative extraction techniques, such as nanoparticle synthesis and supercritical carbon dioxide extraction, to evaluate the antitumor potential of S. costus extracts [14,42,43]. One study optimized the extraction of S. costus oil using supercritical fluid extraction at different pressures, achieving significant inhibition with an IC50 value of 0.46 μg/mL on MCF-7 cells [42]. In contrast, magnesium oxide nanoparticles synthesized from S. costus methanol extracts exhibited relatively lower antiproliferative effects on MCF-7 cells, with IC50 values of 80 μg/mL and 26.7 μg/mL for magnesium oxide and palladium nanoparticles, respectively [14,42].
Choi, (2009) tested dehydrocostuslactone on MDA-MB-231, SK-BR-3, and MDA-MB-453 cell lines, reporting IC50 values ranging from 25.6 to 43.2 μM/mL, while costunolide tested on MCF-7 cells showed an IC50 value of 30.16 μM/mL [39].
A similar pattern of inhibition, depending on plant parts, solvents, and extraction methods, was observed in other cancer types, including colon, liver, gastric, esophageal, and pancreatic cancers [14,28,28,30,37,43,43-45]. Although hexane and chloroform extracts generally showed potent inhibition, one study found no significant cytotoxicity with hexane root extracts against colon (HCT-116, HCT-29) and prostate cancer (PC-3, LNCaP, DU145), as the hexane extracts did not affect cell viability [21,38].
However, neuroblastoma cell lines (XF498, SH-AY5Y, and B103) showed susceptibility to both methanol and ethanol extracts of S. costus, with IC50 values ranging from 15-20 μg/mL and 0.43-1.70 μM/mL when exposed to ethanol and methanol extracts, respectively [29,46].
Other cancer types that demonstrated sensitivity to S. costus extracts include ovarian, colorectal, skin, and soft tissue cancers [15,22,24].
Proliferation inhibition: The mechanisms of action of S. costus involved the modulation of the PI3K/AKT/mTOR pathway, as well as the regulation of the MAPK/ERK pathway. Additionally, S. costus impacts the WNT/β-Catenin pathway by reducing β-catenin levels and depicts the inhibition of the NF-κB pathway. Furthermore, the regulation of cell cycle proteins, such as CDKs and cyclins leading to induction of cell cycle arrest.
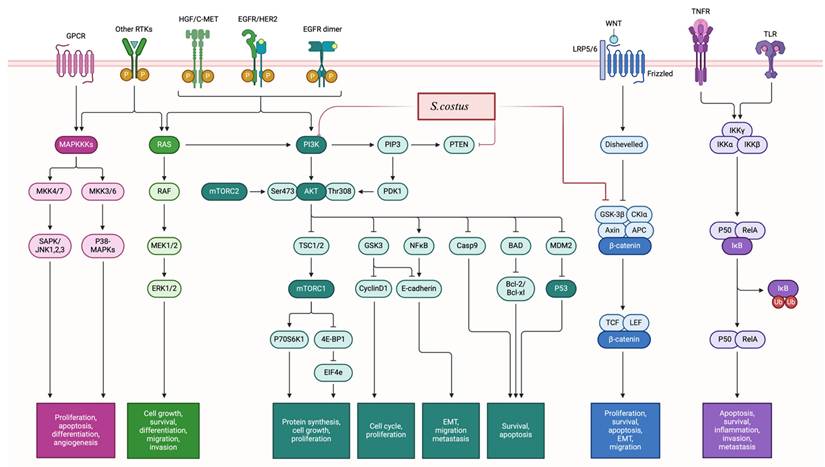
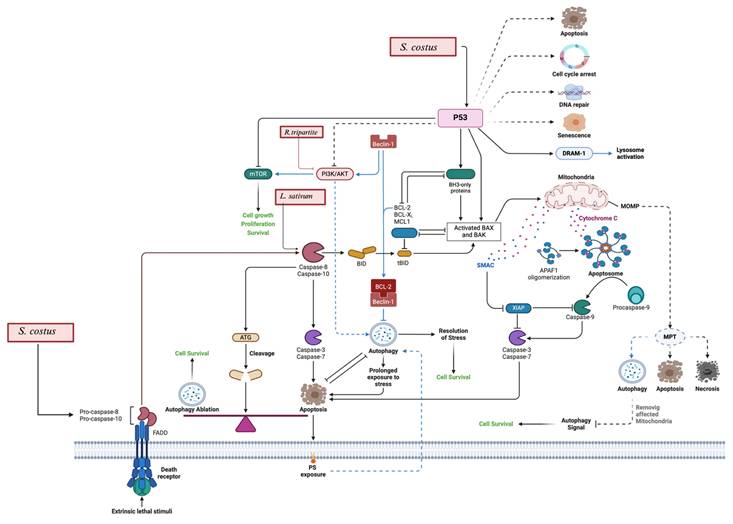
The cytotoxic potential of S. costus has been extensively investigated, and it has been observed that its anticancer effects are mediated through multiple mechanisms (Figure 3), including the induction of apoptosis, cell cycle arrest, modulation of the androgen receptor and autophagy processes, decreased proliferation, and alterations in cellular signaling cascades by modulating phosphorylation processes. In particular, autophagy appears to be promoted through upregulation of LC3I and LC3II levels (high LC3II/LC3I ratio) and beclin1, while mTOR phosphorylation is inhibited [32,33,47]. The interplay between autophagy and apoptosis is notable; inhibition of one can stimulate the other, suggesting a dual mechanism of action.
Ten in vivo studies have evaluated the anticancer activity of S. costus. The investigated type of cancer included breast cancer (MCF-7, MDA-MB-231), liver cancer (SMMC-7721), lung cancer (LC-540), leukemia, laryngeal carcinoma, esophageal cancer, gastric cancer (MKN-28) [47-51] (Table 3). Most studies utilized either the roots or isolated compounds (costunolide and dehydrocostus lactone), with ethanol and hexane as organic solvents for the extraction.
One study found that hexane root extract of S.costus inhibited hepatocellular carcinoma (HCC) with an inhibition rate of 55.71% [52]. Likewise, ethanol extracts showed anti-leukemic effects by reducing white blood cell counts to normal levels [50].
The isolated compounds also exhibit anticancer activity, the lowest dose (costunolide at 10 mg/kg/day) was tested on immunodeficient female NCr nude homozygous mice with breast and colon cancer, showing reduction of the tumor volume. The highest dose (dehydrocostus lactone at 40 mg/kg/day for 28 days) was tested also for esophageal cancer in the Eca109-b mouse model, and the results showed inhibition of the tumor growth [27]. Several studies attempt to investigate the mechanisms, the in vivo anticancer activity of S. costus is related mainly to induction of apoptosis by activating pro-apoptotic proteins such as p53 and Bax, while inhibiting anti-apoptotic proteins like Bcl-2, leading to programmed cell death, cell cycle arrest, modulating certain signaling pathways, such as EGFR, which reduces the proliferation and invasion of cancer cells, and suppress PI3K/Akt and MEK/P38 pathways, reduction the inflammatory process by suppression the level of TNF-α and NF-κB, and induction of reactive oxygen species.
Comprehensive analysis of S. costus reported in vivo studies: treatment protocols and observed effects.
| Cancer Type | Animal Model | Part Used | Extraction Method | Dose | Proposed Mechanisms | Results Description | References |
|---|---|---|---|---|---|---|---|
| Breast Cancer | |||||||
| MCF-7 | Adult Sprague Dawley (SD) rats (sex-female; weight-160 ±20 g; age-6-8 weeks | Roots | Ethanol by sonication | (100, 250 and 500 mg/kg BW) | Not determined | Inhibits the pulmonary metastases breast cancer | [51] |
| Six-week-old nude (Nu/Nu) mice | Not applicable | Single isolated metabolite (costunolide) | 20 μM Three times a week for 30 days | Suppress breast cancer growth and metastases by inhibiting TNFα-induced NF-κB activation | Inhibits tumor growth and prevent migration | [47] | |
| MDA-MB-23 | Female BALB/c nude mice (4 weeks old) | Roots | Hexane by sonication | 20 mg/kg/day | Cell cycle arrest and apoptosis regulations | Inhibits tumor growth | [88] |
| Liver Cancer | |||||||
| Not determined | Albino Swiss mice (aged 8-10 weeks, with an average body weight of 28±1.5 g) | Roots | Ethanol | 400, 600, 800mg/Kg | Cell cycle arrest and apoptosis regulations | Inhibits tumor growth | [48] |
| SMMC-7721 | Male nude mice (4 weeks old; BALB/c-nude) | Roots | Hexane | 15 mg/kg/day | Apoptosis and anti-metastatic activity. | Inhibits tumor growth | [52] |
| Lung Cancer | |||||||
| LC-540 | Adult Sprague Dawley (SD) rats (sex-female; weight-160 ±20 g; age-6-8 weeks) | Roots | Ethanol | 100, 250 and 500 mg/kg BW | Not determined | Inhibits tumor growth | [89] |
| Leukemia | |||||||
| Not determined | Adult male albino rats (180-220g); Male nude mice (4 weeks old; BALB/c-nude) | Roots | Ethanol | (300mg/Kg/day) orally for 4 weeks | Not determined | Inhibits tumor growth | [50] |
| Laryngeal Cancer | |||||||
| Not determined | Female nude mice (BALB/c nu/nu, 4-5 weeks old, 18-19 g) | Roots | Ethanol by maceration | 10, 15 mg/kg | Inhibition of PI3K/Akt/Bad pathway | Inhibits tumor growth | [90] |
| Esophageal Cancer | |||||||
| Not determined | SPF-grade female BALB/c nude mice aged 4-5 weeks | Roots | Ethanol by maceration, Petroleum Ether by liquid-liquid extraction | (0, 20, and 40 mg/kg/day) for 28 days | Migration Inhibition, Prefoliation Prevention, Apoptosis Induction; Inhibition of the cell migration and expression of MMp-2 and MPP-9, modulation of the autophagy process, Induction of ROS | Inhibits tumor growth and migration | [27] |
| Gastric Cancer | |||||||
| MKN-28 | Female BALB/c nude mice each weighing 20 g ± 2 g | Not applicable | Single isolated metabolite (dehydrocostus lactone) | 15, 30 mg/kg/day | Inhibition of autophagy | Inhibits tumor growth | [49] |
*Not applicable: The studies did not extract the compound, they tested the isolated compounds, which are mainly (dehydrocostus lactone or costunolide.
*Not determined: The studies did not determine the type of cell line, or did not investigate the mechanism of action of anticancer activity.
3.2. Lepidium sativum
Fourteen studies have investigated the anticancer activity of L. sativum on various cancer types, such as liver (HuH-7 and HEPG-2), breast cancer (MCF-7), colon cancer (DLD-1, HCT-116, HT-15, HT-29, SW480, HTB-38, and Caco2), cervical cancer (HeLA 2), lung cancer (A-549 cell line), prostate cancer cells (P-C3), endometrium cancer (ECC-1), tongue squamous carcinoma (CAL-27), melanoma cancer (A-375 cell line), neuroblastoma (IMR-32), ovarian cancer (OV17R), leukemia (Jurkat E6-1)[53-66]. The studies utilized various plant parts, including leaves, roots and seeds, with th most employed seeds being the most commonly used part.
Liver cancer was the most commonly studied cancer type. Studies reported similar IC50 values across different cell lines, plant parts, and extraction solvents [58,64], particularly when polar solvents were used. This pattern was also observed in other cancer types, including breast, colon, cervical, lung, prostate, endometrial, tongue squamous cell carcinoma, and leukemia [54,55,57,60,66]. However, the results showed variability in cytotoxic activity.
In a study by Abd-elmegeed et al. (2023), phenolic compounds, such as rutin, benzoic acid, cinnamic acid, and vanillin were isolated and evaluated, revealing IC50 values ranging from 28.8 μg/mL to 64.32 μg/mL [53]. Furthermore, Ibrahim et al. (2023) demonstrated that L. sativum ethanol extracts that are treated with glucosinolates showed inhibition of cancer cells, prostate cancer (PC-3), colon cancer (coca2), lung cancer (A-549), and liver cancer (HepG2) with IC50 ranges 38.5-92.6 mg/mL, without effect normal cell lines [59].
Nanoparticles synthesis using L. sativum extracts has also been explored. Meer et al. (2022), and Efati et al. (2023) reported enhanced cytotoxic potential of L. ativum extracts against colorectal adenocarcinoma (SW480) and colon adenocarcinoma (HT-29 and Caco-2) at different degrees of temperature, the lowest IC50 (13.14 µg/mL) was detected to SW480 with ZnO at 350 °C [57,63]. In contrast, Amina et al., (2021) study of Ag-MgO nanoparticles found no enhancement of L. sativum extracts [55].
Several studies have investigated the mechanism of action underlying the anticancer effects of L. sativum. These studies demonstrated that the anticancer activity is related mainly to induction of apoptosis and cell cycle arrest. L. sativum extracts showed upregulation of pro-apoptotic proteins such as BAX, p53, and caspases 3/7, alongside the downregulation of anti-apoptotic proteins like Bcl-2 (Figure 4) [57]. Additionally, upregulation of SMAD2 and SMAD3 expression has been observed in the live cancer cell upon exposure to L. sativum extracts. Moreover, L. sativum extracts showed induction of cell cycle arrest at the S phase, thereby inhibiting their proliferation (Table 4) [59].
3.3. Rhus tripartite
The cytotoxic potential of R. tripartite leaves and roots was examined in four studies, using diverse cancer cell lines, including acute myeloid leukemia (THP-1), myelogenous leukemia (K-562), colon cancer (DLD-1, Caco-2), breast cancer (MCF-7), lung cancer (A-549) (Table 6) [67-71]. The extraction process used butanol, methanol, ethanol, and ethyl acetate as organic solvents. The lowest IC50 value (39.83 μg/mL) was reported for methanol extracts against colon adenocarcinoma DLD-1 cell line [68].
In contrast, aqueous extracts showed lower cytotoxic activity with IC50 values 195.37 μg/mL and 200 μg/mL against A-549 and DLD-1) cell lines, respectively. Similarly, in Tlili et al. (2019) reported IC50 values less than 50 μg/mL for methanol extracts against CaCo-2 and K-562 cell lines [71].
The mechanism of action for R. tripartite extractions is thoroughly explained by Tlili et al., 2021, which involves the inhibition of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT mammalian target of rapamycin (mTOR) pathway that eventually results in the induction of apoptosis and the suppression of tumor growth, as shown previously in Figure 4 [70].
Apoptosis and autophagy: S. costus and L. sativum activate p53, which leads to DNA damage response, cell cycle arrest, and apoptosis by upregulating pro-apoptotic proteins like Bax and Bak while down regulating anti-apoptotic proteins such as Bcl-2 and Mcl-1. Additionally, S. costus influences autophagy pathways, including Beclin-1 and the interaction of death receptors, leading to autophagic cell death. It includes also the apoptosis by ROS generation, which leads to mitochondrial dysfunction and apoptosis. R. tripartite is also involved in the modulation of the PI3K/AKT/mTOR pathway.
Comprehensive analysis of in vitro anticancer studies on L. sativum: cell lines, extracts, and mechanisms of action.
| Cancer Type | Plant Part | Extraction Method | IC50 | Proposed Mechanism | Results Description | Reference |
|---|---|---|---|---|---|---|
| Liver Cancer | ||||||
| HepG-2 | Seeds | Methylene chloride, n-Hexane, Ethyl Acetate, Butanol, Methanol | 45 - 63.8 µg/mL | Apoptosis and downregulation of EGFR | Decrease cellular proliferation | [64] |
| Aqueous with ZnO nanoparticles | 100 µg/mL | Induction of ROS | Decrease cellular proliferation | [63] | ||
| Seeds and leaf | Ethanol and Aqueous | 382.2 mg /mL | Not determined | Decrease cellular proliferation | [58] | |
| Leaves and roots | Ethanol extracts with glucosinolate | 38.5 - 81.2 µg/mL | Apoptotic regulation, caspase activity, cell cycle effects | Decrease cellular proliferation, the glucosinolate extracts enhance the selectivity, it did not affect normal cell lines | [59] | |
| HuH-7 | Seeds | Methylene chloride, n-Hexane, Ethyl Acetate, Butanol, Methanol; | 59 - 63.5 µg/mL | Gene expression modulation (EGFR, BCL2, SMAD3, BAX, P53) | Decrease cellular proliferation | [64] |
| Breast Cancer | ||||||
| MCF-7 | Seeds | Aqueous | 70% concentration of L. sativum inhibited the growth by 64.03% | Not determined | Decrease cellular proliferation | [62] |
| Crude and soxhlet Methanol | 88.49 - 136.75 µg/mL | Not determined | Decrease cellular proliferation | [65] | ||
| Leaves and Roots | Ethanol extracts with glucosinolates | 61 - 71.1 µg/mL | Apoptosis regulation, caspase activity, cell cycle effects | Decrease cellular proliferation, the glucosinolate extracts enhance the selectivity, it did not affect normal cell lines | [59] | |
| Colon Cancer | ||||||
| CaCo-2 | Seeds | Aqueous extract with ZnO-NPs | 18.45 - 105.9 µg/mL | Gene expression modulation (p53, Bax, Bcl-2 | Decrease cellular proliferation | [57] |
| Leaves | Ethanol extracts with glucosinolates | 56.6 - 89.9 µg/mL | Apoptotic regulation, caspase activity, cell cycle effects | Decrease cellular proliferation, the glucosinolate extracts enhance the selectivity, it did not affect normal cell lines | [59] | |
| DLD-1 | Above-Ground Parts | Methanol by maceration | 100 µg/mL (DLD-1) | 100 µg/mL | Decrease cellular proliferation | [66] |
| HT-29 | Seeds | n-Hexane, Chloroform, Ethyl Acetate Methanol by soxhlet apparatus | 100 µg/mL | Not determined | Decrease cellular proliferation | [60] |
| HT-15 | Seeds | n-Hexane, Chloroform, Ethyl Acetate Methanol by soxhlet apparatus | 100 µg/mL | Not determined | Decrease cellular proliferation | [60] |
| Cervical Cancer | ||||||
| HeLA - 2 | Seeds | Soxhlet extraction with silver nanoparticles | 135 - 220.35 μg/mL | ROS generation | Decrease cellular proliferation, and nanoparticles formulation enhances the anticancer activity | [55] |
| Leaves | Ethanolic maceration extracts | 100 µg/mL (at second day) | Apoptosis induction | Decrease cellular proliferation | [61] | |
| Lung Cancer | ||||||
| A-549 | Seeds | n-Hexane, Chloroform, Ethyl Acetate Methanol by soxhlet apparatus | 100 µg/mL | Not determined | Decrease cellular proliferation | [60] |
| Leaves and Roots | Ethanol extracts with glucosinolates | 42.3 - 92.6 µg/mL | Apoptotic regulation, caspase activity, cell cycle effects | Decrease cellular proliferation, the glucosinolate extracts enhance the selectivity, it did not affect normal cell lines | [59] | |
| Prostate Cancer | ||||||
| PC-3 | Seeds and leaf Calli | Aqueous and Ethanol extracts | 113.6 mg/mL | Not determined | Decrease cellular proliferation | [58] |
| Leaves and Roots | Ethanol extracts with glucosinolates | 51.4 - 72.4 µg/mL | Apoptotic regulation, caspase activity, cell cycle effects | Decrease cellular proliferation, the glucosinolate extracts enhance the selectivity, it did not affect normal cell lines | [59] | |
| Endometrium Cancer | ||||||
| ECC-1 | Above-Ground Parts | Methanol extracts by maceration | 353 µg/mL | Not determined | Decrease cellular proliferation | [66] |
| Tongue Squamous Carcinoma | ||||||
| CAL-27 | Leaves | Aqueous extract | 100 µg/mL | ROS generation induction | Decrease cellular proliferation | [54] |
| Melanoma | ||||||
| A-375 | Leaves and roots | Ethanol extracts with glucosinolates | Not determined | Apoptotic regulation, caspase activity, cell cycle effects | Decrease cellular proliferation, the glucosinolate extracts enhance the selectivity, it did not affect normal cell lines | [59] |
| Neuroblastoma | ||||||
| IMR-32 | Seeds | Various solvents by soxhlet apparatus | 100 µg/mL | Not determined | Decrease cellular proliferation | [60] |
| Ovarian Adenocarcinoma | ||||||
| OV17R | Seeds | Various extracts and HPLC | 28.8 - 64.32 μg/mL | Not determined | Decrease cellular proliferation | [53] |
| Leukemia | ||||||
| Jurkat E6-1 | Seeds | Tertiary Alkaloid extract | 75.25 mg/mL | Apoptosis via DNA laddering, caspase-3 activity | Decrease cellular proliferation | [56] |
| Colorectal Cancer | ||||||
| SW-480 | Seeds | Aqueous extract\ green synthesis of ZnO-NP | 13.14 - 100 μg/mL | Gene expression modulation (EGFR, BCL2, SMAD3, BAX, P53) | Decrease cellular proliferation | [57] |
*Not determined: The studies did not investigate the mechanism of action of anticancer activity.
Comprehensive analysis of L. sativum reported in in vivo studies: treatment protocols and observed effects.
| Cancer Type | Animal Model | Part Used | Extraction Method | Dose | Proposed Mechanisms | Results Description | References |
|---|---|---|---|---|---|---|---|
| Ehrlich ascites carcinoma (EAC) | |||||||
| Ehrlich ascites carcinoma (EAC) | Female Swiss albino mice | Seeds | Dichloromethane and Ethyl Acetate. | 500 mg/kg | Decreased chromosomal aberration and DNA fragmentation induced by EAC in mice | The mice have an increased lifespan by 37.14%. | [82] |
Comprehensive analysis of in vitro anticancer studies on R. tripartite: cell lines, extracts, and mechanisms of action.
| Cancer Type | Part Used | Extraction Method | IC50 | Proposed Mechanisms | Results Description | References |
|---|---|---|---|---|---|---|
| Acute Myeloid Leukemia | ||||||
| THP-1 | Leaves | Acetone, Methanol buy maceration | 63 μg/mL | Apoptosis induction by inhibiting PI3K/AKT/mTOR signaling pathway. | Decrease cellular proliferation | [70] |
| K-562 | Aerial Parts | Acetone, Methanol buy maceration | 42.89 μg/mL | Not determined | Decrease cellular proliferation | [71] |
| Colon Adenocarcinoma | ||||||
| DLD-1 | Roots | Hexane, Dichloromethane, Methanol, Water by soxhlet extraction | 39.83 - 200 μg/mL | Not determined | Decrease cellular proliferation | [68] |
| CaCo-2 | Aerial parts | Acetone, Methanol buy maceration | 44.87 μg/mL | Not determined | Decrease cellular proliferation | [71] |
| Breast Adenocarcinoma | ||||||
| MCF-7 | Roots | Ethanol by maceration, then sequential partitioning: Hexane, Ethyl acetate, n-butanol | 100 μg/mL | Not determined | Decrease cellular proliferation | [69] |
| Lung Cancer | ||||||
| A-549 | Roots | Hexane, Dichloromethane, Methanol, Water by soxhlet extraction | 60.69 - 205.52 μg/mL | Not determined | Decrease cellular proliferation | [68] |
*Not determined: The studies did not investigate the mechanism of action of anticancer activity.
3.4. Pyrus communis
Cytotoxic potential of P. communis has been examined in two studies on various cancer types, including lung cancer (A549, WI-38), prostate cancer (LNCaP), urinary bladder cancer (HCV29T), kidney cancer (A-498), mouse myelogenous leukemia carcinoma (M-NFS-60), ovary cancer (CHO-K1) [72,73].
Both studies utilized the fruits of P. communis. The study that utilized hydroinstillation extraction method showed higher cytotoxicity, with IC50 values ranging values: from 30.9 to 105 μg/mL. In contrast, the study employing the UPLC-PDA-MS extraction method showed lower cytotoxicity, with IC50 values ranging from: 0.5 - to 3.2 mg/mL (Table 7) [72,73].
3.5. Chenopodium murale
Only one in vitro study has evaluated C. murale's anticancer activity in breast cancer (MCF-7) and liver cancer (HCAM), with leaves extracted using an ethanol solvent. The investigation found that the extraction had weak cytotoxic activity, with IC50 of 1504 µg/mL for breast cancer and 1267 µg/mL for liver cancer cells (Table 8) [74].
3.6. Erucaria hispanica
E. hispanica was investigated in one study involving four different cancer cell lines, breast (MCF7), liver (HEPG2), cervix (HELA) and colon (HCT116) cancers, utilizing methanol extracts of aerial parts of E. hispanica [10].The results showed IC50values 18 μg/mL, 20.8 μg/mL, 14.7 μg/mL and 21.4 μg/mL respectively (Table 9).
3.7. Trigonella hamosa
A single study has investigated to explore the anticancer activity of methanol extracts from T. hamosa aerial parts. The reported IC50 values were 6.71 μg/mL for breast cancer (MDA-MB-231), 4.93 μg/mL for lung cancer (A549), and 13.74 μg/mL for colon cancer (HTC-166) (Table 10) [75].
Comprehensive analysis of in vitro anticancer studies on P. communis: cell lines, extracts, and mechanisms of action.
| Cancer Type | Part Used | Extraction Method | IC50 | Proposed Mechanism | Results Description | References |
|---|---|---|---|---|---|---|
| Lung Cancer | ||||||
| A-549 | Fruits | Hydroinstillation | 30.9 μg/mL | Not determined | Decrease cellular proliferation | [72] |
| Fruits | UPLC-PDA-MS | 0.5 - 2.5 mg/mL | Not determined | Decrease cellular proliferation | [73] | |
| WI-38 | Fruits | Hydroinstillation | 55.9 μg/ml | Not determined | Decrease cellular proliferation | [72] |
| Colon Cancer | ||||||
| HT-29 | Fruits | UPLC-PDA-MS | 0.5 - 2.5 mg/mL | Not determined | Decrease cellular proliferation | [73] |
| Breast Cancer | ||||||
| MCF-7 | Fruits | UPLC-PDA-MS | 0.4 - 2.4 mg/mL | Not determined | Decrease cellular proliferation | [73] |
| Prostate Cancer | ||||||
| LNCaP | Fruits | UPLC-PDA-MS | 0.5 - 1.4 mg/mL | Not determined | Decrease cellular proliferation | [73] |
| Urinary Cancer | ||||||
| HCV29T | Fruits | UPLC-PDA-MS | 0.5 - 1.5 mg/mL | Not determined | Decrease cellular proliferation | [73] |
| Kidney Cancer | ||||||
| A498 | Fruits | UPLC-PDA-MS | 1.8 - 3.2 mg/mL | Not determined | Decrease cellular proliferation | [73] |
| Ovary Cancer | ||||||
| CHO-K1 | Fruits | Hydroinstillation | 105 μg/mL | Not determined | Decrease cellular proliferation | [72] |
| Leukemia | ||||||
| M-NFS-60 | Fruits | Hydroinstillation | 56.5 μg/mL | Not determined | Decrease cellular proliferation | [72] |
*Not determined: The study did not investigate the mechanism of action of anticancer activity.
Comprehensive analysis of in vitro anticancer studies on C. murale: cell lines, extracts, and mechanisms of action.
| Cancer Type | Part used | Extraction Method | IC50 | Proposed Mechanisms | Results Description | References |
|---|---|---|---|---|---|---|
| Breast Cancer | ||||||
| MCF-7 | Leaves | Ethanol extracts microwave assisted extraction. | 1504 µg/mL | Not determined | Decrease cellular proliferation | [74] |
| Liver Cancer | ||||||
| HCAM | Leaves | Ethanol extracts microwave assisted extraction. | 1267 µg/mL | Not determined | Decrease cellular proliferation | [74] |
*Not determined: The studies did not investigate the mechanism of action anticancer activity.
Comprehensive analysis of in vitro anticancer studies on E. hispanica: cell lines, extracts, and mechanisms of action.
| Cancer Type | Part Used | Extraction and Method | IC50 | Proposed Mechanism | Results Description | References |
|---|---|---|---|---|---|---|
| Breast Cancer | ||||||
| MCF-7 | Ground, Aerial parts | Methanol | 18 μg/mL | Not determined | Decrease cellular proliferation | [10] |
| Liver Cancer | ||||||
| HePG-2 | Ground, Aerial parts | Methanol | 20.8 μg/mL | Not determined | Decrease cellular proliferation | [10] |
| Cervical Cancer | ||||||
| HeLA-2 | Ground, Aerial parts | Methanol | 14.7 μg/mL | Not determined | Decrease cellular proliferation | [10] |
| Colon Cancer | ||||||
| HCT-116 | Ground, Aerial parts | Methanol | 21.4 μg/mL | Not determined | Decrease cellular proliferation | [10] |
* Not determined: The studies did not investigate the mechanism of action of anticancer activity.
Comprehensive analysis of in vitro anticancer studies on T. hamosa: cell lines, extracts, and mechanisms of action.
| Cancer Type | Part Used | Extraction and Method | IC50 | Proposed Mechanism | Results Description | References |
|---|---|---|---|---|---|---|
| Breast Cancer | ||||||
| MDA-MB-231 | Aerial Parts | Methanol | 28.9 μM | Not determined | Decrease cellular proliferation | [75] |
| Lung Cancer | ||||||
| A-549 | Aerial Parts | Methanol | 21.2 μM | Not determined | Decrease cellular proliferation | [75] |
| Colon Cancer | ||||||
| HCT-116 | Aerial Parts | Methanol | 59.1 μM | Not determined | Decrease cellular proliferation | [75] |
*Not determined: The studies did not investigate the mechanism of action of anticancer activity.
4. Discussion
Medicinal plants have long served as a valuable source for the discovery of novel therapeutic agents, particularly in the treatment of diseases such as cancer [76]. Cancer remains a complex and life-threatening condition, contributing to rising mortality rates globally. Additionally, the development of resistance to existing treatments further complicates cancer management and presents significant challenges [77]. Consequently, there is a pressing need to identify new therapeutic agents that can enhance current treatment strategies and address resistance issues. Several medicinal plants have shown promising therapeutic properties, including anticancer activity.
This systematic review critically assessed the available evidence on the anticancer potential of selected medicinal plants. A total of sixty-nine studies were identified that investigated the anticancer effects of for S. costus, L. sativum, Rhus tripartite, C. murale, P. communis, E. hispanica, T. hamosa. In contrast, A. ochroleuca, and G. odoratum have not yet been evaluated for their anticancer potential.
Among these, S. costus was the most extensively studied. It has been tested against various cancer types, most notably breast (MCF-7, MDA-MB-231, SK-BR-3), liver (HepG2), and colon cancers (HCT-116, HT-29). The cytotoxic activity of S. costus varied depending on the extract type and cell line. For instance, non-polar organic solvents like hexane and chloroform demonstrated high potency, with IC50 0.4 µg/mL - 2.1 µg/mL [30]. However, this potency did not consistently extend to all cancers—prostate cancer cell lines (PC-3, LNCaP, DU145), for example, showed resistance to hexane extracts [21,38]. In comparison, extracts prepared with polar solvents (methanol, ethanol, butanol) generally showed lower potency (IC50 values:10 µg/mL to > 100 µg/mL), suggesting solvent polarity significantly influences the bioactivity of phytoconstituents present in S. costus [37,45,78]. In contrast, other cancer types showed to be sensitive, such as ovarian, colorectal, skin, and soft tissue, regardless of polarity of the solvents or extraction method.
Skin cancer (SK-MEL-2) cells showed exceptional sensitivity to costunolide and dehydrocostus lacton, two well-characterized sesquiterpene lactones isolated from S. costus, with IC50 ranging from 4.7 µM to 60 µM. These compounds demonstrated consistent tumor-suppressive activity across various studies. Advanced extraction techniques like supercritical CO₂ extraction yielded highly potent results, with IC₅₀ values of 0.44-0.74 μg/mL against HCT-116, MCF-7, and HepG2 cells [42]. Nanoparticle formulations also enhanced cytotoxicity: methanolic S. costus-derived palladium nanoparticles showed IC₅₀ values of 7.8-26.7 μg/mL, whereas magnesium oxide nanoparticles were moderately effective (IC₅₀ = 80 μg/mL) [14,43]. These findings highlight that supercritical extraction and specific nanoparticle formulations can optimize S. costus's therapeutic potential.
In vivo studies support the anticancer potential of S. costus: hexane extracts demonstrated a 55.71% inhibition rate on hepatocellular carcinoma (HCC) [52]. While ethanol extracts effectively normalized white blood cell counts in leukemia [50]. Costunolide (10 mg/kg/day) reduced breast and colon tumor volumes in mice, while dehydrocostuslactone (40 mg/kg/day) inhibited esophageal tumor growth [78]. These findings reinforce the therapeutic relevance of S. costus.
Mechanistically, S. costus exhibits multiple modes of action, including inhibition of the Epidermal Growth Factor Receptor (EGFR) and PI3K/Akt signaling pathways, as well as suppression of inflammation, invasion, and metastasis. EGFR dysregulation is linked to tumor progression. Thereover, targeting EGFR suppresses cancer cell proliferation [79]. Additionally, S. costus downregulates TNF-α and NF-κB, key mediators of tumor metastasis and chronic inflammation [80]. The ability to modulate multiple pathways makes S. costus a promising multi-targeted anticancer agent.
L. sativum has been evaluated in fourteen studies on different cancers, highlighting its potential versatility. The anticancer potency varied depending on extraction solvent and methodology. For instance, Nazir et al., (2021) observed differential activity across A-549 and HepG2 cell lines using various organic solvents [64]. Furthermore, the selectivity inhibition of the cancer cells has been tested in a study by Ibrahim et al. (2023), who utilized glucosinolates with L. sativum ethanol extracts [59]. The IC50 values for these glucosinolate-treated extracts ranged from 38.5 to 92.6 µg/mL and were noted to have no adverse effects on normal cell lines. While some nanoparticle-based formulations improved bioactivity [57], others, like Ag-MgO nanoparticles, showed no enhancement [55], suggesting that nanoparticle composition critically influences therapeutic efficacy.
Although most L. sativum studies were in vitro, one in vivo study demonstrated a 37.14% increase in lifespan in mice with Ehrlich ascites carcinoma treated with L. sativum seed extracts [81]. L. sativum extracts induced apoptosis and cell cycle arrest—key processes in cancer treatment [59]. Apoptosis, a programmed cell death, is regulated at the genetic level, ensuring the orderly and efficient removal of damaged cells [82]. Thus, the ability of L. sativum extracts to induce the apoptosis underlying its potential as a therapeutic agent.
R. tripartite has demonstrated cytotoxic effects. Methanol extracts exhibited the lowest IC50 values, particularly against the DLD-1 colon adenocarcinoma cell line (39.83 μg/mL) [68]. Aqueous extracts, on the other hand, showed less cytotoxicity, reaffirming the importance of solvent selection. The primary mechanism identified involves inhibition of the PI3K/AKT/mTOR pathway [70]. The PI3K/AKT/mTOR pathway plays a crucial role in survival, growth, and proliferation of the cells. Inhibition of this pathway results in suppression of the tumor progression [83].
The cytotoxic potential of P. communis was moderate and varied between studies, even though both used fruit extracts. IC₅₀ values ranged from 30.9 to 105 μg/mL in one study [72]; While the other reported lower cytotoxicity (IC₅₀: 0.4-3.2 mg/mL), possibly due to differences in extraction methods (hydroinstillation vs. UPLC-PDA-MS) [73]. While the findings indicate promising activity, the limited number of studies necessitates further investigation to validate their efficacy against cancer.
In contrast, C. murale demonstrated weak anticancer activity, with IC₅₀ values of 1504 μg/mL (MCF-7) and 1267 μg/mL (liver cancer) [74].These values indicate a relatively weak cytotoxic potential, which may limit its applicability in cancer therapy. However, further research using different extraction approaches or targeting other cancer types may yield better results.
E. hispanica showed moderate cytotoxic activity in one study, with IC50 values ranging from 14.7 μg/mL to 21.4 μg/mL across different cancer cell lines[10]. Similarly, T. hamosa demonstrated promising cytotoxicity (IC₅₀ = 6.7-13.7 μg/mL), though only one study has investigated its potential [75]. The limited data highlights the necessity for further investigation into these plants to better understand their anticancer potential, considering the influence of plant parts, different solvents and mechanism of action.
This systematic review has several important limitations. First, the literature search was conducted exclusively using Google Scholar, which may not provide the same level of indexing rigor or coverage as more specialized scientific databases. This may have led to the omission of relevant studies. Second, the review only included studies published in English, introducing potential language bias. Another major limitation is the lack of formal risk of bias assessment of the included studies, which undermines the ability to critically evaluate the quality and reliability of the evidence. Furthermore, there was considerable heterogeneity in study designs, plant parts used, extraction methods, solvents, cancer types, and assay conditions, which makes it difficult to compare findings or draw definitive conclusions. Lastly, while some in vivo studies were included, the review is heavily weighted toward in vitro data, limiting the applicability of findings to clinical or physiological settings.
5. Conclusion
Cancer remains a complex, multifactorial disease that is difficult to treat. For decades, using plants as a source of potential therapeutic agents has been a key approach. This systematic review highlights promising anticancer properties of several plant extracts, particularly S. costus, L. sativum, and R. tripartite, due to their ability to induce apoptosis. Extensive in vitro and in vivo studies on S. costus have demonstrated its significant cytotoxic potential. For S. costus in vitro studies, the polarity of the extraction solvents played a significant role in cytotoxic potency. L. sativum and R. tripartita also demonstrated notable activity, other plants—such as P. communis, C. murale, E. hispanica, and T. hamosa—require further exploration. Importantly, A. ochroleuca and G. odoratum have not been studied at all in this context and represent important gaps in literature.
Overall, this review underscores the therapeutic promise of several medicinal plants in cancer treatment and emphasizes the need for standardized methodologies, in vivo studies, and deeper mechanistic investigations to fully harness their potential.
Abbreviations
ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; DHE: dehydrocostuslactone; EGF: epidermal growth factor; ER: endoplasmic reticulum; GL: glucosinolates of leaf cell suspension; GLM: glucosinolates of leaf cell suspension treated with L-methionine; GLT: glucosinolates of leaf cell suspension treated with L-tyrosine; GSH: glutathione; LAMP: lysosome-associated membrane protein; LC3II: microtubule-associated protein 1 light chain 3-II lipidation chain indicator; MDA: malondialdehyde; MEK: mitogen-activated protein kinase kinase MAPKK; MMP: matrix metalloproteinase; MS: mass spectrometry; NF-κB: nuclear factor-kappa B; OL: petroleum ether extract of leaf cell suspension; OR: petroleum ether extract of root cell suspension; PARP: poly ADP-ribose polymerase; PDA: photodiode array detector; PI3K/AKT/mTOR: phosphatidylinositol 3-kinase PI3K protein kinase B AKT mammalian target of rapamycin mTOR; RNS: reactive nitrogen species; ROS: reactive oxygen species; TIMP: tissue inhibitor of metalloproteinases; TNF-α: tumor necrosis factor-alpha; UPLC: ultra-performance liquid chromatography; VEGF: vascular endothelial growth factor.
Acknowledgements
The authors want to express their sincerest gratitude to King Abdullah International Medical Research Center (KAIMRC) for supporting this research project (grant number #SPR24/007/5).
Funding
This research project (grant number #SPR24/007/5) was financially supported by King Abdullah International Medical Research Center (KAIMRC).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Majolo F, de Oliveira Becker Delwing LK, Marmitt DJ, Bustamante-Filho IC, Goettert MI. Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochem. Lett. 2019;31:196-207
2. Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005;100:72-9
3. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2024;74:229-63
4. Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017;7:339-48
5. Abdel-Aziz MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. SI Nanomater. Energy Environ. Appl. 2014;18:356-63
6. Abdelwahab SI, Taha MME, Alhazmi HA, Ahsan W, Rehman ZU, Bratty MA. et al. Phytochemical profiling of Costus (Saussurea lappa Clarke) root essential oil, and its antimicrobial and toxicological effects. Trop. J. Pharm. Res. 2021;18:2155-60
7. Ahmed SS, Ibrahim ME, El-Sawi SA, Motawe HM. Monitoring and evaluation of some Egyptian wild plants grown in the Eastern Desert of Egypt. J Mater Env. Sci. 2018;9:1692-9
8. Chatoui K, Talbaoui A, Aneb M, Bakri Y, Harhar H, Tabyaoui M. Phytochemical screening, antioxidant and antibacterial activity of Lepidium sativum seeds from Morocco. J Mater Env. 2016 7
9. Ledoux A, Martin B, De Tullio P, Tits M, Wauters JN, Choi YH, et al. Metabolomics analysis of Galium odoratum (L.) Scop.: impact of the plant population origin and growth conditions. 2015
10. Marzouk MM. Flavonoid constituents and cytotoxic activity of Erucaria hispanica (L.) Druce growing wild in Egypt. Arab. J. Chem. 2016;9:S411-5
11. Rao H, Ahmad S, Y.Aati H, Basit A, Ahmad I, Ahmad Ghalloo B. et al. Phytochemical screening, biological evaluation, and molecular docking studies of aerial parts of Trigonella hamosa (branched Fenugreek). Arab. J. Chem. 2023;16:104795
12. Sánchez-Mendoza ME, Castillo-Henkel C, Navarrete A. Relaxant action mechanism of berberine identified as the active principle of Argemone ochroleuca Sweet in guinea-pig tracheal smooth muscle. J. Pharm. Pharmacol. 2008;60:229-36
13. Sharma K, Pasricha V, Satpathy G, Gupta R K. Evaluation of phytochemical and antioxidant activity of raw Pyrus communis (l), an underexploited fruit. J. Pharmacogn. Phytochem. 2015;3:46-50
14. Al-Radadi NS. Saussurea costus for sustainable and eco-friendly synthesis of palladium nanoparticles and their biological activities. Arab. J. Chem. 2022;15:104294
15. Choi. Evaluation of anticancer activity of dehydrocostuslactone in vitro. Mol. Med. Rep. [Internet] 2009 [cited. 2024 Nov 16];3. Available from: http://www.spandidos-publications.com/mmr/3/1/185
16. Dong G zhi, Shim AR, Hyeon JS, Lee HJ, Ryu JH. Inhibition of Wnt/β-Catenin Pathway by Dehydrocostus Lactone and Costunolide in Colon Cancer Cells: SAUSSUREA LAPPA INHIBITS WNT/Β-CATENIN PATHWAY. Phytother. Res. 2015;29:680-6
17. Gao K, Chen Z, Zhang N, Jiang P. High throughput virtual screening and validation of Plant-Based EGFR L858R kinase inhibitors against Non-Small cell lung Cancer: An integrated approach Utilizing GC-MS, network Pharmacology, Docking, and molecular dynamics. Saudi Pharm. J. 2024;32:102139
18. Hsu YL, Wu LY, Kuo PL. Dehydrocostuslactone, a Medicinal Plant-Derived Sesquiterpene Lactone, Induces Apoptosis Coupled to Endoplasmic Reticulum Stress in Liver Cancer Cells. J. Pharmacol. Exp. Ther. 2009;329:808-19
19. Hu XF, Liu WX, Zhang R, Zhang W, Wang C, Chen M. et al. Essential oil from Saussurea costus inhibits proliferation and migration of Eca109 cells via mitochondrial apoptosis and STAT3 signaling. Asian Pac. J. Trop. Biomed. 2022;12:253-61
20. Hua P, Zhang G, Zhang Y, Sun M, Cui R, Li X. et al. Costunolide induces G1/S phase arrest and activates mitochondrial-mediated apoptotic pathways in SK-MES 1 human lung squamous carcinoma cells. Oncol. Lett. 2016;11:2780-6
21. Kim EJ, Lim SS, Park SY, Shin HK, Kim JS, Park JHY. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem. Toxicol. 2008;46:3651-8
22. Kretschmer N, Rinner B, Stuendl N, Kaltenegger H, Wolf E, Kunert O. et al. Effect of Costunolide and Dehydrocostus Lactone on Cell Cycle, Apoptosis, and ABC Transporter Expression in Human Soft Tissue Sarcoma Cells. Planta Med. 2012;78:1749-56
23. Kumar A, Kumar S, Kumar D, Agnihotri VK. UPLC/MS/MS method for quantification and cytotoxic activity of sesquiterpene lactones isolated from Saussurea lappa. J. Ethnopharmacol. 2014;155:1393-7
24. Moon SM, Yun SJ, Kook JK, Kim HJ, Choi MS, Park BR. et al. Anticancer activity of Saussurea lappa extract by apoptotic pathway in KB human oral cancer cells. Pharm. Biol. 2013;51:1372-7
25. Oh GS, Pae HO, Chung HT, Kwon JW, Lee JH, Kwon TO. et al. Dehydrocostus Lactone Enhances Tumor Necrosis Factor-α-Induced Apoptosis of Human Leukemia HL-60 Cells. Immunopharmacol. Immunotoxicol. 2004;26:163-75
26. Pavan Kumar Ch, Devi A, Ashok Yadav P, Rao Vadaparthi R, Shankaraiah G, Sowjanya P. et al. “Click” reaction mediated synthesis of costunolide and dehydrocostuslactone derivatives and evaluation of their cytotoxic activity. J. Asian Nat. Prod. Res. 2016;18:1063-78
27. Peng Y, Zhou T, Wang S, Bahetjan Y, Li X, Yang X. Dehydrocostus lactone inhibits the proliferation of esophageal cancer cells in vivo and in vitro through ROS-mediated apoptosis and autophagy. Food Chem. Toxicol. 2022;170:113453
28. Peng Z, Wang Y, Gu X, Wen Y, Yan C. A platform for fast screening potential anti-breast cancer compounds in traditional Chinese medicines. Biomed. Chromatogr. 2013;27:1759-66
29. Rahman MdA, Hong JS, Huh SO. Antiproliferative properties of Saussurea lappa Clarke root extract in SH-SY5Y neuroblastoma cells via intrinsic apoptotic pathway. Anim. Cells Syst. 2015;19:119-26
30. Shati AA, Alkahtani MA, Alfaifi MY, Elbehairi SEI, Elsaid FG, Prasanna R. et al. Secondary Metabolites of Saussurea costus Leaf Extract Induce Apoptosis in Breast, Liver, and Colon Cancer Cells by Caspase-3-Dependent Intrinsic Pathway. BioMed Res. Int. 2020;2020:1-11
31. Su J, H JoKD, Jeong HG, Gyu NC, Gyu GS. Inhibition of Cellular Proliferation by p53 dependent Apoptosis and G2M Cell Cycle Arrest of Saussurea lappa CLARKE in AGS Gastric Cancer Cell Lines. J. Physiol. Pathol. Korean Med. 2004;18:1186-91
32. Tabata K, Nishimura Y, Takeda T, Kurita M, Uchiyama T, Suzuki T. Sesquiterpene lactones derived from Saussurea lappa induce apoptosis and inhibit invasion and migration in neuroblastoma cells. J. Pharmacol. Sci. 2015;127:397-403
33. Tian X, Song HS, Cho YM, Park B, Song YJ, Jang S. et al. Anticancer effect of Saussurea lappa extract via dual control of apoptosis and autophagy in prostate cancer cells. Medicine (Baltimore). 2017;96:e7606
34. Kim HR, Kim JM, Kim MS, Hwang JK, Park YJ, Yang SH. et al. Saussurea lappa extract suppresses TPA-induced cell invasion via inhibition of NF-κB-dependent MMP-9 expression in MCF-7 breast cancer cells. BMC Complement. Altern. Med. 2014;14:170
35. Mohsen E, El-Far AH, Godugu K, Elsayed F, Mousa SA, Younis IY. SPME and solvent-based GC-MS metabolite profiling of Egyptian marketed Saussurea costus (Falc.) Lipsch. concerning its anticancer activity. Phytomedicine Plus. 2022;2:100209
36. Alotaibi AA, Bepari A, Assiri RA, Niazi SK, Nayaka S, Rudrappa M. et al. Saussurea lappa Exhibits Anti-Oncogenic Effect in Hepatocellular Carcinoma, HepG2 Cancer Cell Line by Bcl-2 Mediated Apoptotic Pathway and Mitochondrial Cytochrome C Release. Curr. Issues Mol. Biol. 2021;43:1114-32
37. Bhushan A, Rani D, Tabassum M, Kumar S, Gupta PN, Gairola S. et al. HPLC-PDA Method for Quantification of Bioactive Compounds in Crude Extract and Fractions of Aucklandia costus Falc. and Cytotoxicity Studies against Cancer Cells. Molecules. 2023;28:4815
38. Kim EJ, Hong JE, Lim SS, Kwon GT, Kim J, Kim JS. et al. The Hexane Extract of Saussurea lappa and Its Active Principle, Dehydrocostus Lactone, Inhibit Prostate Cancer Cell Migration. J. Med. Food. 2012;15:24-32
39. Choi YJ, Choi YK, Ko SG, Cheon C, Kim TY. Investigation of Molecular Mechanisms Involved in Sensitivity to the Anti-Cancer Activity of Costunolide in Breast Cancer Cells. Int. J. Mol. Sci. 2023;24:4009
40. Alhakamy NA, Badr-Eldin SM, Ahmed OAA, Aldawsari HM, Okbazghi SZ, Alfaleh MA. et al. Green Nanoemulsion Stabilized by In Situ Self-Assembled Natural Oil/Native Cyclodextrin Complexes: An Eco-Friendly Approach for Enhancing Anticancer Activity of Costunolide against Lung Cancer Cells. Pharmaceutics. 2022;14:227
41. Sun S H, Ko SG. Effects of Costunolide Derived from Saussurea lappa Clarke on Apoptosis in AGS Stomach Cancer Cell Lines. J. Korean Med. 2006;27:48-95
42. Ahmed HY, Kareem SM, Atef A, Safwat NA, Shehata RM, Yosri M. et al. Optimization of Supercritical Carbon Dioxide Extraction of Saussurea costus Oil and Its Antimicrobial, Antioxidant, and Anticancer Activities. Antioxidants. 2022 11
43. Amina M, Al Musayeib NM, Alarfaj NA, El-Tohamy MF, Oraby HF, Al Hamoud GA. et al. Biogenic green synthesis of MgO nanoparticles using Saussurea costus biomasses for a comprehensive detection of their antimicrobial, cytotoxicity against MCF-7 breast cancer cells and photocatalysis potentials. PLOS ONE. 2020;15:e0237567
44. Kumar R, Bhardwaj P, Virmani DN, Asrani RK, Patel SK, Gupta VK, et al. Modulation of Apoptotic Signaling Pathways by Saussurea Costus (Falc.) Lipsch Root Ethanolic Extract on Human Breast Cancer Mcf-7 Cells [Internet]. 2023 [cited 2024 Nov 16];Available from: https://www.ssrn.com/abstract=4513922
45. Ko SG, Koh SH, Jun CY, Nam CG, Bae HS, Shin MK. Induction of Apoptosis by Saussurea lappa and Pharbitis nil on AGS Gastric Cancer Cells. Biol. Pharm. Bull. 2004;27:1604-10
46. Jin M, Ryu JH, Ryu SY, Chung KS. Cytotoxic Effects on Human Cancer Cells and Apoptosis of a Sesquiterpene Lactone from Saussure lappa. Biomol. Ther. 2000;8:22-6
47. Choi YK, Cho SG, Woo SM, Yun YJ, Jo J, Kim W. et al. Saussurea lappa Clarke-Derived Costunolide Prevents TNF α -Induced Breast Cancer Cell Migration and Invasion by Inhibiting NF- κ B Activity. Evid. Based Complement. Alternat. Med. 2013;2013:1-10
48. Al-Zayadi ZA, Shanan HK, Akool ASK. Evaluation of the Anticancer Effect of Saussurea costus Root Extract Against Induced Hepatic and Renal Cancer in White Mice: A Histopathological Study. Adv. Anim. Vet. Sci. [Internet] 2023 [cited. 2024 Nov 16];11. Available from: http://researcherslinks.com/current-issues/Evaluation-Anticancer-Effect-Saussurea-costus-Root-Extract-Against/33/1/6297/html
49. Chen Y, Shen J, Yuan M, Li H, Li Y, Zheng S. et al. Dehydrocostus lactone suppresses gastric cancer progression by targeting ACLY to inhibit fatty acid synthesis and autophagic flux. J. Adv. Res. [Internet]. 2024 Available from: https://www.sciencedirect.com/science/article/pii/S2090123224000407
50. Elshabrawy A, Salem M, Ahmed F, Nabeeh A, Abdel-Wahhab K. The Therapeutic and Synergistic Effect of Saussurea costus Extract against Induced Leukemia in Adult Male Rats. Egypt. Acad. J. Biol. Sci. C Physiol. Mol. Biol. 2023;15:1-19
51. Kumar R, Bhardwaj P, Soni M, Singh R, Choudhary S, Virmani N. et al. Modulation of mammary tumour progression using murine model by ethanol root extract of Saussurea costus (falc.) lipsch. J. Ethnopharmacol. 2024;319:117302
52. Lin X, Peng Z, Fu X, Liu C, Xu Y, Ji W. et al. Volatile oil from Saussurea lappa exerts antitumor efficacy by inhibiting epithelial growth factor receptor tyrosine kinase-mediated signaling pathway in hepatocellular carcinoma. Oncotarget. 2016;7:79761-73
53. Abd-elmegeed ASS, saad Abd-alrahman H, Mohamed AA, Ghaber BM. Biological properties and identification of some active ingredients in Anastatica hierochuntica and Lepidium sativum, grown in Egypt. Int. J. Sci. Res. Arch. 2023;10:435-45
54. AlObaidi LA. Study the anticancer effect of Lepidium sativum leaves extract on squamous cell carcinoma (CAL-27) cell lines. J. Nat. Sci. Res. 2014;4:48-52
55. Amina M, Al Musayeib NM, Al-Hamoud GA, Al-Dbass A, El-Ansary A, Ali MA. Prospective of biosynthesized L.satiVum oil/PEG/Ag-MgO bionanocomposite film for its antibacterial and anticancer potential. Saudi J. Biol. Sci. 2021;28:5971-85
56. Basaiyye SS, Kashyap S, Krishnamurthi K, Sivanesan S. Induction of apoptosis in leukemic cells by the alkaloid extract of garden cress (Lepidium sativum L.). J. Integr. Med. 2019;17:221-8
57. Efati Z, Shahangian SS, Darroudi M, Amiri H, Hashemy SI, Aghamaali MR. Green chemistry synthesized zinc oxide nanoparticles in Lepidium sativum L. seed extract and evaluation of their anticancer activity in human colorectal cancer cells. Ceram. Int. 2023;49:32568-76
58. El-Haggar M, El-Hosseiny L, Ghazy NM, El-Fiky FK, El-Hawiet A. Phytochemical investigation, antimicrobial and cytotoxic activities of suspension cultures of Lepidium sativum L. South Afr. J. Bot. 2021;138:500-5
59. Ibrahim MM, Mounier MM, Bekheet SA. Targeting apoptotic anticancer response with natural glucosinolates from cell suspension culture of Lepidium sativum. J. Genet. Eng. Biotechnol. 2023;21:53
60. Indumathy R, Aruna A. Cytotoxic potential of various extracts of Lepidium sativum (Linn.). An in-vitro evaluation. Int J Pharm Pharm Sc. 2015;2:1-5
61. Jahani S, Heidari Z, Azami M, Moudi B. Comparison of Anticancer Effects of Hydroalcoholic Extracts of Camellia sinensis and Lepidium sativum L on HeLa Cell Line. Int. J. Cancer Manag. [Internet] 2020 [cited. 2024 Nov 16];13. Available from: https://brieflands.com/articles/ijcm-98913.html
62. Mahassni SH, Al-Reemi RM. Cytotoxic effect of an aqueous extract of Lepidium sativum L. seeds on human breast cancer cells. 2013.
63. Meer B, Andleeb A, Iqbal J, Ashraf H, Meer K, Ali JS. et al. Bio-Assisted Synthesis and Characterization of Zinc Oxide Nanoparticles from Lepidium sativum and Their Potent Antioxidant, Antibacterial and Anticancer Activities. Biomolecules. 2022;12:855
64. Nazir S, El-Sherif AA, Abdel-Ghani NT, Ibrahim MAA, Hegazy MEF, Atia MAM. Lepidium sativum Secondary Metabolites (Essential Oils): In Vitro and In Silico Studies on Human Hepatocellular Carcinoma Cell Lines. Plants. 2021;10:1863
65. Rajasekaran R, Suresh PK. Evaluation of Cell Death Potential of Lepidium sativum Seed Extracts in MCF-7 Cells and Molecular Docking-based Correlation of Identified Bioactive Components with Human Caspase-6 Protein. Indian J. Pharm. Educ. Res. 2022;56:166-74
66. Selek S, Koyuncu I, Caglar HG, Bektas I, Yilmaz MA, Gonel A. et al. The evaluation of antioxidant and anticancer effects of Lepidium Sativum Subsp Spinescens L. methanol extract on cancer cells. Cell. Mol. Biol. 2018;64:72-80
67. Alqahtani AS, Abdel-Mageed WM, Shahat AA, Parvez MK, Al-Dosari MS, Malik A. et al. Proanthocyanidins from the stem bark of Rhus tripartita ameliorate methylgloxal-induced endothelial cell apoptosis. J. Food Drug Anal. 2019;27:758-65
68. Ben Miled H, Saada M, Jallali I, Ben Barka Z, Tlili M, Alimi H. et al. Variability of antioxidant and biological activities of Rhus tripartitum related to phenolic compounds. EXCLI J. 16Doc439 ISSN 1611-2156 [Internet] 2017 [cited. 2024 Nov 10];Available from: https://www.excli.de/vol16/Ben_Miled_31032017_proof.pdf
69. Rekik I, Ben Ameur R, Ayadi W, Soussi A, Gargouri A, Allouche N. Anti-oxidant, anti-diabetic and anti-lipidemic activities of root bark extracts from Rhus tripartitum and cytotoxicity evaluation of isolated compounds. South Afr. J. Bot. 2022;147:71-80
70. Tlili H, Macovei A, Buonocore D, Lanzafame M, Najjaa H, Lombardi A. et al. The polyphenol/saponin-rich Rhus tripartita extract has an apoptotic effect on THP-1 cells through the PI3K/AKT/mTOR signaling pathway. BMC Complement. Med. Ther. 2021;21:153
71. Tlili H, Hanen N, Ben Arfa A, Neffati M, Boubakri A, Buonocore D. et al. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLOS ONE. 2019;14:e0213049
72. El-Hawary SS, El-Tantawi ME, Kirollos FN, Hammam WE. Chemical Composition, in Vitro Cytotoxic and Antimicrobial Activities of Volatile Constituents from Pyrus communis L. and Malus domestica Borkh. Fruits cultivated in Egypt. J. Essent. Oil Bear. Plants. 2018;21:1642-51
73. Kolniak-Ostek J, Kłopotowska D, Rutkowski KP, Skorupińska A, Kruczyńska DE. Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus communis L.) Fruits. Molecules. 2020;25:4444
74. Hameed MF, Al-Shawi AAA. Phytochemical Analysis and Anticancer Evaluation of Iraqi Herb Chenopodium Murale Extracted by Microwave-assisted Extraction. 2021 [cited. 2024 Nov 10];Available from: http://rgdoi.net/10.13140/RG.2.2.33581.20969
75. Khalil HE, Ibrahim HIM, Ahmed EA, Emeka PM, Alhaider IA. Orientin, a Bio-Flavonoid from Trigonella hamosa L, Regulates COX-2/PGE-2 in A549 Cell Lines via miR-26b and miR-146a. Pharmaceuticals. 2022;15:154
76. Nasim N, Sandeep IS, Mohanty S. Plant-derived natural products for drug discovery: current approaches and prospects. The Nucleus. 2022;65:399-411
77. Lei Z, Tian Q, Teng Q, Wurpel JND, Zeng L, Pan Y. et al. Understanding and targeting resistance mechanisms in cancer. MedComm. 2023;4:e265
78. Binobead MA, Aziz IM, Ibrahim SM, Aljowaie RM. Chemical composition and bioactivities of the methanol root extracts of Saussurea costus. Open Chem. 2024;22:20240002
79. Levantini E, Maroni G, Del Re M, Tenen DG. EGFR signaling pathway as therapeutic target in human cancers. Target. Cell. Signal. Pathw. 2022;85:253-75
80. Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: molecular insights and therapeutic approaches. Cell. Oncol. 2020;43:1-18
81. El Sayed RAA, Hanafy ZEM, Abd El Fattah HF, Mohamed AK. Possible antioxidant and anticancer effects of plant extracts from Anastatica hierochuntica, Lepidium sativum and Carica papaya against Ehrlich ascites carcinoma cells. Cancer Biol. 2020;10:1-16
82. Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8:603-19
83. Li Q, Li Z, Luo T, Shi H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. Mol. Biomed. 2022;3:47
84. Patel AA, Amanullah M, Elsaid FG, Soliman T, Eissa M, Alothaid H. In-Vitro evaluation of anti-cancer and genotoxic potential of medicinal herb Saussurea lappa extract in human cancer cell lines. Eur. J. Mol. Clin. Med. 2020 7
85. Ansari S, Hasan K, Bhat S. Anticancer, antioxidant, and hepatoprotective activity of Saussurea lappa, C.B. clarke (qust) on human hepatoma cell line. J. Cancer Res. Ther. 2021;17:499-503
86. Yang M, Zhang J, Li Y, Han X, Gao K, Fang J. Bioassay-guided isolation of dehydrocostus lactone from Saussurea lappa: A new targeted cytosolic thioredoxin reductase anticancer agent. Arch. Biochem. Biophys. 2016;607:20-6
87. Okubo S, Ohta T, Fujita H, Shoyama Y, Uto T. Costunolide and dehydrocostuslactone from Saussurea lappa root inhibit autophagy in hepatocellular carcinoma cells. J. Nat. Med. 2021;75:240-5
88. Peng Z, Wang Y, Fan J, Lin X, Liu C, Xu Y. et al. Costunolide and dehydrocostuslactone combination treatment inhibit breast cancer by inducing cell cycle arrest and apoptosis through c-Myc/p53 and AKT/14-3-3 pathway. Sci. Rep. 2017;7:41254
89. Kumar R, Tripathi BN, Rana J, Bhardwaj P, Singla A, Asrani RK, et al. Prevention of DMBA-induced Mammary Tumors from Pulmonary Metastases by Saussurea costus (Falc.) Lipsc Root Ethanolic Extract in Sprague Dawley Rats. Indian J. Anim. Res. [Internet] 2024 [cited 2024 Nov 16];Available from: http://arccjournals.com/journal/indian-journal-of-animal-research/B-5170
90. Zhang R, Hao J, Wu Q, Guo K, Wang C, Zhang WK. et al. Dehydrocostus lactone inhibits cell proliferation and induces apoptosis by PI3K/Akt/Bad and ERS signalling pathway in human laryngeal carcinoma. J. Cell. Mol. Med. 2020;24:6028-42
Author contact
![]() Corresponding author: Dr. Sahar Alghamdi, ghamdisaedu.sa.
Corresponding author: Dr. Sahar Alghamdi, ghamdisaedu.sa.

 Global reach, higher impact
Global reach, higher impact