Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(13):3972-3990. doi:10.7150/jca.116362 This issue Cite
Review
Skin Cancer: Epidemiology, Screening and Clinical Features of Acral Lentiginous Melanoma (ALM), Melanoma In Situ (MIS), Nodular Melanoma (NM) and Superficial Spreading Melanoma (SSM)
1. Institute of Medicine, School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, No. 155, Sec. 2, Li-Nong Street, Beitou District, Taipei 112304, Taiwan.
2. Department of Surgery, Division of Plastic and Reconstructive Surgery, Taichung Veterans General Hospital, 1650 Taiwan Boulevard Sect. 4, Taichung 407219, Taiwan.
3. Department of Dermatology, Dalin Tzu Chi General Hospital, No. 2, Min-Sheng Rd., Dalin Town, Chiayi 62247, Taiwan.
4. Department of Mechanical Engineering, National Chung Cheng University, 168, University Rd., Min Hsiung, Chia Yi 62102, Taiwan.
5. Department of Biotechnology, Karpagam Academy of Higher Education, Salem - Kochi Hwy, Eachanari, Coimbatore, Tamil Nadu 641021, India.
6. Department of General Surgery, Kaohsiung Armed Forces General Hospital, 2, Zhongzheng 1st.Rd., Lingya District, Kaohsiung City 80284, Taiwan.
7. Department of Medical Research, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, No. 2, Minsheng Road, Dalin, Chiayi 62247, Taiwan.
8. Director of Technology Development, Hitspectra Intelligent Technology Co., Ltd., Kaohsiung 80661, Taiwan.
Received 2025-4-24; Accepted 2025-7-9; Published 2025-9-21
Abstract
Melanoma, a highly aggressive form of skin cancer, presents considerable challenges in early detection and accurate diagnosis, particularly across its diverse subtypes such as acral lentiginous melanoma (ALM), melanoma in situ (MIS), nodular melanoma (NM), and superficial spreading melanoma (SSM). This study assesses the epidemiology, clinical characteristics, and screening techniques related to various melanoma subtypes, emphasizing their distinct features and risk factors. Moreover, the use of machine learning (ML) methodologies to categorize melanoma subtypes and the thorough examination of advancements in AI-based melanoma diagnosis, primarily emphasizing convolutional neural networks (CNN) and transfer learning approaches. Evaluate the efficacy of several deep learning models in classifying melanoma subtypes while addressing significant obstacles, including class imbalance and model generalization. Furthermore, it contemplates the integration of multimodal data, including genetic information and patient demographics, to enhance diagnostic accuracy. This comprehensive review assesses the epidemiology, clinical characteristics, and machine learning techniques utilized for the classification and detection of different melanoma subtypes, emphasizing recent advancements in AI-driven methods and their clinical significance.
Keywords: Skin Cancer, Acral Lentiginous Melanoma, Melanoma in situ, Nodular Melanoma, and Superficial Spreading Melanoma, Machine Learning, Artificial Intelligence
Introduction
In humans, skin cancer is the most common cancer, with millions of cases diagnosed annually, particularly in the white population [1]. Ultraviolet radiation (UVR) and radiotherapy or immunosuppressive therapy caused by environmental exposure, that results in skin cancer [2]. The white populations are caused in 90 - 95% by UV radiation in skin cancer and therefore the population-attributable factors are considered to be predominantly [3]. Generally, skin cancer is classified into melanoma and non-melanoma skin cancer (NMSC) will be developed by the derived cell [4]. Melanoma highly deadly type of skin cancer because it causes most deaths and minority populations, melanoma occurs more commonly in unusual anatomic locations compared to white populations [5, 6]. Compared to melanomas, non-melanomas are the largest common kind of skin cancer [7]. Melanoma is a dangerous type of skin cancer, with a global death rate of 14%, and to the World Health Organization, nearly 7,650 deaths from melanoma were anticipated in 2022, and 99,780 new melanoma cases in the USA [8, 9]. There are 1,958,310 newly diagnosed cancer cases and 609,820 cancer deaths in the United States in 2023 [10]. In the United States, 5.4 million new cases of skin cancer in every year [11]. In 2020, the estimated 19.3 million new causes with a 95% uncertainty interval ranging between 19.0 to 19.6 million cancers and almost 10.0 million deaths from cancer, with a 95% uncertainty interval of 9.7 to 10.2 million [12].
Diagnosis of cutaneous cancer usually begins with a skin assessment, dermoscopy, patient history, and surgical biopsy [13]. The management of skin cancer has a long and successful history in radiation therapy (RT), which is a complementary method in cutaneous oncology [14]. The ABCBE mnemonic stands for asymmetry (A), border irregularity (B), color variability (C), diameter (D), and evolution (E) or any change. Additionally, the morphology, location of the body, and arrangement of lesions may also provide information about skin malignancy [15]. There are four main types of skin melanoma: acral lentiginous melanoma (ALM), melanoma in situ (MIS), nodular melanoma (NM), and superficial spreading melanoma [16].
ALM is a subtype that is normally diagnosed at later stages due to poor attention to lesions arising on extremities and medical diagnostic mistakes [17]. ALM occurs on acral skin, including the nail beds, soles, and palms [18]. It is challenging to clinically diagnose ALM, particularly in its early stages, due to the subtle clinical and histopathologic changes [19]. ALM subtypes are the most frequent types of malignant among Asian people and are found in people with dark skin tone, particularly in the soles of the feet [20]. In individuals with Asian, black, or dark brown skin, the most common is ALM [21]. ALM is rare in all ethnicities because other melanoma types are even less common in African Americans, Hispanics, and Asians, ALM represents a common melanoma seen in ethnic groups [22]. ALM is the majority type of melanoma in several African, South American, and Asian countries it represents a moderately low percentage of melanoma cases in some countries. In populations of European descent, such as the United Kingdom, Australia, and the United States [23]. MIS is a special challenge in histopathology, clinical management, and treatment [24]. The incidence of MIS is increasing more rapidly than any invasive or in situ cancer in the US, and it represents the earliest form of melanoma where the malignancy is localized to the epidermis [25, 26]. The prevalence of MIS is increasing as the population ages and risk factors, including immunosuppression and sun exposure, are becoming more common, making treatment increasingly necessary, as long-term cumulative sun exposure is linked to the development of MIS [27, 28]. NM is the most popular type of melanoma, typically diagnosed between 40 and 50 years old, and similarly common in each sex. The trunk and neck are the most common locations for occurrences [29, 30]. NM most commonly appears on the chest or back and tends to grow vertically in the skin, deeply penetrating if not removed [31]. NM has a poor prognosis, and it includes 12% - 30% of all diagnosed melanomas, with the largest incidence rates in Australia and New Zealand [32, 33]. SSM is the usual subtype of melanoma among fair-skin people, corresponding to 70% of cases, and it is a specific histologic subtype of cutaneous melanoma [34, 35]. It begins an initial radial growth phase, characterized by a growth limited to the skin layer, then a depth growth phase that involves the presence of invasion [36]. Between 1978 and 2007, the incidence and survival of SSM have increased, while the incidence and survival rates for NM have changed [37].
This research offers a comprehensive review that synthesizes existing knowledge about the utilization of machine learning and artificial intelligence approaches for the identification and classification of melanoma subtypes. This review critically evaluates existing methodologies, including deep learning models like convolutional neural networks and transfer learning approaches, to elucidate their merits, limitations, and practical issues, in contrast to original experimental findings. The report synthesizes recent research findings to present an overview of technical breakthroughs and emerging trends, while noting gaps and opportunities for further inquiry. This comprehensive analysis aims to aid physicians, researchers, and AI practitioners in comprehending the advancing domain of AI-enhanced melanoma diagnosis and to facilitate the creation of more precise and universally applicable diagnostic instruments.
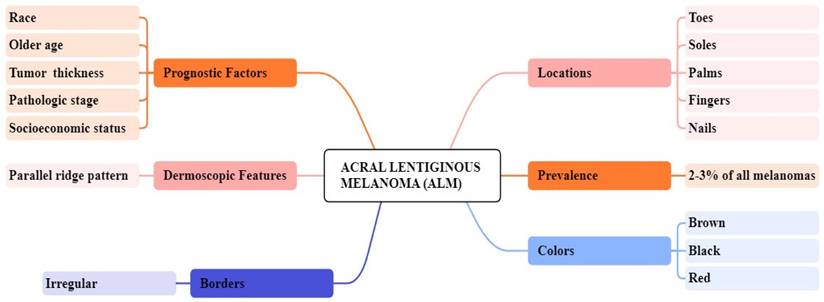
Clinical Features of ALM, MIS, NM, SSM
Acral lentiginous melanoma (ALM)
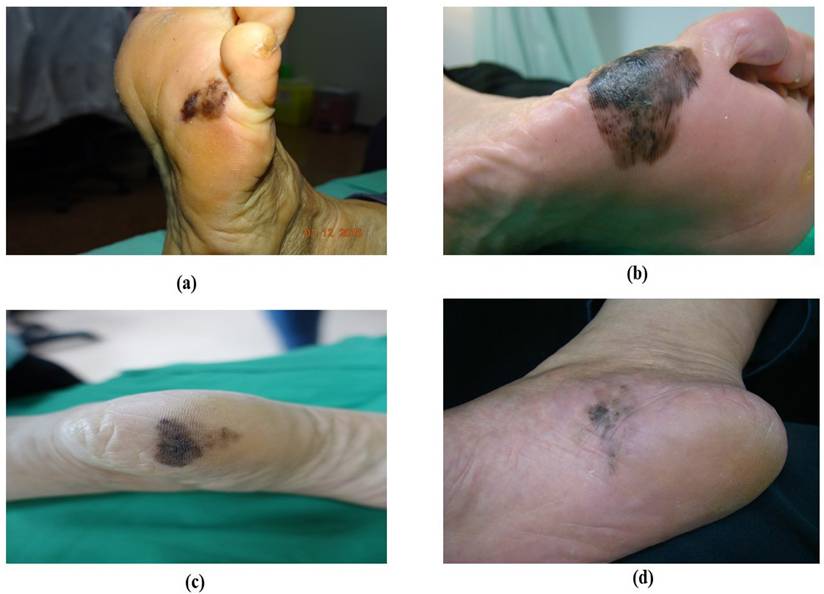
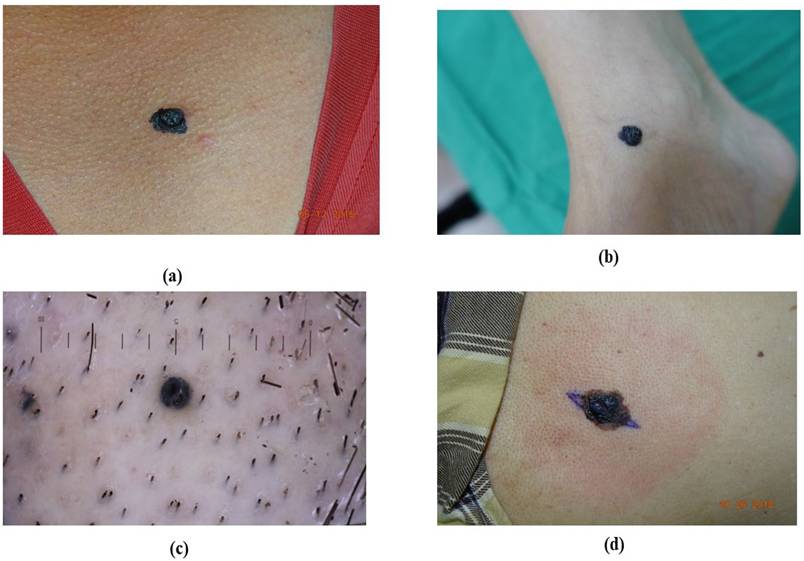
ALM represents about 2 to 3% of all melanoma cases and its uncommon form of melanoma is related to later diagnosis and lower survival percentages [38, 39]. It is found beneath the nail plate or sole, toes, fingers, and hairless skin of palms. The colors that appear on ALM are brown, black, and red as shown in Figure 1 [40]. Clinically, ALM shows an initial radial growth phase, appearing as variegated pigmentation, and an uneven brown to-black macule [41]. The unique dermatoscopic characteristic of ALM is the linear ridge pattern, which is characterized by linear pigmentation along the bands of volar skin as shown in Figure 2 [42]. Prognostic factors include older age, pathologic stage, tumor thickness, socioeconomic status, and race [43]. Histopathologically, ALM is distinguished by lentiginous proliferation during the radial growth phase. Although rare, ALM represents around 10% of all melanomas [44]. Subungual variants are rarer in the white-skinned population, with additional clinical features including clinical hypomelanosis, a family record of melanoma, hair color, and any previous history of non-melanoma skin cancer [45, 46].
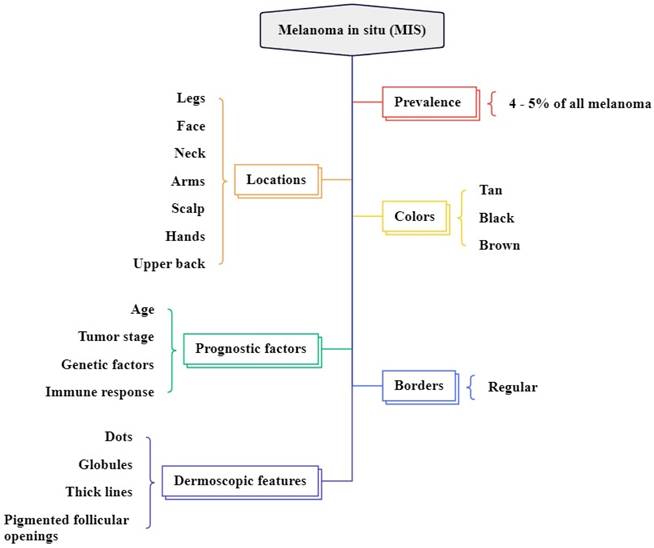
Melanoma in situ (MIS)
MIS accounts for 4% to 15% of all melanoma types and commonly arises in chronically sun-damaged areas of the skin, particularly in older individuals, and begins as a brown or tan macule as shown in Figure 3 [47, 48]. It is often found on the neck, face, and scalp of advanced-age patients with major sun-induced skin damage, and can also occur in non-head and neck regions like the legs, forearms, and back of the hands as shown in Figure 4 [49, 50]. The colors are variable shades of dark brown, tan, brown, and black [51]. The dermatoscopic features are asymmetrically pigmented with follicular openings, dots, and globules aggregated around adnexal openings, thick pigmented lines, and an annular granular pattern [30]. In difference to other standard types of MIS, the borders of lentigo maligna (LM) are regularly defined histologically and clinically, as the lesion usually combines with the surrounding area of long-term sun damage [52].
Acral lentiginous melanoma based on their appearance. (a) brownish ALM at the left sole, (b) blackish ALM at the right sole, (c) blackish ALM at the left sole (d) blackish ALM at the sole
Overview of ALM clinical features
Melanoma in situ (MIS) based on their color. (a) brownish MIS (b) blackish MIS (c) pinkish MIS, (d) reddish MIS
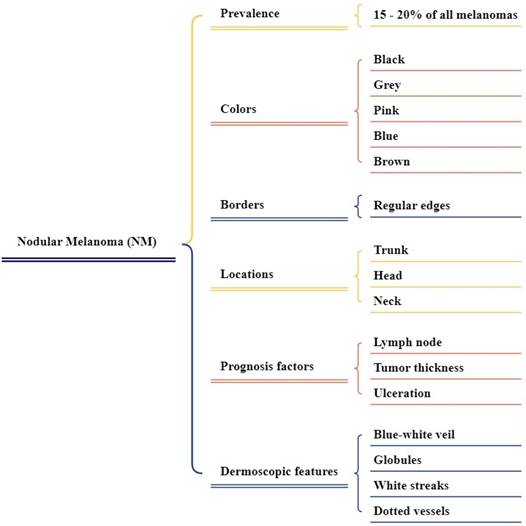
Nodular melanoma (NM)
NM is the second greatest well-known type in light-skinned individuals, representing around 15 to 20% of melanoma cases. It is most generally located on the trunk, neck, or head, more common in males as shown in Figure 5 [53]. The colors found in NM vary from brown, blue, black, grey, and pink, including other shades of these color or their combinations. The surface of NM can be rough, scaly, or smooth as shown in Figure 6 [54]. Dermatoscopic features of NM include a blue-white veil, white streaks, isolated globules, and dotted vessels or uneven linear [55]. NM indicates the lack of the early radial growth phase, which begins with perpendicular growth, and lesions are usually symmetric, with small diameters, regular borders, and uniform color [56, 57]. In the Early stage, the injury is usually an irregular black or blue nodule with even edges [58]. NM lesions are more common and lighter colored than other common melanoma subtypes [59].
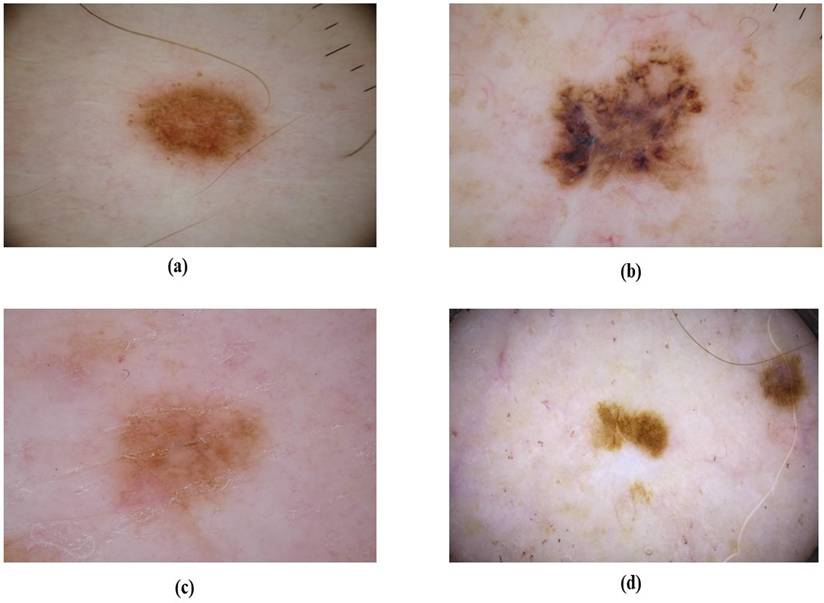
Superficial spreading melanoma (SSM)
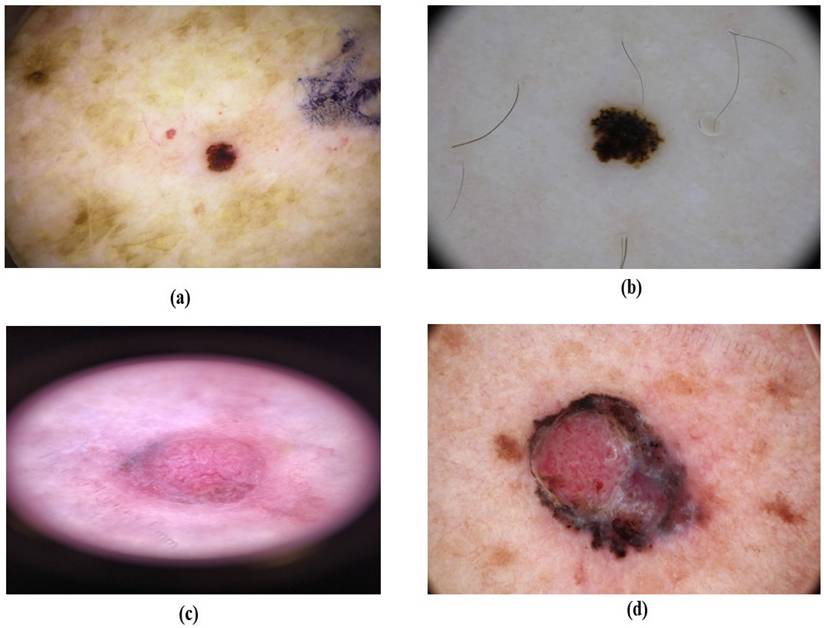
SSM represents between 50% to 70% of melanoma cases and most commonly on the trunk in males and on the lower extremities in females [60, 61]. SSM typically presents as a macule or plaque with an uneven border and variable pigmentation, its measures range from several millimeters to various centimeters, often displaying multiple colors like blue, red, gray, black, and white as shown in Figure 7 [62]. Dermatoscopic features are a blue-white layer, peripheral black dots, several brown spots, and irregular vascular structures as shown in Figure 8 [63]. Histopathological features of SSM include asymmetry, lack of cellular maturation, and poor circumscription [64].
Screening and Diagnosis of ALM, MIS, NM, SSM
Screening and Diagnosis of ALM
Etiology
The case of ALM remains uncertain, as familial cases have not been reported so far, there is diffused evidence indicating that some genetic risk factors may be present. For instance, a major longitudinal study observed that patients with degree relatives diagnosed with ALM had an increased risk of any major melanoma subtypes, indicating some shared genetic factors among various melanoma types [65]. In some studies, dummy or shearing stress was suggested as a cause of the occurrence of the ALM this becomes clear because ALM is most commonly found in load-bearing zones of the foot, such as the heel, forefoot, and lateral side [66, 67]. Ghanavatian et al., followed up on this and noted that adjusted for surface area, ALM occurrence was inversely proportional associated with atypical and benign acral nevi presence in these weight-bearing areas. The incidence of this phenomenon is much lower among men than women [68].
Overview of MIS clinical features
Nodular melanoma (NM) based on their location (a) NM at neck, (b) NM at leg, (c) NM at head, (d) NM at chest.
Overview of NM clinical features.
Superficial Spreading Melanoma (SSM) based on their shape(a) reddish SSM with round, (b) brownish SSM at irregular borders, (c) reddish SSM at irregular round, (d) brownish SSM at small irregular borders.
Overview of SSM clinical features.
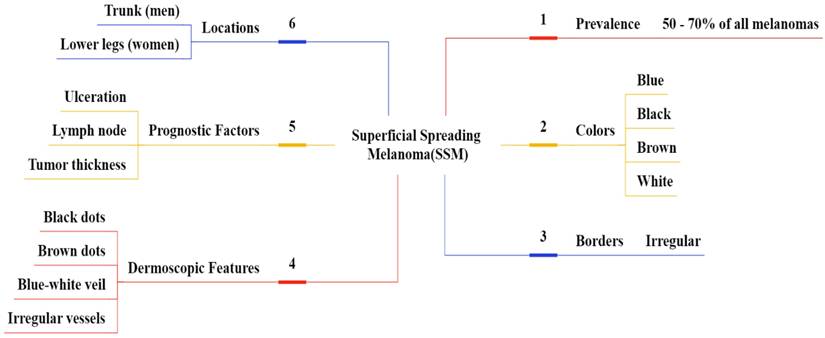
Epidemiology
ALM is in the range of 2 to 3% among newly identified melanomas, and the average age of diagnosis is 62.8 years. Changing ALM distribution seems to positively influence women's age, which generally begins when reaching 80 years old, and suddenly it increases within this age range. Male and female populations affected by ALM are reported to be approximately equal. However, females are reported relatively better than males in the diagnosis stage of ALM. Besides, among different subtypes of melanoma, ALM is much more prevalent among non-whites than other types [69]. There appears to be a considerable difference in the frequency of ALM when compared to the other types of melanoma, across different ethnic-racial groups. Because of the shortage of available cases, the global patterns of ALM pancreatic cancer epidemiology have not been established, although it, in fact, usually, corresponds to the ethnicity of people residing within the territory. As an example, ALM comprises 55-58% of all the developmental melanoma types in Taiwan and Korea while in the United States, it is only about 2% [70]. This difference, for the most part, concerns the whiter population who sustain higher rates of sun-derived melanomas. ALM is present in 1-8% of rare melanoma populations of European origin, and responsible for more than 50% of all cutaneous malignant melanoma cases [23].
Dermoscopy
The accurate clinical diagnosis of ALM has been greatly improved due to the dermoscopic analysis. Dermoscopic findings may be more crucial than histological images in the early stages of ALM [71]. In contrast, classical dermoscopic patterns of acral melanocytic nevi include the parallel furrow pattern, lattice-like pattern, and fibrillar pattern, which are found in over 75% of benign acral lesions [72]. While acquired acral nevi measure several millimeters in size, typically present symmetrically, and are flesh-colored, congenital acral nevi cover areas up to a few centimeters of the skin surface, often have an asymmetric pattern, and show blue-gray color or globules [73].
Screening and diagnosis of MIS
Etiology
MIS is an early, non-invasive form of melanoma in which the tumor is uncertain to the epidermis [49]. UVR exposure from sunlight and tanning beds is an important environmental risk since it increases the risk of melanoma [74]. The development of melanoma is usually caused by genetic mutations in genes such as BRAF, NRAS, and others [75].
Epidemiology
MIS has increased the case strongly among white populations during the past few decades [76]. The Surveillance, Epidemiology, and End Results (SEER) database indicates that MIS is one of the cancers that is developing very quickly, with an annual increase of 9.5% [77]. In 2018, there will be nearly 2500 invasive and 1700 in-suit cases of melanoma in New Zealand, based on estimations [76]. There are indications that many cases of melanoma are overdiagnosed, with overtreatment causing harm to the patients, apart from increasing medical costs for individuals and healthcare systems. Current estimates about the rate of overdiagnosis in Australia may be outdated, and the financial implications for the healthcare system have not yet been thoroughly examined [78]. Over the past 40 years, the incidence of malignant melanoma has steadily increased, at a rate of approximately 5% a year. The current global incidence is reported at 10.9/100,000 persons with the lifetime risk of developing melanoma for Americans estimated to be 1 in 75. Though the overall survival rates specific to melanoma have improved over the last two decades, the prognosis for patients with advanced disease has not demonstrated any advancement compared to 20 years ago [79].
Pathophysiology
MIS is the initial stage of melanoma where the atypical melanocytes are confined within the epidermis and have not yet increased depth dermal layers [49]. Genetic mutation, especially in the BRAF, NRAS, and CDKN2A genes, plays a very significant part in the development of melanoma. The genetic material coding for cyclin D1 (CCND1) plays a role through its interaction with normal cell cycle regulation [80]. Among these mutations, the most important is undoubtedly the BRAF V600E mutation, which leads to the activation of the mitogen-activated protein kinase-MAPK pathway that basically pushes melanocyte proliferation and survival [81].
Dermoscopy
Recent literature suggests that dermatoscopy has increased the diagnostic precision of PSLs. The technique utilizes multiple criteria are specific patterns and structures both for melanocytic and non-melanocytic lesions, general asymmetry, and variability in color and structure [82]. Schiffner et al., described four characteristic dermoscopic criteria as diagnostic patterns of LM pigment asymmetric follicular openings, dark rhomboidal structures, slate-colored globules, and slate dots. These characteristics combined achieve a sensitivity of CI of 89% and a specificity of 96% [82].
Screening and diagnosis of NM
Etiology
The major risk factors include multiple dysplastic nevi, family history, fair skin that tends to burn, and sun exposure some studies also indicate that UV radiation is an important component of melanoma risk [83, 84]. NM has been associated with genetic factors, specifically mutations in the CDKN2A and CDK4 genes, but it also reflects the impact of such environmental factors as sun exposure and dysplastic nevi [85].
Epidemiological
Epidemiological studies have shown that fast-growing NM is predominately found in men over the age of 50 presenting without the conventional risk factors for other types of cutaneous melanoma (CM), such as multiple nevi, freckles, or sun damage. Unfortunately, this group with high risk is largely not included in skin cancer screening programs. Moreover, these screening measures seem to have little impact on the early recognition of NM most tumors develop quickly de novo in other areas of the skin and are generally self-detected [86]. Over the past few decades, invasive melanoma incidence has increased progressively in the white populations of the USA, United Kingdom, Australia, and New Zealand. Incidence rates are projected to continue to rise through 2031 in the majority of these populations. In contrast, over the same time, melanoma mortality has also been rising, but at a rate far lower than the incidence increase [87].
Pathophysiology
Melanoma has its origin in melanocytes at the dermal-epidermal junction where they undergo a malignant transformation. Although this cancer can arise from a pre-existing nevus, it often arises de novo. In summary, melanoma evolution is generally divided into two phases: the radial and vertical growth phases. The radial growth phase displays a horizontal alignment of neoplastic melanocytes at the intraepidermal plane which could extend to the papillary dermis as well [88]. The vertical growth phase is characterized by dermal invasion and nodule formation of a tumor [89].
Dermoscopy
NM is challenging for dermoscopy because the pattern asymmetry is less significant than in SSM. However, pigmented NM usually found an uneven color [90]. Argenziano et al., found a novel indicator of NM characterized by the blue-black color within the lesion. It is proposed that the blue-black color is due to a blend of pigments in the mid-deep dermis resulting in the blue and the epidermis resulting in the black. There was at least moderate agreement between pathologists for any lesion surface with less than 10% blue and black areas being significantly pigmented NM, the authors wrote. Moreover, Pizzichetta et al., related to ulceration and homogeneous disorganized patterns, homogeneous blue pigmented structureless areas, three or more colors, a mix of polymorphous vessels, and milky-red globules, as well as symmetrical shapes, were some other features significantly associated with NM [91].
Screening and diagnosis of SSM
Etiology
The etiology of malignant melanoma is mainly associated with exposure to UV light, which can be regarded as a primary risk factor, especially in the presence of the susceptibility of phenotype. Furthermore, the risk of melanoma rising due to aging can be attributed to exposure to other environmental agents than only UV light. In the pathogenesis of melanoma, break periods between the initiation of exposure to the environment and the appearance of the tumor, along with many more variables, have interfered [92].
Epidemiology
SSM is the most common type of melanoma and accounts for approximately 70% of the total incidence of melanoma in the world [93]. This melanoma originates because of the malignant transformation of melanocytes which are cells synthesizing the photoprotective melanin pigment [94]. The development of a precancerous lesion is usually observed to occur gradually over several years, before a more rapid transformation in the preceding months until diagnosis. Though SSM commonly occurs on the back in men and on the legs in women, it can occur on any part of the body [95]. Studies report that BRAF-mutant melanomas are more common in younger patients and mainly refer to the SSM subtype, the trunk region, and patterns of intermittent ultraviolet sun exposure [96-98]. The incidence of melanoma has significantly increased over the years on a worldwide basis. In 2019, it is estimated that there were 57,220 new cases of melanoma in males and 39,260 in females in the United States, which accounts for 5.5% of the total cancer incidence and was responsible for 7,230 deaths, or 1.2% of all cancer-related deaths. The average annual number of new cases is around 132,000. According to the 2019 report from The American Cancer Society, it is estimated that 192,310 patients were diagnosed with melanoma in the U.S [99].
Pathophysiology
In SSM, large numbers of single melanocytes are situated in the epidermis. These melanocytes may be arranged in clusters along the dermal-epidermal junction and frequently are present in the midportion and upper layers of the epidermis as well [100]. BRAF and NRAS gene mutations occur at an early point in melanoma pathogenesis and are consistently sustained during further tumor progression, taking part in the pathogenesis of invasive melanoma but also in cooperation with other mutations [101]. Several studies confirmed that immune evasion mechanisms-antigen downregulation and resistance to immune cell attacks- play an important role in the uncontrollable growth of melanoma cells [102].
Dermoscopy
Dermoscopy is an early diagnostic technique that is non-invasive and complements the accuracy [103]. One review reported that a clinical examination with the addition of dermoscopy reaches 90% specificity-95% CI: 57-98% and 90% sensitivity-95% CI: 80-95%, and went on to show just how much dermoscopy increases the accuracy of the clinical examination in identifying primary melanoma [104]. Recently, dermoscopy benefitted from the technical evolution of imaging and digital cameras. The use of these new technologies allowed the creation of the so-called video-dermoscopy, paving the way for the application of this diagnostic technique for telemedicine approaches, simplifying the sharing of clinical images, and facilitating follow-up of unclear lesions [105]. This comprehensive review is progress in current applications for patients, primary care providers, dermatologists, and dermatopathologists. The authors discuss various applications of image and molecular processing applied in skin cancer and point out the potential to apply AI in the self-screening of patients and improving diagnostic accuracy in non-dermatologists [106].
Treatments
Surgery
Surgical treatment remains the gold standard in melanoma management. It consists in the complete excision of the scar after an excisional biopsy performed at the diagnostic stage - micro staging I - with a sufficiently large margin of healthy tissue, depending on the depth of infiltration of the lesion [107]. Other lymph nodes are removed if melanoma cells are detected in the lymph nodes. Surgery is another better therapy for metastatic melanoma, but it is a supportive therapy and is often used in conjunction with other treatments as a primary treatment [108].
Chemotherapy
Chemotherapy was the initial treatment approach for advanced melanoma. Various chemotherapy combinations have been studied to improve the clinical responses, but no significant improvement in overall survival (OS) was detected [109]. Apoptosis resistance is thought to be the main reason melanoma does not respond to treatment and while it remains one of the main treatments used in hospitals for patients with advanced, refractory, or recurrent melanoma, other treatments have replaced many chemotherapy regimens [110, 111].
Targeted therapy
Approximately 70% of patients with cutaneous melanoma have genetic mutations in key genes that control cell growth and cancer progression. These oncogenic mutations may be associated with melanoma cell proliferation and a malignant phenotype [109]. The targeted therapy approach uses use small molecule drugs or antibodies to disrupt the mutant protein and thereby reduce the severity of the disease [112].
Immunotherapy
Melanoma is a cancer that generally tends to respond relatively to immune modulation [113]. Various factors have been identified to explain melanoma cell sensitivity to activation by the immune system. These factors include increased tumor mutational burden caused by UV light exposure, the generation of cancer-testis antigens, and mimicry of melanocyte lineage proteins that pathogen-associated antigens [114].
Machine Learning Applications in Skin Cancer Detection
Acral lentiginous melanoma (ALM)
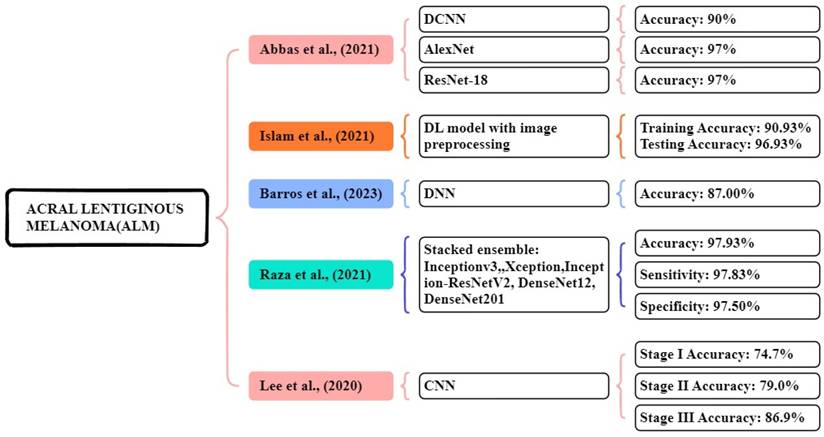
In the study proposed by Abbas et al., [115] a seven-layered DCNN model was developed using a dataset of 724 dermoscopic images collected from the Hospital at Yonsei University Health Organization in South Korea. This experiment compared and analyzed the results of the three DL models. The proposed seven-layered DCNN model achieved an accuracy of over 90%. Additionally, they applied transfer learning models like Resnet18 and AlexNet for comparison, which achieved nearly 97% accuracy. This research shows that DL models diagnose the early stage of melanoma. In this study, Islam et al., [116] proposed a CNN model that uses image preprocessing techniques, using the HAM10000 dataset, for this experiment consisting of 10,015 image files of different skin growths. The results of the study were compared with other existing models like AlexNet, ResNet, Inception V4, and VGG-16. The proposed CNN model achieved a 90.93% accuracy in training and 96.93% accuracy in testing respectively. This model works better at classification compared to other models.
Barros et al., [117] focus on supervised and self-supervised models, using datasets from ISIC data archive, Atlases, PAD-UFES-2, and Derm7pt. The models are BYOL, SwAV, MoCo, InfoMin, supervised, and SimCLR. In the DDI dataset, all models show poor results, the supervised model achieved low results, and MoCo achieved high results with 55.8% accuracy and a 12.5% f1 score. The Fitzpatrick 17k dataset shows good results, the supervised model achieved the best accuracy of 63.4% and f1-score of 41.8%. Each of the supervised and self-supervised learning approaches performs similarly on the dataset. In the PAD-UFES-20 dataset, both model types performed similarly with the BYOL model achieving a high accuracy of 59.2% and the f1 score of 27.5% respectively.
Raza al., [118] focus dataset containing 724 dermoscopy images collected from Dongsan Clinic at KeiMyung University, Korea, and Severance Medical Clinic, at Yonsei University, Korea. They used stacked ensemble methods Inceptionv3, Xception, InceptionResNet-V2, DenseNet201, and DenseNet121 these models perform high accuracy of imageNet. The proposed stacking ensemble of the optimized models achieved an accuracy, sensitivity, and specificity of 97.93%, 97.83%, and 97.50% respectively. In a study focused by Lee et al., [119] used a trained dataset of 1072 dermoscopic image acral benign nevi. The system has three stages: stage I based on dermoscopic images, stage II clinical information, and stage III evaluation and probability estimated by CNN. The CNN achieved an accuracy of detecting ALM stage I-74.7%, stage II-79.0 %, and stage III-86.9% respectively. Figure 9 illustrates the methods for the early and accurate diagnosis of the ALM in these studies.
Melanoma in situ (MIS)
Patil et al., [120] focus on two techniques CNN and multilayer perceptron (MLP). Using a dataset retrieved from https://dermnetnz.org/. The models are CNN-MLP architecture for multi-class classification. Their model achieved accuracy, recall, f1-score, and precision of 95.12%, 94.73%, 95.37%, and 96.05% respectively. Hussein et al., [121] implemented a CNN method for classifying melanoma skin cancer. The publicly available datasets of skin lesion images consist of 1,800 images of two types of moles. The proposed CNN model delivered a 99.99% accuracy, 99.9% precision, 99.9% Recall, and 99.99% F1 score respectively. Javaid et al., [122] focus on SVM, random forest, and quadratic discriminant for classification, using the publicly available ISIC-ISBI 2016 collection of skin images as a dataset. The SVM, random forest, and quadratic discriminant system achieved an accuracy of 88.17%, 90.84%, and 93.89%. The random forest classifier achieved high accuracy compared to other classifiers.
Machine Learning Overview of ALM.
Machine Learning Overview of MIS.
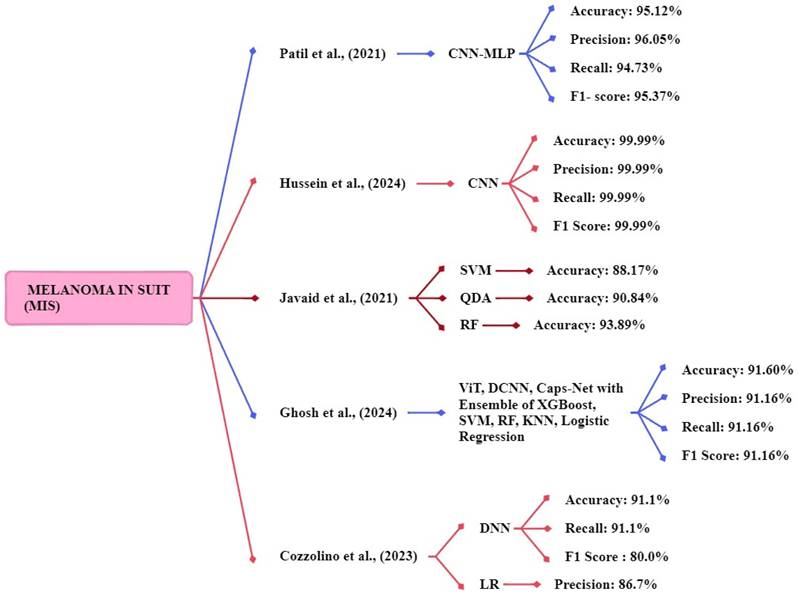
Ghosh et al., [123] proposed an ensemble model that used a dataset consisting of evaluation images of 1000 and training images of 9600. The study uses ViT, DCNN, and Caps-Net to extract features from the skin image and ensemble model with five ML methods XGBoost, SVM, RF, KNN, and logistic regression. The proposed ensemble method achieved an accuracy, f1 score, precision, and recall of 91.6%, 91.16%, 91.16%, and 91.16% respectively. Cozzolino et al., [124] developed models such as LR, SVM, RF, gradient boosting (GB), kNN, and DNN. The DNN models achieved the best accuracy of 91.1%, a recall rate of 91.1%, and an f1-score of 80.0%. The logistic regression model achieved the best precision of 86.7% respectively. Figure 10 shows the methods for the early and accurate diagnosis of the MIS in these studies.
Nodular melanoma (NM)
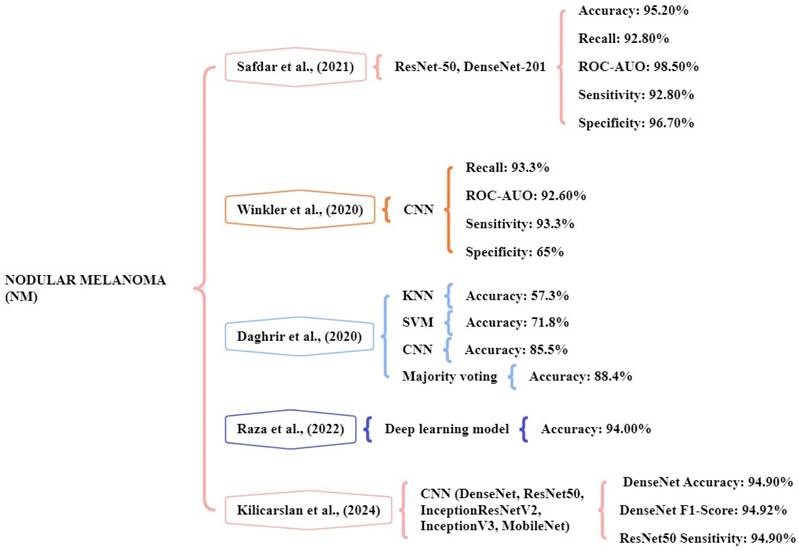
The study performed by Safdar et al., [125] indicated that ensemble models like DenseNet-201 and ResNet-50 significantly increased classification rates. The datasets of multiple skin lesions including Med-Node, DermIs, and PH2 have been collected for the identification and classification of lesions. The database comprises a total of 2301 images consisting of 1611 training images and 690 testing images. The ensemble models achieved an accuracy of 95.20%, ROC- AUO of 98.50%, sensitivity of 92.80%, and specificity of 96.70% in multiple dermoscopy image datasets. Winkler et al., [126] the study focus on a CNN to detect the different melanoma subtypes. Six dermoscopic image sets are used for the classifications. The CNN showed a high-level performance for NM achieving a sensitivity of over 93.3%, ROC-AUO of 92.60%, and specificity above 65% respectively.
Daghrir et al., [127] focuses on different models namely KNN, SVM, CNN, and majority voting. The system utilized a public dataset of the ISIC archive, which contains 23000 images of melanoma. The system achieved an accuracy kNN of 57.3%, SVM of 71.8%, CNN of 85.5%, and majority voting of 88.4% were obtained. The majority voting methods will achieve high accuracy in detecting melanoma. Raza et al., [128] experimented with the CNN model by using the dataset of 17,805 training images using the DL model. Their CNN model achieved an accuracy of 94% for the melanoma skin cancer classification task. Kilicarslan et al., [129] the study tested five DL models Densenet, ResNet50, InceptionResNetV2, InceptionV3, and MobileNet with seven optimizers. The melanoma skin cancer dataset is used and it consists of RGB images 10,605, benign 5500, and malignant 5105. The DenseNet-SGD optimizer model delivered the best accuracy of 94.90%, f-score of 94.92%, and sensitivity of 94.03%. Figure 11 represents the methods for the early and accurate diagnosis of NM in these studies.
Machine learning overview of NM.
Superficial spreading melanoma (SSM)
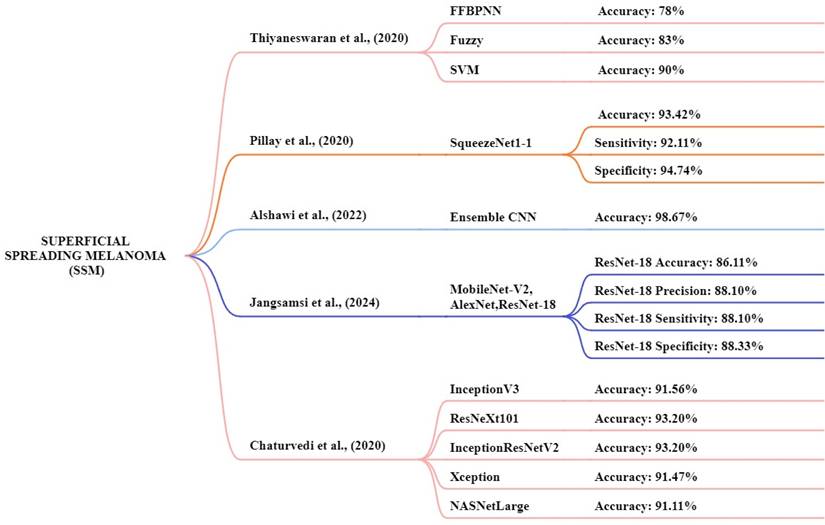
Thiyaneswaran et al., [130] proposed models consisting of three different approaches feed-forward back propagation neural network, fuzzy logic, and SVM. The PH2 and ISIC database images are used for this analysis. The images of the ISIC datasets were compared with those of existing processes, including Inception-V3, ResNet50, Inception ResNet V2, and DenseNet-201. This study achieved an accuracy of the models like fuzzy, SVM, and FFBPNN of 78%, 83%, and 90% respectively. The FFBPNN model achieved the highest accuracy for melanoma classification. Pillay et al. [131] the study focus on transfer learning by testing 14 pre-trained models to classification and diagnosis of skin cancer. The datasets used are MED-NODE, DermIS, and DermQuest to determine the model performance and this experiment consists of 376 macroscopic images. The squeezenet1-1 method achieved a high-performance accuracy of 93.42%, a sensitivity of 92.11%, and a specificity of 94.74% respectively. Kaur et al., [132] this study focus on an automated melanoma classifier using an LCNet model. The dermoscopic image datasets ISIC 2017, ISIC 2016, and ISIC 2020 were used for this study and the ISIC 2020 dataset achieved high results. The proposed LCNet model achieved an accuracy, f1-score, precision, and recall of 90.42%, 90.42%, 90.39%, and 90.41% for ISIC 2020, respectively.
Jangsamsi et al., [133] this research focuses on comparing three DL models AlexNet, ResNet-18, and MobileNet-V2 using a comprising dataset from the MED-NODE dermatology database. The dataset consists of 358 images of skin cancer. The ResNet-18 model achieved high accuracy, precision, sensitivity, and specificity at 86.11%, 88.10%, 88.10%, and 88.33% respectively. Chaturvedi1 et al., [134] focus CNN models like InceptionV3, ResNeXt101, InceptionResNetV2, Xception, and NASNetLarge. In this study, they utilized a HAM10000 dataset. The dataset includes 10,015 dermoscopic images of various skin cancer types. The models achieved an accuracy of 91.56%, 93.20%, 93.20%, 91.47%, and 91.11% respectively. The high-performance accuracy is ResNetXt101 and InceptionResNetV2. Figure 12 presented the techniques for the early and accurate diagnosis of SSM in these studies.
Despite significant progress in AI-based melanoma classification, some limitations remain in the analyzed models. A primary limitation is the relatively small size and uneven attributes of most training datasets, which may hinder model robustness and increase the risk of overfitting. Furthermore, most models have been developed and validated using data from limited demographic and ethnic populations, which raises concerns regarding their generalizability and effectiveness in diverse clinical settings. Variations in imaging devices, acquisition techniques, and data fidelity intensify the difficulties of model transferability. To address these constraints, it is imperative to collect larger, more diverse datasets and establish consistent review protocols to ensure that AI technologies are trustworthy, equitable, and usable for all populations. Notwithstanding the encouraging progress in AI-based melanoma detection and diagnosis, some potential obstacles persist prior to achieving broad clinical implementation. Regulatory approval processes must guarantee the safety, efficacy, and resilience of AI models across varied populations and clinical environments. Ethical considerations around data privacy, algorithmic bias, and informed consent necessitate meticulous attention to uphold patient trust and ensure equitable healthcare delivery. Furthermore, thorough clinical validation via prospective trials and real-world investigations is crucial to establish the generalizability and dependability of AI solutions. Confronting these obstacles will be essential to properly leveraging the capabilities of AI technologies in dermatology and eventually enhancing patient outcomes. A crucial issue affecting the clinical adoption of AI tools is the capacity to comprehend model decisions. Methods like Gradient-weighted Class Activation Mapping (Grad-CAM) and saliency maps offer visual elucidations of deep learning model predictions by emphasizing image areas that significantly impact classification results. These interpretability methodologies boost clinician trust through transparency, facilitate validation and error analysis, and eventually support the safer and more successful integration of AI in melanoma diagnosis.
Machine learning overview of SSM.
Discussion
Newly emerging technologies for the early detection of skin cancer
Hyperspectral and multispectral imaging methods use optical equipment to measure several wavelength bands that are not found in the visible spectrum [135]. Light absorption and reflectance properties of different parts of the skin allow these imaging methods to give a detailed overview of both structural and chemical properties of the tissues. Using this enhanced data allows for better separation of malignant from benign lesions than was previously possible with typical imaging methods. Hyperspectral and multispectral imaging improve the way lesions are examined and melanoma is detected, as they can find small skintone and shape changes in a clinical setting [136]. Similarly, biosensors and molecular diagnostic systems are advanced ways to find early signs of skin cancer by looking at certain melanoma biomarkers [137]. They combine biomolecules such as antibodies, nucleic acids or aptamers, to detect cancerous DNA, proteins or microRNAs in bodily fluids. When biosensors are combined with microfluidics and nanotech, this greatly increases the speed, sensitivity and available locations for diagnostic tests and may enhance traditional histopathology. Monitoring melanoma progress and therapy effects in real time, thanks to molecular diagnostics, makes it possible to personalize care for each patient. A liquid biopsy is now often used to detect and track melanoma by studying circulating tumor cells (CTCs), circulating tumor DNA (ctDNA) and extracellular vesicles found in the blood or in various bodily fluids [138]. This approach allows doctors to analyze the tumor's molecular features without taking out pieces of the tumor. With liquid biopsy, metastasis can be detected early, the variability of tumors can be studied and how therapy is working can be monitored in real time [139]. Manufacturers have greatly improved the accuracy of liquid biopsy for melanoma thanks to new technology. Nanotechnology-enhanced imaging methods, in conjunction with optical coherence tomography (OCT), provide high-resolution, depth-resolved viewing of skin microstructures, hence enhancing the identification of early melanoma lesions [140]. Nanoparticles can be designed as contrast agents or molecular probes to selectively target certain tumor markers, hence improving OCT signal contrast and specificity. OCT delivers cross-sectional images of the skin with micrometer-level resolution, facilitating noninvasive evaluation of lesion depth and morphology in real time [141]. The integration of nanotechnology with OCT presents significant potential for enhancing traditional imaging techniques and enabling more precise, early-stage melanoma detection.
Established and emerging methods for differentiating benign and malignant lesions
Diagnosing benign from malignant skin lesions is easier because of these types of diagnostics which rely on analysis of specific genomic, proteomic and metabolomic profiles [142]. Experts use genetic markers and especially changes in BRAF and NRAS genes, to learn more about how melanoma develops and progresses. In proteomic studies, characteristic changes in proteins are observed in cancer cells, while metabolomic analysis finds differing metabolic ways in cancer. The use of biomarkers provides more clarity about lesions than just their appearance, helping with early and correct diagnosis. By using high-throughput sequencing and mass spectrometry technology, molecular diagnostics can become more accurate and help with choosing personal approaches to care and treatment. RCM, MPM and hyperspectral imaging allow advanced visualization of the skin's structure and cellular shapes without invading the skin. RCM lets experts see inside the skin in real time and at nearly the same quality as a biopsy, helping to identify cancerous cells without a biopsy. With endogenous fluorophores, MPM captures images that highlight the energy use and organization of tissues. Hyperspectral imaging gives a wide range of frequencies, so it can detect the biochemical differences between tumors and normal tissues that help tell them apart. They add to the reliability of diagnosis and obviate unnecessary invasive treatments, accompanying traditional medical and dermoscopic evaluations. Combining the findings of a clinical exam, images and molecular information often results in more accurate diagnosis of skin lesions and reduces incorrect positive or negative test results. Employing multimodal data fusion, we are able to merge morphological, functional and molecular information to assess the lesion status carefully [143]. The use of machine learning and similar algorithms supports the efficient processing of different types of data which helps build dependable diagnostic models. When an integrated approach is used, doctors can create unique treatment plans that benefit each patient's care.
Newly emerging therapeutics for the treatment and prevention of skin cancer
Concentrating on important systems in the body that fuel tumor growth and life has brought major changes to melanoma treatment. Using BRAF and MEK inhibitors which are small molecule inhibitors, helps to stop diseases related to melanoma in patients with these gene abnormalities [144]. On top of that, monoclonal antibodies designed to interfere with signaling or attack antigens on cancer cells are highly effective and cause less harm to the body. As a result of using targeted strategies, melanoma patients have improved reaction to treatment and live longer than those who use conventional chemotherapy. Immunotherapy is now often given in melanoma treatment, relying on the patient's immune system to seek out and kill cancer. Anti-CTLA-4 and anti-PD-1 antibodies are immune checkpoint inhibitors that boost immune cells and encourage them to fight cancer [145]. Cancer vaccines and therapies such as transferring tumor-infiltrating lymphocytes are meant to enhance how the immune system recognizes cancer cells. Numerous patients now benefit from a longer and more successful treatment due to the standard-setting value of these medications in advanced melanoma. It uses light therapies called photodynamic therapy and lasers to treat selected cancerous cells without harming healthy tissues nearby. The approach involves using drugs that build up in cancer cells which are then exposed to light of the right color to make toxic oxygen species. Laser therapy uses direct and focused light power to carefully remove malignant tumors [146]. Especially with early or outward skin cancers, these methods are useful as either main or complementary treatments to surgery. Skincare precautions are designed to decrease the risk of skin cancer with prevention drugs, better habits and new vaccines. Nicotinamide and retinoids are drugs that have shown they help decrease the risk of non-melanoma skin cancers. Public health programs underscore sun protection practices, such as the application of sunscreen and the avoidance of UV exposure, as essential strategies for melanoma prevention. Furthermore, vaccination research is investigating preventative and therapeutic strategies aimed at melanoma-associated antigens to elicit enduring immune protection. These methods combined form a comprehensive strategy for skin cancer prevention.
Conclusion
Melanoma includes subtypes like ALM, MIS, NM, and SSM which present significant challenges for early detection and accurate diagnosis. The systematic review paper has considered various machine-learning techniques for detecting and classifying skin cancer. All those techniques are noninvasive. Recent advancements in deep learning, particularly CNN are highly effective in improving diagnostic accuracy. By integrating advanced imaging techniques with dermoscopic, healthcare providers can diagnose melanoma earlier and improve accuracy, thereby providing instant treatment and better patient care. This technique has greatly improved the accuracy of skin cancer detection, the diagnostic process, and clinicians diagnosing high-risk lesions. There is strong potential for further development, particularly in enhancing AI models by integrating a broader range of multimodal input data, including genetic markers, patient histories, and advanced imaging techniques such as hyperspectral and multispectral imaging. Challenges will survive, especially in addressing class imbalance when the dataset expands to include various skin types and melanoma subtypes. The integration of AI tools into telemedicine presents a valuable opportunity to increase early screening, especially in rural areas. The effective incorporation of AI-driven melanoma detection tools into clinical practice will mostly rely on thorough prospective validation trials carried out in actual healthcare environments. These studies are crucial for thoroughly evaluating model performance, safety, and usefulness among varied patient populations and clinical workflows. Furthermore, the continual enhancement of AI algorithms via persistent learning and feedback systems will be essential to uphold their precision and pertinence. Subsequent research ought to investigate multi-institutional cooperation to promote data sharing and standardization, thus improving model robustness and generalizability. These initiatives, alongside a focus on ethical, regulatory, and practical factors, will facilitate AI's integration as a crucial element in tailored melanoma management. In conclusion, the research in AI, advanced imaging technologies, and telemedicine provides strong improvements in melanoma detection and diagnosis. The advancement is considered to improve early skin cancer detection and provide more personalized treatment options, improve patient outcomes and further advance the management of skin cancer.
Acknowledgements
Funding
This research was supported by the National Science and Technology Council, the Republic of China, under grants NSTC 113-2221-E-194-011-MY3. This study was financially or partially supported by the Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation-National Chung Cheng University Joint Research Program and Kaohsiung Armed Forces General Hospital Research Program KAFGH_D_114014 in Taiwan.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Dalin Tzu Chi General Hospital (B11302024). Written informed consent was waived in this study because of the retrospective, anonymized nature of study design.
Data availability statement
The data presented in this study are available in this article upon considerable request to the corresponding author (H.-C.W.).
Author contributions
Conceptualization, T.-L.L., S.-C.H., C.-T.L., and H.-C.W.; methodology, A.M., H.-C.W., C.-T.L., and S.-C.H.; software, C.-T.L., R.K., H.-C.W. and A.M.; validation, S.-C.H., R.K., T.-L.L., and H.-C.W.; formal analysis, S.-C.H., R.K., A.C., T.-L.L. and H.-C.W.; investigation, S.-C.H., and A.M.; resources, S.-C.H., T.-L.L. and H.-C.W.; data curation, A.M., R.K., H.-C.W. and A.C.; writing—Original draft preparation, A.M.; writing—Review and editing, A.M. and H.-C.W.; supervision, A.C., and H.-C.W.; project administration, H.-C.W. All authors have read and agreed to the published version of the manuscript.
Competing Interests
Author Hsiang-Chen Wang was employed by the company Hitspectra Intelligent Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Simoes MCF, Sousa JJS, Pais AACC. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015;357:8-42
2. Schierbeck J, Vestergaard T, Bygum A. Skin cancer associated genodermatoses: a literature review. Acta Derm Venereol. 2019;99:360-9
3. Leiter U, Keim U, Garbe CJS, vitamin D, cancer s. Epidemiology of skin cancer: update 2019. 2020: 123-39.
4. Roky AH, Islam MM, Ahasan AMF, Mostaq MS, Mahmud MZ, Amin MN. et al. Overview of skin cancer types and prevalence rates across continents. Cancer Pathog Ther. 2024;2:E01-E36
5. Refianti R, Mutiara AB, Priyandini RP, Applications. Classification of melanoma skin cancer using convolutional neural network. 2019; 10.
6. Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:1-19
7. Zambrano-Román M, Padilla-Gutiérrez JR, Valle Y, Muñoz-Valle JF, Valdés-Alvarado E. Non-melanoma skin cancer: a genetic update and future perspectives. Cancers (Basel). 2022;14:2371
8. Sari EY, Kiscahyadi INB, Gracia M, Gunawan AAS. Melanoma Skin Cancer Classification using Machine Learning: A Systematic Literature Review. 2022 2nd International Conference on Information Technology and Education (ICIT&E): IEEE. 2022 p. 212-6
9. Gamage L, Isuranga U, De Silva S, Meedeniya D. Melanoma skin cancer classification with explainability. 2023 3rd International Conference on Advanced Research in Computing (ICARC): IEEE. 2023 p. 30-5
10. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023 73
11. Khan NH, Mir M, Qian L, Baloch M, Khan MFA, Ngowi EE. et al. Skin cancer biology and barriers to treatment: Recent applications of polymeric micro/nanostructures. J Adv Res. 2022;36:223-47
12. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A. et al. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;149:778-89
13. Orthaber K, Pristovnik M, Skok K, Perić B, Maver U. Skin cancer and its treatment: novel treatment approaches with emphasis on nanotechnology. Journal of Nanomaterials. 2017. 2017
14. Pashazadeh A, Boese A, Friebe M. Radiation therapy techniques in the treatment of skin cancer: an overview of the current status and outlook. J Dermatolog Treat. 2019;30:831-839
15. Lee TK. Measuring border irregularity and shape of cutaneous melanocytic lesions: Citeseer; 2001
16. Dildar M, Akram S, Irfan M, Khan HU, Ramzan M, Mahmood AR. et al. Skin cancer detection: a review using deep learning techniques. Int J Environ Res Public Health. 2021;18:5479
17. Boriani F, O'leary F, Tohill M, Orlando A, Sciences P. Acral Lentiginous Melanoma-misdiagnosis, referral delay and 5 years specific survival according to site. Eur Rev Med Pharmacol Sci. 2014 18
18. Goydos JS, Shoen SL. Acral lentiginous melanoma. Cancer Treat Res. 2016:321-9
19. Han B, Hur K, Ohn J, Lim SS, Mun J-H. Acral lentiginous melanoma in situ: dermoscopic features and management strategy. Sci Rep. 2020;10:20503
20. Kim SY, Yun SJ. Cutaneous melanoma in Asians. Chonnam Med J. 2016;52:185-93
21. Daniels CP, Huo M, Xu W, Porceddu SV. Melanoma. Radiation Therapy for Sarcomas and Skin Cancers: A Practical Guide on Treatment Techniques: Springer. 2022 p. 295-346
22. Fernandez-Flores A, Cassarino DS. Histopathological diagnosis of acral lentiginous melanoma in early stages. Ann Diagn Pathol. 2017;26:64-9
23. Basurto-Lozada P, Molina-Aguilar C, Castaneda-Garcia C, Vázquez-Cruz ME, Garcia-Salinas OI, Álvarez-Cano A. et al. Acral lentiginous melanoma: Basic facts, biological characteristics and research perspectives of an understudied disease. Pigment Cell Melanoma Res. 2021;34:59-71
24. Higgins II HW, Lee KC, Galan A, Leffell D. Melanoma in situ: Part II. Histopathology, treatment, and clinical management. J AM Acad Dermatol. 2015;73:193-203
25. Patel VR, Roberson ML, Pignone MP, Adamson AS. Risk of mortality after a diagnosis of melanoma in situ. JAMA Dermatol. 2023;159:703-10
26. Toren KL, Parlette EC. Managing melanoma in situ. Seminars in cutaneous medicine and surgery: WB Saunders. 2010 p. 258-63
27. Joyce KM, Joyce CW, Jones DM, Donnellan P, Hussey AJ, Regan PJ. et al. An assessment of histological margins and recurrence of melanoma in situ. Plast Reconstr Surg Glob Open. 2015;3:e301
28. Lens M. Current clinical overview of cutaneous melanoma. Br J Nurs. 2008;17:300-5
29. Hernandez LE, Frech FS, Mohsin N, Dreyfuss I, Nouri K. Nodular melanoma: a review of pathogenesis, presentation, diagnosis, and treatment. J Dermatol Skin Sci. 2021;3:25-30
30. Šitum M, Buljan M, Kolić M, Vučić M. Melanoma-clinical, dermatoscopical, and histopathological morphological characteristics. Acta Dermatovenerol Croat. 2014;22:2 -
31. Tseng H-W, Li W-T, Hsieh J-F. Targeted Agents for the Treatment of Melanoma: An Overview. 2013.
32. Longo C, Pampena R, Moscarella E, Chester J, Starace M, Cinotti E. et al. Dermoscopy of melanoma according to different body sites: Head and neck, trunk, limbs, nail, mucosal and acral. J Eur Acad Dermatol Venereol. 2023;37:1718-30
33. Khazaei Z, Ghorat F, Jarrahi A, Adineh H, Sohrabivafa M, Goodarzi E. Global incidence and mortality of skin cancer by histological subtype and its relationship with the human development index (HDI); an ecology study in 2018. 2019; 6: e13.
34. Longo C, Casari A, Pellacani G. Superficial spreading melanoma. 2012: 151-78.
35. Egger ME, Stepp LO, Callender GG, Quillo AR, Martin II RC, Scoggins CR. et al. Outcomes and prognostic factors in superficial spreading melanoma. Am J Surg. 2013;206:861-8
36. Trindade FM, de Freitas MLP, Bittencourt FV. Dermoscopic evaluation of superficial spreading melanoma. An Bras Dermatol. 2021;96:139-47
37. Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. 2012; 148: 30-6.
38. Behbahani S, Malerba S, Samie F. Racial and ethnic differences in the clinical presentation and outcomes of acral lentiginous melanoma. Br J Dermatol. 2021;184:158-60
39. Holman DM, King JB, White A, Singh SD, Lichtenfeld JL. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. 2023; 175: 107692.
40. Lee T-L, Lin M-H, Liao Y-H, Liau J-Y, Sheen Y-S. Clinicopathological characteristics and prognosis in significantly thick acral lentiginous melanoma in Taiwan. J Formos Med Assoc. 2022;121:2338-44
41. Park HS, Cho KH. Acral lentiginous melanoma in situ: a diagnostic and management challenge. Cancers (Basel). 2010;2:642-52
42. Teramoto Y, Martinez-Said H, Guo J, Garbe C. Acral Lentiginous Melanoma. Br J Dermatol. 2020:897-924
43. Bian SX, Hwang L, Hwang J, Ragab O, In GK, Peng D. et al. Acral lentiginous melanoma—population, treatment, and survival using the NCDB from 2004 to 2015. Pigment Cell Melanoma Res. 2021;34:1049-61
44. Phan A, Touzet S, Dalle S, Ronger-Savlé S, Balme B, Thomas L. Acral lentiginous melanoma: histopathological prognostic features of 121 cases. Br J Dermatol. 2007;157:311-8
45. Phan A, Touzet S, Dalle S, Ronger-Savlé S, Balme B, Thomas L. Acral lentiginous melanoma: a clinicoprognostic study of 126 cases. Br J Dermatol. 2006;155:561-9
46. Howard M, Xie C, Wee E, Wolfe R, McLean C, Kelly JW. et al. Acral lentiginous melanoma: Clinicopathologic and survival differences according to tumour location. Australas J Dermatol. 2020;61:312-7
47. Swetter SM. Dermatological perspectives of malignant melanoma. Surg Clin North Am. 2003;83:77-95
48. Massi G, Leboit PE, Massi G, Leboit PE, Melanoma. Lentigo Maligna Pattern of Melanoma in Situ and Melanoma. 2004: 413-8.
49. Friedman EB, Scolyer RA, Williams GJ, Thompson JF. Melanoma in situ: a critical review and re-evaluation of current excision margin recommendations. Adv Ther. 2021;38:3506-30
50. Duarte AF, Sousa-Pinto B, Barros AM, Haneke E, Correia O. Lentigo maligna-not always a face and neck disease of the elderly. Dermatology. 2018;234:37-42
51. McKenna JK, Florell SR, D Goldman G, Bowen GM. Lentigo maligna/lentigo maligna melanoma: current state of diagnosis and treatment. Dermatol Surg. 2006;32:493-504
52. Shiau CJ, Thompson JF, Scolyer RA. Controversies and evolving concepts in the diagnosis, classification and management of lentigo maligna. 2013; 8: 195-214.
53. Kibbi N, Kluger H, Choi JN. Melanoma: clinical presentations. Cancer Treat Res. 2016:107-29
54. Cabo H, Lallas A. Comprehensive atlas of dermatoscopy cases: Springer; 2018
55. Argenziano G, Longo C, Cameron A, Cavicchini S, Gourhant JY, Lallas A. et al. Blue-black rule: a simple dermoscopic clue to recognize pigmented nodular melanoma. Br J Dermatol. 2011;165:1251-5
56. Pizzichetta M, Kittler H, Stanganelli I, Bono R, Cavicchini S, De Giorgi V. et al. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-14
57. Kalkhoran S, Milne O, Zalaudek I, Puig S, Malvehy J, Kelly JW. et al. Historical, clinical, and dermoscopic characteristics of thin nodular melanoma. Arch Dermatol. 2010;146:311-8
58. Manci EA, Balch CM, Murad TM, Soong SJ. Polypoid melanoma, a virulent variant of the nodular growth pattern. Am J Clin Pathol. 1981;75:810-5
59. Menzies SW, Moloney FJ, Byth K, Avramidis M, Argenziano G, Zalaudek I. et al. Dermoscopic evaluation of nodular melanoma. JAMA Dermatol. 2013;149:699-709
60. Netscher DT, Leong M, Orengo I, Yang D, Berg C, Krishnan BJP. et al. Cutaneous malignancies: melanoma and nonmelanoma types. Plast Reconstr Surg. 2011;127:37e-56e
61. Stanienda-Sokół K, Salwowska N, Sławińska M, Wicherska-Pawłowska K, Lorenc A, Wcisło-Dziadecka D. et al. Primary locations of malignant melanoma lesions depending on patients' gender and age. Asian Pac J Cancer Prev. 2017;18:3081
62. Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28:1005-11
63. Bhat YJ, Jha AK. Dermatoscopy of inflammatory diseases in skin of color. Indian Dermatol Online J. 2021;12:45-57
64. Payette MJ, Katz III M, Grant-Kels JM. Melanoma prognostic factors found in the dermatopathology report. Clin Dermatol. 2009;27:53-74
65. Avilés-Izquierdo JA, Vírseda-González D, Izquierdo del Monte MG, Rodríguez-Lomba E. Who Will Have a Second Melanoma? A Prospective Longitudinal Study in Patients without Any Genetic Predisposition. Dermatology. 2023;239:403-8
66. Yilmaz A, Kalebasi M, Samoylenko Y, Guvenilir ME, Uvet H. Benchmarking of lightweight deep learning architectures for skin cancer classification using ISIC 2017 dataset. 2021.
67. Minagawa A, Omodaka T, Okuyama R. Melanomas and mechanical stress points on the plantar surface of the foot. N Engl J Med. 2016;374:2404-6
68. Hall KH, Rapini RP. Acral lentiginous melanoma. 2020.
69. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015, an analysis of the SEER registry. J Surg Res. 2020;251:329-39
70. Dugan MM, Perez MC, Karapetyan L, Zager JS. Management of acral lentiginous melanoma: current updates and future directions. Front Oncol. 2024 14
71. Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Opinions Oncol. 2018;19:1-11
72. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34
73. Chuah S, Tsilika K, Chiaverini C, Fontas E, Ortonne J, Lacour J. et al. Dermoscopic features of congenital acral melanocytic naevi in children: a prospective comparative and follow-up study. Br J Dermatol. 2015;172:88-93
74. Kanavy HE, Gerstenblith MR. Ultraviolet radiation and melanoma. Seminars in cutaneous medicine and surgery: WB Saunders. 2011 p. 222-8
75. Gosman LM, Țăpoi D-A, Costache M. Cutaneous melanoma: a review of multifactorial pathogenesis, immunohistochemistry, and emerging biomarkers for early detection and management. Int J Mol Sci. 2023;24:15881
76. Toender A, Kjær SK, Jensen A. Increased incidence of melanoma in situ in Denmark from 1997 to 2011: results from a nationwide population-based study. Melanoma Res. 2014;24:488-95
77. Higgins II HW, Lee KC, Galan A, Leffell D. Melanoma in situ: Part I. Epidemiology, screening, and clinical features. J Am Acad Dermatol. 2015;73:181-90
78. Lindsay D, Bell KJ, Olsen CM, Whiteman DC, Pathirana T, Collins LG. Estimating the magnitude and healthcare costs of melanoma in situ and thin invasive melanoma overdiagnosis in Australia. Br J Dermatol. 2024: ljae296.
79. Hasney C, Butcher RB, Amedee RG. Malignant melanoma of the head and neck: A brief review of pathophysiology, current staging, and management. Ochsner J. 2008;8:181-5
80. Amaral T, Sinnberg T, Meier F, Krepler C, Levesque M, Niessner H. et al. The mitogen-activated protein kinase pathway in melanoma part I-activation and primary resistance mechanisms to BRAF inhibition. Eur J Cancer. 2017;73:85-92
81. Chan XY, Singh A, Osman N, Piva T. Role played by signalling pathways in overcoming BRAF inhibitor resistance in melanoma. Int J Mol Sci. 2017;18:1527
82. Schiffner R, Schiffner-Rohe J, Vogt T, Landthaler M, Wlotzke U, Cognetta AB. et al. Improvement of early recognition of lentigo maligna using dermatoscopy. J Am Acad Dermatol. 2000;42:25-32
83. Erkurt MA, Aydogdu I, Kuku I, Kaya E, Basaran Y. Nodular melanoma presenting with rapid progression and widespread metastases: a case report. J Med Case Rep. 2009;3:1-4
84. Liu Y, Sheikh MS. Melanoma: molecular pathogenesis and therapeutic management. Mol Cell Pharmacol. 2014;6:228
85. Tucker MA, Goldstein AM. Melanoma etiology: where are we? Oncogene. 2003;22:3042-52
86. Corneli P, Zalaudek I, Magaton Rizzi G, di Meo N. Improving the early diagnosis of early nodular melanoma: can we do better? Expert Rev Anticancer Ther. 2018;18:1007-12
87. Dessinioti C, Geller A, Whiteman D, Garbe C, Grob J, Kelly J. et al. Not all melanomas are created equal: a review and call for more research into nodular melanoma. Br J Dermatol. 2021;185:700-10
88. Crowson AN, Magro CM, Mihm Jr MC. Prognosticators of melanoma, the melanoma report, and the sentinel lymph node. Mod Pathol. 2006;19:S71-S87
89. Laga AC, Murphy GF. Cellular heterogeneity in vertical growth phase melanoma. Arch Pathol Lab Med. 2010;134:1750-7
90. Rose S, Argenziano G, Marghoob A. Melanomas difficult to diagnose via dermoscopy. G Ital Dermatol Venereol. 2010;145:111
91. Kato J, Horimoto K, Sato S, Minowa T, Uhara H. Dermoscopy of melanoma and non-melanoma skin cancers. Front Med (Lausanne). 2019;6:180
92. Meyskens Jr FL, Farmer PJ, Anton-Culver H. Etiologic pathogenesis of melanoma: a unifying hypothesis for the missing attributable risk. Clin Cancer Res. 2004;10:2581-3
93. Gheoca Mutu D-E, Avino A, Balcangiu-Stroescu A-E, Mehedințu M, Bălan DG, Brîndușe LA. et al. Histopathological evaluation of cutaneous malignant melanoma: A retrospective study. Exp Ther Med. 2022;23:402
94. Jenkins RW, Fisher DE. Treatment of advanced melanoma in 2020 and beyond. J Invest Dermatol. 2021;141:23-31
95. Halpern AC, Marghoob AA, Sober AJ, Mar V, Marchetti MA. Clinical presentations of melanoma. Cancer Treat Res. 2020:107-44
96. Ammirati CT, Hruza G. Clinical presentations of cutaneous melanoma. Facial Plast Surg Clin North Am. 2003;11:9-22
97. Broekaert SM, Roy R, Okamoto I, Van Den Oord J, Bauer J, Garbe C. et al. Genetic and morphologic features for melanoma classification. Pigment Cell Melanoma Res. 2010;23:763-70
98. Liu W, Kelly JW, Trivett M, Murray WK, Dowling JP, Wolfe R. et al. Distinct clinical and pathological features are associated with the BRAFT1799A (V600E) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-5
99. Shamshad MF, Moatsim FSA, Zubair RE, Zia S, Rizvi SI, Shamail F. et al. A Comprehensive Review on Treatment Modalities of Malignant Melanoma. 2023; 1: 90-102.
100. Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod Pathol. 2006;19:S34-S40
101. Lázár V. Characterization of distinct molecular genetic alterations in BRAF and NRAS mutated human primary melanoma. Magy Onkol. 2013;57:96-9
102. Kallingal A, Olszewski M, Maciejewska N, Brankiewicz W, Baginski M. Cancer immune escape: the role of antigen presentation machinery. J Cancer Res Clin Oncol. 2023;149:8131-41
103. Braun RP, Saurat J-H, French LE. Dermoscopy of pigmented lesions: a valuable tool in the diagnosis of melanoma. Swiss Med Wkly. 2004;134:83-90
104. Vestergaard M, Macaskill P, Holt P, Menzies S. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-76
105. Blundo A, Cignoni A, Banfi T, Ciuti G. Comparative analysis of diagnostic techniques for melanoma detection: a systematic review of diagnostic test accuracy studies and meta-analysis. Front Med (Lausanne). 2021;8:637069
106. Wei ML, Tada M, So A, Torres R. Artificial intelligence and skin cancer. Front Med (Lausanne). 2024;11:1331895
107. Stachyra-Strawa P, Ciesielka M, Janiszewski M, Grzybowska-Szatkowska L. The role of immunotherapy and molecular-targeted therapy in the treatment of melanoma. Oncol Rep. 2021;46:158
108. Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019;20:1366-79
109. Domingues B, Lopes JM, Soares P, Pópulo H. Melanoma treatment in review. Immunotargets Ther. 2018:35-49
110. Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138-51
111. Wilson MA, Schuchter LM. Chemotherapy for melanoma. Cancer Treat Res. 2016:209-29
112. Dhanyamraju PK, Patel TN. Melanoma therapeutics: a literature review. J Biomed Res. 2022;36:77
113. Leonardi GC, Candido S, Falzone L, Spandidos DA, Libra M. Cutaneous melanoma and the immunotherapy revolution. Int J Oncol. 2020;57:609-18
114. Naik PP. Current trends of immunotherapy in the treatment of cutaneous melanoma: a review. Dermatol Ther (Heidelb). 2021;11:1481-96
115. Abbas Q, Ramzan F, Ghani MU. Acral melanoma detection using dermoscopic images and convolutional neural networks. Vis Comput Ind Biomet Art. 2021;4:1-12
116. Islam MK, Ali MS, Ali MM, Haque MF, Das AA, Hossain MM. et al. Melanoma skin lesions classification using deep convolutional neural network with transfer learning. 2021 1st International Conference on Artificial Intelligence and Data Analytics (CAIDA): IEEE. 2021 p. 48-53
117. Barros L, Chaves L, Avila S. Assessing the Generalizability of Deep Neural Networks-Based Models for Black Skin Lesions. Iberoamerican Congress on Pattern Recognition: Springer. 2023 p. 1-14
118. Raza R, Zulfiqar F, Tariq S, Anwar GB, Sargano AB, Habib Z. Melanoma classification from dermoscopy images using ensemble of convolutional neural networks. Mathematics. 2022;10:26
119. Lee S, Chu Y, Yoo S, Choi S, Choe S, Koh S. et al. Augmented decision-making for acral lentiginous melanoma detection using deep convolutional neural networks. J Eur Acad Dermatol Venereol. 2020;34:1842-50
120. Patil R, Bellary S. Ensemble learning for detection of types of melanoma. 2021 International Conference on Computing, Communication and Green Engineering (CCGE): IEEE. 2021 p. 1-6
121. Hussien MA, Alasadi AHH. Classification of melanoma skin cancer using deep learning approach. 2024; 22: 129-37.
122. Javaid A, Sadiq M, Akram F. Skin cancer classification using image processing and machine learning. 2021 international Bhurban conference on applied sciences and technologies (IBCAST): IEEE. 2021 p. 439-44
123. Ghosh S, Dhar S, Yoddha R, Kumar S, Thakur AK, Jana ND. Melanoma Skin Cancer Detection Using Ensemble of Machine Learning Models Considering Deep Feature Embeddings. 2024; 235: 3007-15.
124. Cozzolino C, Buja A, Rugge M, Miatton A, Zorzi M, Vecchiato A. et al. Machine learning to predict overall short-term mortality in cutaneous melanoma. Discov Oncol. 2023;14:13
125. Safdar K, Akbar S, Shoukat A. A majority voting based ensemble approach of deep learning classifiers for automated melanoma detection. 2021 International Conference on Innovative Computing (ICIC): IEEE. 2021 p. 1-6
126. Winkler JK, Sies K, Fink C, Toberer F, Enk A, Deinlein T. et al. Melanoma recognition by a deep learning convolutional neural network—performance in different melanoma subtypes and localisations. Eur J Cancer. 2020;127:21-9
127. Daghrir J, Tlig L, Bouchouicha M, Sayadi M. Melanoma skin cancer detection using deep learning and classical machine learning techniques: A hybrid approach. 2020 5th international conference on advanced technologies for signal and image processing (ATSIP): IEEE. 2020 p. 1-5
128. Shah SAR, Ahmed I, Mujtaba G, Kim M-H, Kim C, Noh S-Y. Early detection of melanoma skin cancer using image processing and deep learning. Advances in Intelligent Information Hiding and Multimedia Signal Processing: Proceeding of the IIH-MSP 2021 & FITAT 2021, Kaohsiung, Taiwan, Volume 2: Springer. 2022 p. 275-84
129. Kılıçarslan S, Aydın HA, Adem K, Yılmaz EK. Impact of optimizers functions on detection of Melanoma using transfer learning architectures. 2024: 1-21.
130. Thiyaneswaran B, Anguraj K, Kumarganesh S, Thangaraj K. Early detection of melanoma images using gray level co-occurrence matrix features and machine learning techniques for effective clinical diagnosis. 2021; 31: 682-94.
131. Pillay V, Hirasen D, Viriri S, Gwetu M. Melanoma skin cancer classification using transfer learning. Advances in Computational Collective Intelligence: 12th International Conference, ICCCI 2020, Da Nang, Vietnam, November 30-December 3, 2020, Proceedings 12: Springer. 2020 p. 287-97
132. Kaur R, GholamHosseini H, Sinha R, Lindén M. Melanoma classification using a novel deep convolutional neural network with dermoscopic images. Sensors (Basel). 2022;22:1134
133. Jangsamsi K, Samosorn K, Hirunuran P, Sakdapetchsiri P, Mahikul W, Smithrithee R. et al. Web Application for Screening Malignant Melanoma in Digital Images Using Deep Learning. 2024 IEEE International Conference on Big Data and Smart Computing (BigComp): IEEE. 2024 p. 406-11
134. Chaturvedi SS, Tembhurne JV, Diwan T. A multi-class skin cancer classification using deep convolutional neural networks. 2020; 79: 28477-98.
135. Cheng M-F, Mukundan A, Karmakar R, Valappil MAE, Jouhar J, Wang H-C. Modern Trends and Recent Applications of Hyperspectral Imaging: A Review. Technologies. 2025;13:170
136. Ilișanu M-A, Moldoveanu F, Moldoveanu A. Multispectral imaging for skin diseases assessment—state of the art and perspectives. Sensors. 2023;23:3888
137. Hamza MN, Islam MT, Lavadiya S, Koziel S, Din IU, de Souza Sanches BC. Designing a high-sensitivity dual-band nano-biosensor based on petahertz MTMs to provide a perfect absorber for early-stage nonmelanoma skin cancer diagnostic. IEEE Sensors Journal. 2024;24:18418-27
138. Li W, Wang H, Zhao Z, Gao H, Liu C, Zhu L. et al. Emerging nanotechnologies for liquid biopsy: The detection of circulating tumor cells and extracellular vesicles. Advanced Materials. 2019;31:1805344
139. Esposito A, Criscitiello C, Locatelli M, Milano M, Curigliano G. Liquid biopsies for solid tumors: Understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacology & therapeutics. 2016;157:120-4
140. Chuchvara N, Rao B, Liu X. Manually scanned single fiber optical coherence tomography for skin cancer characterization. Scientific reports. 2021;11:15570
141. Boostani M, Bozsányi S, Suppa M, Cantisani C, Lőrincz K, Bánvölgyi A. et al. Novel imaging techniques for tumor margin detection in basal cell carcinoma: a systematic scoping review of FDA and EMA-approved imaging modalities. International Journal of Dermatology. 2025;64:287-301
142. Hagyousif YA, Sharaf BM, Zenati RA, El-Huneidi W, Bustanji Y, Abu-Gharbieh E. et al. Skin cancer metabolic profile assessed by different analytical platforms. International Journal of Molecular Sciences. 2023;24:1604
143. Cui C, Yang H, Wang Y, Zhao S, Asad Z, Coburn LA. et al. Deep multimodal fusion of image and non-image data in disease diagnosis and prognosis: a review. Progress in Biomedical Engineering. 2023;5:022001
144. Smalley KS, Sondak VK. Inhibition of BRAF and MEK in BRAF-mutant melanoma. The Lancet. 2015;386:410-2
145. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Frontiers in oncology. 2018;8:86
146. Marmur ES, Schmults CD, Goldberg DJ. A review of laser and photodynamic therapy for the treatment of nonmelanoma skin cancer. Dermatologic Surgery. 2004;30:264-71
Author contact
![]() Corresponding authors: wenyen85com (W.-Y.C.); hcwangedu.tw (H.-C.W.).
Corresponding authors: wenyen85com (W.-Y.C.); hcwangedu.tw (H.-C.W.).

 Global reach, higher impact
Global reach, higher impact