Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(10):3270-3282. doi:10.7150/jca.111680 This issue Cite
Review
The Dual Roles of Circular RNAs in Breast Cancer Distant Metastasis and Their Clinical Applications
1. State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China.
2. Guangzhou Kangda Vocational Technical College, Guangzhou, 510555, China.
3. The Sixth Affiliated Hospital, School of Medicine, South China University of Technology, Foshan, China.
* These authors contributed equally to this work.
Received 2025-2-7; Accepted 2025-3-27; Published 2025-7-11
Abstract
Circular RNAs (circRNAs) are emerging as crucial regulators of the progression and metastasis of breast cancer. This review examines the dual roles of circRNAs, which act as both oncogenes and tumor suppressors, in breast cancer metastasis. CircRNAs play crucial roles in processes such as the epithelial‒mesenchymal transition (EMT), angiogenesis, immune evasion, and metabolic adaptation, facilitating cancer cell invasion, survival, and colonization in distant organs such as the brain, liver, bone, and lungs. Pro-metastatic circRNAs, such as circKIF4A and circBACH1, promote metastasis by modulating signaling pathways such as the STAT3 and PI3K/AKT pathways, whereas tumor-suppressive circRNAs, including circFOXO3 and circNFIB, inhibit metastatic progression through mechanisms such as VEGF downregulation and the suppression of arachidonic acid metabolism. Although circRNAs hold promise as biomarkers and therapeutic targets, their clinical application is impeded by challenges such as targeted delivery, off-target effects, and context-dependent roles. This review highlights the current understanding of circRNA-mediated regulation of breast cancer metastasis and emphasizes future directions, including the integration of multiomics technologies and advanced delivery systems, to increase the diagnostic and therapeutic utility of circRNAs.
Keywords: Circular RNAs (circRNAs), Breast cancer metastasis, Epithelial‒mesenchymal transition (EMT)
Introduction
Breast cancer is the most common malignancy in women worldwide, accounting for more than 11.6% of all cancer diagnoses [1]. Metastasis is frequently linked to both a high incidence and poor prognosis [2]. Breast cancer can be classified into several molecular subtypes, including hormone receptor-positive (ER+/PR+), human epidermal growth factor receptor 2-positive (HER2+), and triple-negative breast cancer (TNBC). TNBC is considered the most challenging subtype due to its aggressiveness and high risk of metastasis [3, 4]. The World Health Organization (WHO) reports an increasing global incidence of breast cancer, with treatment challenges primarily involving metastasis and drug resistance [5]. In breast cancer, distant metastasis arises when tumor cells disseminate from the primary site to other organs or tissues via the blood or lymphatic system. This phenomenon is often linked to a more aggressive tumor type and notably decreases patient survival rates [6]. Metastasis involves biological mechanisms such as the epithelial‒mesenchymal transition (EMT), angiogenesis, and extracellular matrix (ECM) degradation [7, 8]. Breast cancer frequently metastasizes to the bone, liver, lung, and brain [9, 10]. Bone metastasis typically manifests as osteolytic lesions, which may result in pathological fractures and severe pain [11]; liver metastasis is often associated with tumor invasiveness and high blood flow in the organ [12]; and brain metastasis involves the breakdown of the blood‒brain barrier and generally has a poor prognosis [13].
The mechanisms of distant breast cancer metastasis involve multiple signaling pathways and molecular regulation, including the EMT, ECM degradation, angiogenesis, and immune evasion [7]. CircRNAs, a new form of noncoding RNA, are essential for regulating distant metastasis processes in breast cancer. CircRNAs influence gene expression by sequestering specific microRNAs (miRNAs) or interacting with RNA-binding proteins (RBPs) to affect cellular behavior [14-17]. Circular RNAs (circRNAs) are stable, covalently closed-loop RNA molecules formed via back-splicing and have unique biological functions [18]. The main mechanisms involve acting as competing endogenous RNAs (ceRNAs) to modulate miRNA activity, interacting with RNA-binding proteins (RBPs), and directly encoding proteins [19-21]. CircRNAs play roles in regulating cell proliferation, migration, invasion, angiogenesis, and drug resistance [22]. In breast cancer, aberrant circRNA expression is significantly associated with tumor initiation, progression, and metastasis [23, 24].
In this study, we reviewed the latest advancements in the field and found that circRNAs play a key role in modulating the aggressiveness of breast cancer cells, as well as influencing distant metastasis and therapeutic resistance in breast cancer patients. Additionally, we identified that circRNAs regulate breast tumor angiogenesis, further contributing to cancer metastasis. Furthermore, through an extensive examination of numerous studies, we explored whether and how circRNAs could facilitate a diagnosis and prognostic predictions, as well as enhance the specificity of breast cancer treatment by serving as biomarkers. Many investigations have elucidated the influence of circRNAs on breast cancer metastasis through diverse signaling pathways, which constitutes a key aspect of this review.
Biological Characteristics of CircRNAs in Breast Cancer
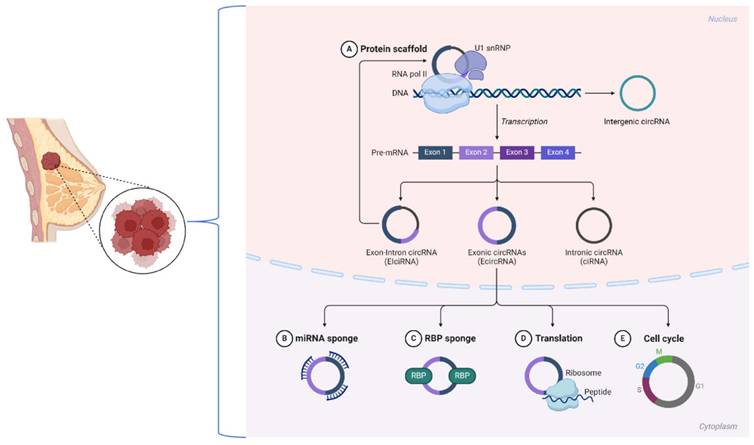
Circular RNAs (circRNAs) are noncoding RNAs with a covalently closed loop structure lacking a 5' cap and a 3' poly(A) tail [25, 26]. Back-splicing, a noncanonical splicing process, is the primary mechanism of circRNA formation, setting them apart from linear RNAs. Most circRNAs are classified into three types based on their sequence origins: ecircRNAs, ciRNAs, and EIciRNAs [27]. Research has shown that in breast cancer, many circRNAs contain known RBP binding sites that direct their circularization. These examples include circKIF4A, which is expressed from exon regions that can promote tumor development through its interactions with important signaling pathways [5, 28].
The closed-loop structure of circRNAs renders them highly stable by resisting exonuclease degradation. This stability enables circRNAs to accumulate in cells and bodily fluids, thus becoming attractive biomarkers for diseases such as breast cancer. In addition, circRNAs exhibit a high degree of conservation across species, which points toward their evolutionary importance [29]. For example, circCDR1as is conserved across mammalian species and has been implicated in regulating oncogenic processes [23]. The stability and conservation of circRNAs, such as circPVT1, are guaranteed in breast cancer tissues, leading to robust expression and the ability to serve as reliable diagnostic and prognostic markers.
Most likely, the best-described function of circRNAs that has been studied to date is acting as ceRNAs, which sponge miRNAs to regulate target genes at the downstream level. In ER-positive breast cancer, circPVT1 functions as a miR-181a-2-3p sponge, stabilizing the ESR1 mRNA and increasing estrogen receptor α (ERα) expression [30]. Thus, this mechanism links circPVT1 to the proliferation of cells and drug resistance in breast cancer.
Some circRNAs, such as EIciRNAs, localize to the nucleus and interact with the transcriptional machinery. For example, circPAIP2 enhances the transcription of its parental gene through interactions with RNA polymerase II [31]. In breast cancer, similar nuclear circRNAs may contribute to aberrant gene regulation, facilitating oncogenesis and metastasis. CircFOXO3 interacts with proteins such as p21 and CDK2 to regulate cell cycle progression and inhibit tumor growth [32].
These mechanisms underscore the diverse functions of circRNAs in breast cancer biology. Recent advancements, including the use of CRISPR-Cas13d-based screening tools [33], have facilitated the identification of functional circRNAs, revealing their critical involvement in tumor progression, immune evasion, and therapy resistance. These findings underscore the potential of targeting circRNAs for therapeutic intervention in breast cancer (Figure 1).
Circular RNAs that Enhance Breast Cancer Cell Invasiveness
Circular RNAs that Promote Breast Cancer Cell Invasion and Migration
Recent research has indicated that circRNAs significantly increase breast cancer cell invasion and migration (Table 1). A study revealed that circKIF4A is overexpressed in TNBC, where it competitively binds to miR-637, modulating the STAT3 signaling pathway to promote brain metastasis and cell migration [3].
CircEZH2 is notably overexpressed in breast cancer patients with liver metastasis. The overexpression of circEZH2 significantly increases breast cancer cell viability and invasiveness. Conversely, when the expression of circEZH2 is knocked down, the resulting effects are precisely the opposite of those observed under overexpression conditions [34]. The expression of circROBO1 is notably increased in breast cancer patients with liver metastases. Silencing circROBO1 significantly inhibits breast cancer cell proliferation, migration, and invasion. Conversely, its overexpression has the opposite effect, promoting these processes [35].
Studies using in vitro and in vivo models have shown that increased CircXPO6 expression promotes tumor progression and metastasis. CircXPO6 interacts with c-Myc to inhibit its ubiquitination and degradation by speckle-type POZ protein (SPOP), enhancing breast cancer cell migration and invasion [36]. CircRAD18, a circRNA that is markedly upregulated in TNBC, has been identified in previous studies as a key regulator of breast cancer progression. By acting as a molecular sponge for miR-208a and miR-3164, it alleviates the inhibitory effects of these miRNAs on the IGF1 and FGF2 target genes, thus facilitating cell proliferation, increasing migration, and preventing apoptosis [37]. Studies have indicated that circEPSTI1, a circRNA whose expression is markedly elevated in TNBC, acts as a miRNA sponge by interacting with miR-4753 and miR-6809. Through this interaction, it regulates BCL11A expression, thereby influencing TNBC cell proliferation and apoptosis [38].
In TNBC, overexpressed CircPLK1 can act as a ceRNA to regulate cell growth and invasion through the CircPLK1‒miR-296‒5p‒PLK1 axis [39]. CircGFRA1, which is significantly upregulated in TNBC, functions as a ceRNA by sequestering miR-34a, thereby modulating GFRA1 expression. Silencing circGFRA1 markedly reduces cell proliferation and triggers apoptosis in TNBC [40]. CircRRM2 is upregulated in breast cancer tissues, where it acts as a ceRNA to sponge miR-31-5p/miR-27b-3p and activate IGF2BP1, leading to increased tumor cell invasion and migration [41].
CircCRIM1, which is secreted via adipocyte-derived exosomes, inhibits miR-503-5p and activates the OGA protein, thereby accelerating TNBC progression and metastasis [42]. CircCD44 enhances cell proliferation, migration, and invasion by functioning as a molecular sponge for miR-502-5p. This interaction influences insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2), resulting in changes in cell behavior [43, 44]. Moreover, circBACH1 is upregulated in chemotherapy-induced breast cancer exosomes, increasing tumor cell invasion and drug resistance via the miR-217/G3BP2 axis [45].
Biological mechanisms of circRNAs in breast cancer.
CircRNAs play dual roles in the invasion and migration of breast cancer cells
| Gene symbol | Location | CircBase ID (allas) | miRNA sponge | Target gene/pathway | |
|---|---|---|---|---|---|
| Promote | CircKIF4A | chrX:69549254-69553539 | hsa_circ_0007255 | miR-637 | circKIF4A/miR-637/STAT3 |
| CircEZH2 | chr7:148529725-148544397 | hsa_circ_0008324 | miR-217-5p | FUS/circEZH2/KLF5/CXCR4 | |
| CircMYBL2 | chr20:42338602-42345122 | hsa_circ_0060467 | miR-1205 | circMYBL2/miR-1205/E2F1 | |
| CircROBO1 | chr3:78763546-78796050 | hsa_circ_0124696 | miR-217-5p | circROBO1/KLF5/FUS | |
| CircXPO6 | chr16:28112778-28113266 | hsa_circ_0038773 | c-Myc | ||
| CircRAD18 | chr3:8977554-8990254 | hsa_circ_0002453 | miR-208a/3164 | circRAD18/miR-208a/3164-IGF1/FGF2 | |
| CircEPSTI1 | chr13:43528083- 43544806 | hsa_circ_000479 | miR-4753/miR-6809 | circEPSTI1/miR-4753/6809-BCL11A | |
| CircPLK1 | chr16:23691404-23701688 | hsa_circ_0038632 | miR‑1294 | ||
| CircGFRA1 | chr10:117,849,251-117,856,275, | hsa_circ_005239 | miR-34a | miR-34a | |
| CircMMP2 | chr16:55522454-55523736 | hsa_circ_0039408 | circMMP2(6,7)/β-catenin/PRMT5 | ||
| CircRRM2 | chr2:10267001-10269281 | hsa_circ_0052582 | miR-31-5p/miR-27b-3p | IGF2BP1 | |
| CircCRIM1 | chr2:36764604-36764689 | hsa_circ_0007408 | miR-503-5p | miR-503-5p/O-GlcNAcase (OGA)/FBP1 | |
| CircCD44 | chr11:35226058-35227790 | hsa_circ_0021735 | miR-502-5p | CircCD44/miR-502-5p/KRAS | |
| CircBACH1 | chr21:30698379-30702014 | hsa_circ_0061395 | miR-217 | CircBACH1/miR-217/G3BP2 | |
| CircFOXO3 | chr6:108984657-108986092 | hsa_circ_0006404 | CircFOXO3/WHSC1-H3K36me2-ZEB2 | ||
| CircRAD54L2 | chr3:51575513-51586079 | hsa_circ_0001306 | miR-888 | CircRAD54L2/miR-888s/PDK1 | |
| CircRREB1 | chr6:7176887-7189555 | hsa_circ_0001573 | Erk1/2 | ||
| CircBRAF | chr7:140476711-140508795 | hsa_circ_0007178 | KDM4B and IGF2BP3 | ||
| CircCFL1 | chr11:65622881-65623563 | hsa_circ_0000328 | CircCFL1 /HDAC1/c-Myc/mutp53 | ||
| CircTFF1 | chr21:43782390-43786644 | hsa_circ_0061825 | miR-326 | TFF1 | |
| Inhibit | CircLIFR-007 | chr5:38481587-38481740 | hsa_circ_0129040 | YAP | |
| CircNFIB | chr9:14146687-14179779 | hsa_circ_0086376 | AA | ||
| CircDUSP1 | chr5:172195092-172196135 | hsa_circ_0075043 | miR-761 | circDUSP/miR-761/DACT2 | |
| CircRPAP2 | chr1:92798947-92846430 | hsa_circ_0000091 | circRPAP2/SRSF1/PTK2 | ||
| CircKDM4B | chr19:5047486-5082515 | hsa_circ_0002926 | miR-675 | circKDM4B /miR-675/NEDD4L | |
| CircSEMA4B | chr15:90760670-90764997 | hsa_circ_0000650 | miR-330-3p | circSEMA4B /miR-330-3p/PI3K/AKT | |
| CircAGFG1 | chr2:228356262-228389631 | hsa_circ_0058514 | miR-195-5p | circAGFG1/miR-195-5p/CCNE1 | |
| CircRPPH1 | chr14:20811305-20811534 | hsa_circ_0000515 | miR-542-3p | circRPPH1/miR-542-3p/ARHGAP1 | |
| CircNDST1 | chr5:149918789-149919826 | hsa_circ_0006943 | PI3K-Akt | ||
| CircGLIS3 | chr9:4117767-4,125,941 | hsa_circ_0007368 | miR-146b-3p | circGLIS3/miR-146b-3p/AIF1L | |
| CircPLK1 | chr16:23691404-23701688 | hsa_circ_0038632 | miR-296-5p | circPLK1/miR-296-5p/PLK1 | |
| CircAHNAK1 | chr11:62297840-62298224 | hsa_circ_0000320 | miR-421 | circAHNAK1 /miR-421/RASA1 | |
| CircFBXW7 | chr4:153332454-153333681 | hsa_circ_0001451 | miR-197-3p | circFBXW7/miR-197-3p/FBXW7-185aa | |
| CircEIF3H | chr8:117668094-117671219 | hsa_circ_0005231 | HSPD1/RBM8A/G3BP1 |
Although CircFOXO3 is generally downregulated, its overexpression significantly suppresses TNBC cell proliferation and metastasis through the modulation of the WHSC1-H3K36me2-ZEB2 axis [46]. CircRAD54L2 functions as an miR-888 family sponge, upregulating PDK1 expression, which facilitates breast cancer cell invasion and migration [47]. circRREB1 directly interacts with GNB4 to activate the Erk1/2 signaling pathway, enhancing tumor cell migration and invasion [48]. Notably, circCFL1 acts as a scaffold to increase HDAC1 binding to c-Myc, stabilizing the c-Myc protein and activating mutant TP53 expression. This process promotes TNBC stemness and immune evasion [49]. CircTFF1 is markedly increased in breast cancer tissues, and its silencing inhibits the progression of breast cancer cells. circTFF1 acts as a molecular sponge for miR-326, facilitating breast cancer progression via the miR-326/TFF1 pathway. These findings identify circTFF1 as a novel oncogene in breast cancer [50].
Circular RNAs that Promote the Distant Metastasis of Breast Cancer
The primary cause of death among breast cancer patients is distant metastasis. CircRNAs play pivotal roles in this process by regulating key molecular pathways, interacting with miRNAs, and modulating the tumor microenvironment. These processes facilitate the spread and growth of breast cancer cells in distant organs, including the brain, liver, bone, and lungs (Figure 2).
Research has shown that circKIF4A is upregulated in breast cancer tissues and is positively correlated with an advanced tumor stage and metastasis. Functionally, circKIF4A acts as a ceRNA, sponging miR-637 to prevent its suppression of STAT3 expression. STAT3, a well-known transcription factor, promotes the EMT, angiogenesis, and immune evasion, facilitating metastatic spread to distant organs [3]. Both in vitro and in vivo studies have shown that silencing circKIF4A significantly reduces STAT3 activation, inhibits the expression of EMT markers such as N-cadherin and vimentin, and increases E-cadherin expression [51]. The prometastatic roles of circKIF4A illustrates how circRNAs can influence key pathways to drive breast cancer metastasis. Targeting these circRNAs with antisense oligonucleotides (ASOs) or small-molecule inhibitors of the STAT3 and PI3K/AKT pathways is a promising approach for mitigating metastatic spread [52].
The stimulatory or inhibitory effects of differentially expressed circRNAs on metastasis.
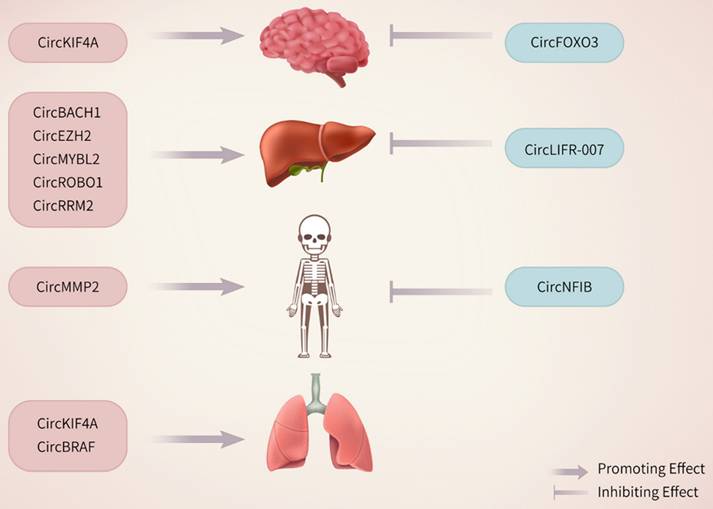
Besides promoting brain metastases, circRNAs also play a pro-oncogenic role in breast cancer liver metastases. The metastatic process involves a highly intricate, multistep cascade in which the EMT acts as a pivotal mechanism, promoting tumor cell detachment, migration, and invasion of distant sites [53]. Zhang et al. showed that KLF5 is crucial for sustaining the EMT state and driving tumor progression in prostate cancer. This process is mediated by the upregulation of C-X-C chemokine receptor type 4 (CXCR4), which significantly promotes bone metastasis and enhances resistance to chemotherapy [54]. High CXCL12 levels in organs such as the liver, bones, lungs, and lymph nodes activate the CXCR4/CXCL12 axis, promoting breast cancer progression and metastasis [55, 56]. Moreover, the CXCR4‒CXCL12 axis, along with phosphorylated mTOR, can induce the EMT program in metastatic breast cancer [57]. CircEZH2 promotes tumor cell proliferation and metastasis in vitro and in vivo by acting as a molecular sponge for miR-217-5p, thereby increasing Krüppel-like factor (KLF5) protein levels. KLF5 enhances FUsed in Sarcoma (FUS) transcription, facilitating the back-splicing required for circEZH2 formation. Furthermore, KLF5 activates CXCR4 transcription, triggering the EMT and driving the liver metastasis of breast cancer [34]. Similarly, CircMYBL2 is overexpressed in both breast cancer cells and liver metastases. It facilitates the proliferation of breast cancer cells and their metastasis to the liver. CircMYBL2 promotes the EMT in breast cancer cells by sponging miR-1205, leading to the upregulation of the transcription factor E2F1, and by interacting with eukaryotic translation initiation factor 4A3 (eIF4A3) [58]. Recent studies have indicated that CircROBO1 increases KLF5 expression by sequestering miR-217-5p. This upregulation of KLF5 increases the transcription of FUS, which in turn promotes the back-splicing of circROBO1. This process establishes a positive feedback loop involving circROBO1, KLF5, and FUS. circROBO1 promotes liver metastasis progression from breast cancer by upregulating KLF5, which suppresses selective autophagy mediated by afadin [35]. CircRNAs significantly influence liver metastasis by regulating metabolic and signaling pathways. CircRRM2 is upregulated in metastatic breast cancer tissues and sponges miR-31-5p and miR-27b-3p, thereby activating IGF2BP1 and forming a positive feedback loop with MYC, which enhances cancer cell invasion and colonization in the liver [41]. Furthermore, circCRIM1, which is secreted through adipocyte-derived exosomes, inhibits miR-503-5p and activates the OGA protein, facilitating TNBC liver metastasis [42].
CircRNAs also play a role in bone metastasis by affecting bone homeostasis. CircMMP2 interacts with β-catenin and PRMT5 to activate the transcription of bone remodeling factors such as S100A4 and LGALS3. These factors promote osteoclast activation and extracellular matrix degradation, creating a bone microenvironment conducive to cancer cell colonization. Targeting circMMP2 has shown efficacy in reducing the bone metastatic burden in preclinical models [59].
Moreover, circRNAs play critical roles in lung metastasis by modulating the EMT and angiogenesis. CircBRAF recruits KDM4B and IGF2BP3, regulating m6A RNA methylation and activating metastasis-related genes such as VCAN and MMP9. This process promotes cancer cell invasiveness and adaptation in the lung microenvironment, facilitating metastasis [60]. The increased expression of these circRNAs in metastatic tissues indicates their potential as noninvasive biomarkers for assessing the metastatic risk and monitoring the treatment response.
Interestingly, while most circRNAs promote metastasis, some exhibit context-dependent roles. For example, circCFL1 stabilizes c-Myc by enhancing its interaction with HDAC1, promoting immune evasion and stemness in TNBC [49]. Conversely, circNFIB suppresses metastasis by reducing arachidonic acid metabolism, which inhibits tumor growth and distant spread [61].
Circular RNAs that Suppress Breast Cancer Cell Invasiveness
Circular RNAs that Inhibit Breast Cancer Cell Invasion and Migration
The majority of circRNAs are known to promote breast cancer cell invasion and metastasis; however, certain circRNAs exhibit tumor-suppressive properties by inhibiting cell invasion, migration, and distant metastasis (Table 1). CircLIFR-007 suppresses breast cancer cell growth and spread in vitro and in vivo. Mechanistically, it promotes YAP phosphorylation by facilitating the cytoplasmic export of hnRNPA1, thereby enhancing the interaction between hnRNPA1 and YAP within the cytoplasm [59]. circNFIB suppresses breast cancer cell proliferation and invasion by regulating phospholipase activity, which reduces arachidonic acid synthesis [61]. CircDUSP1 functions as a molecular sponge for miR-761, increasing DACT2 expression and thereby inhibiting TNBC cell migration, invasion, and EMT. It increases cellular sensitivity to paclitaxel [62]. circDUSP16 is significantly overexpressed in TNBC cells, tissues, and plasma-derived exosomes, and it promotes TNBC cell proliferation, migration, and invasion through the miR-1224-3p/TFDP2 axis. Silencing circDUSP16 suppresses tumor growth in vivo, highlighting its potential as a biomarker and therapeutic target for TNBC [63].
CircRPAP2 interacts with SRSF1, hindering its splicing regulation of PTK2 precursor mRNAs, leading to decreased PTK2 protein levels and subsequently inhibiting breast cancer cell migration and invasion [64].
CircKDM4B acts as a molecular sponge for miR-675, increasing NEDD4L expression. This process inhibits the PI3K/AKT signaling pathway and angiogenesis, significantly reducing breast cancer invasion and metastasis [65].
CircAHNAK1 expression is notably lower in TNBC tissues than in normal tissues. By sponging miR-421, it may suppress TNBC proliferation, migration, and invasion, increasing the expression of the tumor suppressor gene RASA1 [66].
circFBXW7 acts as an miR-197-3p sponge and encodes the FBXW7-185aa protein, thereby inhibiting cell proliferation, migration, and tumor growth by upregulating FBXW7 expression in TNBC cell lines [67]. circSEMA4B encodes the novel protein SEMA4B-211aa, which inhibits AKT phosphorylation, thereby blocking the PI3K/AKT pathway and significantly suppressing breast cancer cell proliferation and migration [68]. Interestingly, although circAGFG1 generally has oncogenic properties in multiple cancers, its expression in breast cancer cells is associated with tumor-suppressive networks, where it regulates the EMT and key metastasis pathways [69].
CircRPPH1 functions as a molecular sponge for miR-542-3p, resulting in increased ARHGAP1 expression, which subsequently suppresses breast cancer cell proliferation, migration, and invasion [70]. Additionally, in vivo experiments have demonstrated that the overexpression of circRPPH1 significantly suppresses tumor growth and metastasis in breast cancer.
CircNDST1 interacts with CSNK2A1 to suppress the PI3K-Akt signaling pathway and EMT, which in turn diminishes the invasiveness and metastatic potential of breast cancer cells [71]. Studies have shown that circNDST1 expression is downregulated in breast cancer tissues and is associated with an improved patient prognosis.
In breast cancer cells, circPLK1 regulates the miR-1294/HMGA1 axis, resulting in reduced cancer cell migration, invasion, and tumor stemness, despite its usual oncogenic role in other cancers [72].
CircEIF3H interacts with HuR and IGF2BP2 to stabilize the HSPD1 and RBM8A mRNAs, thereby reducing the invasive characteristics of breast cancer cells [73].
These findings indicate that circRNAs influence breast cancer invasiveness and metastasis via crucial molecular pathways, positioning them as potential therapeutic targets and biomarkers for breast cancer diagnosis and prognosis.
Circular RNAs that Inhibit Distant Breast Cancer Metastasis
Distant metastasis of breast cancer cells is a major cause of high mortality in patients. CircRNAs play essential roles in breast cancer metastasis by interacting with miRNAs, regulating gene expression, or modulating key signaling pathways, which can suppress distant metastasis (Figure 2).
CircFOXO3 is significantly downregulated in TNBC and acts as a potent suppressor of brain metastasis. It inhibits the nuclear localization of WHSC1, reducing H3K36me2 modifications and suppressing ZEB2 expression. This cascade halts the EMT, ultimately preventing TNBC cell invasion and brain metastasis. The low expression of circFOXO3 in patients correlates with a poor prognosis, highlighting its potential as a prognostic biomarker [46].
CircRNAs, including circLIFR-007, are essential for inhibiting liver metastasis. CircLIFR-007 promotes the nuclear export of hnRNPA1 and increases YAP phosphorylation, resulting in the downregulation of liver metastasis-associated proteins, including SREBF1 and SNAI1. This mechanism notably diminishes the liver metastatic potential of breast cancer cells, improving patient outcomes [74].
CircNFIB inhibits breast cancer metastasis by modulating arachidonic acid metabolism. A previous study showed that circNFIB sponges and suppresses the expression of enzymes that participate in the arachidonic acid metabolic pathway, such as COX-2 [61]. The inhibition of these enzymes results in a reduction in prostaglandin synthesis, which is essential for enhancing metastatic potential, with a primary effect on inducing inflammatory responses. Both in vitro and in vivo studies have shown that elevated circNFIB expression is associated with decreased invasion of tumor cells and reduced metastasis to bone, a frequent site of breast cancer spread. Clinical data further support its role, showing that circNFIB is downregulated in metastatic breast cancer tissues compared with primary tumors [61].
CircRNAs also suppress metastasis by targeting the EMT and angiogenesis pathways. CircKDM4B functions as a molecular sponge for miR-675, resulting in increased NEDD4L expression. This process inhibits the PI3K/AKT signaling pathway, reducing angiogenesis and cell migration. As a result, circKDM4B effectively reduces distant metastasis in TNBC models [65]. circSEMA4B encodes the novel protein SEMA4B-211aa, which inhibits AKT phosphorylation, thereby blocking the PI3K/AKT pathway. This mechanism significantly suppresses breast cancer cell proliferation, migration, and distant spread [68].
Although circAGFG1 typically has oncogenic properties in other cancers, its expression in breast cancer is linked to tumor-suppressive networks. CircAGFG1 modulates key EMT- and metastasis-associated pathways, contributing to the inhibition of distant metastasis in specific breast cancer contexts [75].
The Role of Circular RNAs in Angiogenesis During Breast Cancer Metastasis
Angiogenesis is essential for the distant metastasis of breast cancer. Tumors facilitate new blood vessel formation to secure the oxygen and nutrients essential for cell growth and migration [76].
CircRNAs that promote angiogenesis are critical for supporting metastatic progression by increasing vascularization within primary tumors and metastatic niches. CircRRM2 upregulates proangiogenic factors through the miR-31-5p/miR-27b-3p/IGF2BP1 axis, which enhances endothelial cell proliferation and vascular permeability, supporting the metastatic colonization of distant organs such as the liver. This mechanism is tightly linked to the aggressive nature of breast cancer metastasis [41]. CircBACH1, which is secreted via chemotherapy-induced exosomes, facilitates angiogenesis by regulating the miR-217/G3BP2 signaling axis. By increasing vascular endothelial cell growth, circBACH1 promotes tumor vascularization and metastasis, particularly in drug-resistant breast cancer [45]. CircKIF4A, which is overexpressed in TNBC, indirectly enhances angiogenesis through the activation of the STAT3 pathway, which upregulates key vascular endothelial growth factors and cytokines, enabling rapid tumor progression [3]. These circRNAs act as essential modulators of angiogenesis, indicating their importance in establishing the vascular networks necessary for distant metastasis.
In contrast to proangiogenic circRNAs, a subset of circRNAs inhibits angiogenesis, counteracting metastatic progression. CircSEMA4B encodes a novel protein, SEMA4B-211aa, which inhibits angiogenesis by suppressing AKT phosphorylation and blocking the PI3K/AKT signaling pathway. This disruption of proangiogenic signaling pathways significantly reduces vascular formation and metastatic spread [68]. CircNFIB reduces endothelial cell activation by downregulating arachidonic acid, a key metabolic pathway involved in angiogenesis. Its antiangiogenic properties inhibit tumor growth and metastasis in both in vitro and in vivo models [61]. CircKDM4B functions as a molecular sponge for miR-675, leading to the upregulation of NEDD4L, which in turn inhibits angiogenesis by suppressing the PI3K/AKT pathway. This reduction in the angiogenic capacity is associated with decreased tumor cell migration and distant metastatic potential [65]. These circRNAs highlight the potential of targeting angiogenesis as a therapeutic strategy to limit breast cancer metastasis.
CircRNAs regulate angiogenesis through multiple molecular pathways, demonstrating their versatile roles in shaping the tumor microenvironment. In the VEGF pathway, circRNAs such as circBACH1 and circKIF4A indirectly regulate the expression of VEGF, a central driver of angiogenesis. Targeting these circRNAs could help disrupt VEGF-mediated vascularization. Regarding m6A RNA methylation, CircRNAs such as circBRAF modulate epigenetic mechanisms such as m6A RNA methylation, which influence the expression of angiogenesis-related genes. This regulation highlights the role of circRNAs in the posttranscriptional control of proangiogenic factors [60]. In PI3K/AKT signaling, both pro- and antiangiogenic circRNAs, such as circSEMA4B and circKDM4B, affect the PI3K/AKT pathway, underscoring their importance as therapeutic targets in circRNA-mediated angiogenesis. These mechanisms provide a molecular framework for understanding how circRNAs influence angiogenesis and metastatic progression [68].
The dual roles of circRNAs in angiogenesis highlight their potential as biomarkers and therapeutic targets in breast cancer metastasis. Proangiogenic circRNAs represent viable targets for the inhibition of vasculogenesis within metastatic neoplasms, whereas antiangiogenic circRNAs have potential for the derivation of novel therapeutic paradigms. For example, in the context of therapeutic targeting, the silencing of proangiogenic circRNAs, exemplified by circ_0008673[77] or circHIPK3[78], has the capacity to attenuate vascularization and curtail tumor dissemination.
Regarding biomarker applications, antiangiogenic circRNAs, such as circ_0047303, could function as reliable biomarkers for the evaluation of the angiogenic propensity and metastatic risk in breast cancer patients [79]. In terms of combination therapy, circRNA-centered interventions may be integrated with extant antiangiogenic regimens, such as VEGF inhibitors, to augment therapeutic efficacy and overcome resistance. Advancements in delivery modalities, notably exosome-based circRNA carriers, portend the potential to surmount the challenges associated with circRNA stability and off-target consequences.
The Role of Circular RNAs in Chemotherapy Resistance in Breast Cancer
The efficacy of breast cancer treatments is considerably hindered by chemotherapy resistance. The mechanisms of resistance involve increased expression of drug efflux proteins, increased DNA damage repair, and inhibition of apoptosis [9]. Recent studies have identified circRNAs as key regulators of the development of chemotherapy resistance [80].
Research indicates that circRNAs play a role in the resistance of breast cancer to doxorubicin. The downregulation of circRNAs notably reduces drug resistance and inhibits the proliferation and metastasis of breast cancer cells, suggesting its potential as a therapeutic target [81]. The suppression of circ_0085495 markedly decreases doxorubicin resistance and hinders the proliferation and spread of breast cancer cells [82]. Furthermore, circRNA-CREIT is reportedly downregulated in TNBC cells that are resistant to doxorubicin. The therapeutic potential of circRNA-CREIT is further highlighted by the fact that its encapsulation in exosomes increases doxorubicin sensitivity in TNBC cells [16].
CircPVT1 is overexpressed in breast cancer tissues, driving tumor progression and drug resistance. It exerts this effect by stabilizing the ESR1 mRNA through competition for binding with miR-181a-2-3p, hence leading to an increase in the level of the ERα protein and subsequently activating target genes that are required for cancer cell growth and survival. Additionally, circPVT1 inhibits immune responses by binding to the MAVS protein and perturbing the RIG-I-MAVS complex, thereby inhibiting type I interferon signaling and enhancing immune evasion. Both mechanisms indicate a dual role for circPVT1 in tumorigenesis and therapy resistance. More importantly, therapeutic interventions with ASOs against circPVT1 resulted in the significant inhibition of proliferation and tumor growth, suggesting a new therapeutic intervention for ER-positive breast cancer [30].
In addition to modulating the chemotherapy response, circRNAs are implicated in shaping the tumor microenvironment, which contributes to both chemotherapy resistance and immunotherapy resistance. A study revealed that a high RM score, which is correlated with an immunosuppressive microenvironment, is a key factor contributing to the resistance to interventions such as PD-L1 blockade therapy. Within this regulatory framework, circWWC3 has been shown to upregulate IL-4 expression and subsequent cytokine secretion in breast cancer cells. This paracrine signaling subsequently induces PD-L1 expression in polarized M2 macrophages, thereby facilitating tumor immune evasion through PD-1/PD-L1 checkpoint activation. These findings highlight a potential link between circWWC3-mediated immunomodulation and circRNA-driven therapeutic resistance mechanisms in breast cancer [83].
Leveraging these insights, the integration of circRNA-targeted therapies with traditional chemotherapies or SG inhibitors such as ISRIB represents a promising approach for overcoming resistance and improving treatment outcomes in breast cancer patients.
The Application of Circular RNAs as Biomarkers in Breast Cancer Patients
Advances in molecular biology technology have led scholars to recognize the significant potential of circRNAs in diagnosing, classifying, and assessing the prognosis of patients with breast cancer. The concept of circRNAs as biomarkers has progressed from early basic discovery to applied research [84], and some researchers believe that circRNAs show increased stability and conservation through their unique closed-loop structure [85]. Others have proposed that circRNAs have specific tissue and temporal expression properties, making them important candidates as disease markers. In this work, the main discussion of circRNAs as biomarkers includes information on their feasibility as molecules with disease-specific expression patterns, molecular stability and the ability to be detected in body fluids [86].
The existing studies can be roughly divided into the following two main viewpoints according to different topics. In the first type of research, two representative views are discussed. CircRNA stability and specific expression in blood, urine, and tissue samples make it a potential noninvasive marker for breast cancer diagnosis [87]. For example, elevated levels of circRHOT1 were consistently detected in circulating exosomes isolated from both BC patient sera and in vitro tumor cell cultures, establishing this circular RNA as a promising diagnostic biomarker for breast malignancies [88]. In contrast, another view emphasizes the tissue-specific expression of circRNAs, such as the high expression pattern of circTP63 in estrogen receptor-positive breast cancer [89], which provides support for molecular typing. Thus, the study of circRNAs in breast cancer diagnosis reveals a basic consensus: their stability and specificity provide a new technical direction for early diagnosis. A discussion of how to overcome the limitation of detection sensitivity in the detection process is worthwhile, which is the focus of some research. A CRISPR-Cas13a/Cas12a-based system has been developed to enable simultaneous fluorescence detection of the breast cancer biomarkers circROBO1 and BRCA1[90].
The second category of studies focuses on the potential of circRNAs as prognostic markers and is subdivided into two representative views. CircRNA expression levels are significantly linked to survival and the recurrence risk in breast cancer patients [84]. For example, the downregulation of circCDR1as is associated with poorer outcomes in breast cancer patients. Another idea is that circRNAs may influence patient prognosis by regulating pathways associated with tumor metastasis [25]. For example, circ PDSS1 promotes tumor metastasis by regulating the EMT, and its high expression is significantly associated with a poorer prognosis for patients with metastatic breast cancer [91]. The circKIF4A-miR-375-KIF4A axis drives the progression of TNBC via the ceRNA mechanism. Thus, circKIF4A has potential as both a prognostic biomarker and a therapeutic target for TNBC [51]. In addition, other studies have explained the role of circRNAs in the dynamic monitoring of breast cancer treatment response from the perspective of multiomics integration [25]. The second view further expands the applications of circRNAs and forms a progressive relationship with the first view, namely, the overall application path from diagnosis to prognostic assessment.
Although circRNA research in breast cancer has advanced considerably, the clinical application of circRNAs remains challenging because of issues such as targeted delivery specificity and potential off-target effects [92]. Future advancements in the integration of multiomics technologies with artificial intelligence are anticipated to expedite the translational use of circRNAs in the diagnosis and treatment of breast cancer [84, 93].
CircRNAs participate in breast cancer tumorigenesis and progression through various mechanisms, including acting as ceRNAs, encoding proteins, and regulating gene expression. Their inherent stability and tissue specificity further increase their potential as ideal biomarkers for breast cancer diagnosis, particularly in liquid biopsy applications. Moreover, targeting specific circRNAs holds promise as an effective therapeutic strategy, offering novel avenues for personalized breast cancer treatment [25].
The aforementioned studies provide valuable insights for applying circRNAs in breast cancer patients and advancing their clinical translation. Current research has largely overlooked certain aspects, particularly in terms of study design. A predominant focus on the singular role of circRNAs, such as in diagnosis or prognosis, has been noted, with an insufficient exploration of their potential as multifunctional markers. Second, regarding data analysis methods, most studies employ a single-omics analysis, with a notable deficiency in multiomics approaches to investigate the mechanisms of circRNAs. Third, from an argumentative point of view, the existing studies have focused more on the molecular biological functions of circRNAs and less on their feasibility and ethical challenges in clinical practice.
Discussion
CircRNAs play dual roles in the distant metastasis of breast cancer, acting as both oncogenic drivers and tumor suppressors. This duality reflects the complexity of their biological functions and highlights the need for deeper mechanistic investigations to fully understand their context-dependent roles. CircRNAs influence multiple processes central to metastasis, including the EMT, angiogenesis, immune evasion, and metabolic adaptation. These processes enable breast cancer cells to survive, disseminate, and colonize distant organs, such as the brain, liver, bone, and lungs. Despite significant progress, several challenges remain in translating circRNA research into clinical applications.
One major challenge is the intricate regulatory networks in which circRNAs operate. The same circRNAs may exhibit opposing functions depending on the molecular context, such as interactions with specific miRNAs or RBPs. For example, circAGFG1, which is typically considered oncogenic in other cancers, has been shown to suppress metastasis in breast cancer by modulating EMT-related pathways. This finding highlights the importance of exploring the molecular environment and breast cancer subtype specificity when studying circRNA functions [75]. CircRNAs play pivotal roles in regulating metastasis by targeting key signaling pathways, including PI3K/AKT, STAT3, and m6A RNA methylation, underscoring their central roles in metastasis regulation [3, 60, 94]. However, the exact molecular mechanisms of these interactions are not fully understood, necessitating further research to explore these pathways at the single-cell and tissue-specific levels.
From a clinical perspective, circRNAs are promising diagnostic biomarkers and therapeutic targets in clinical settings because of their stability, tissue specificity, and role in modulating metastatic processes. For example, prometastatic circRNAs such as circKIF4A and circRRM2 may serve as predictive biomarkers for high-risk patients, whereas tumor-suppressive circRNAs such as circFOXO3 and circSEMA4B could be harnessed to develop novel therapeutic interventions [3, 41, 68, 95]. Nonetheless, issues such as targeted delivery and off-target effects impede their clinical application. Delivery systems capable of specifically targeting circRNAs to metastatic niches, such as exosome-based carriers, need to be further developed and optimized. Additionally, understanding the role of circRNAs in therapy resistance, such as the contribution of circBACH1 to chemotherapy-induced angiogenesis, will be crucial for overcoming the current treatment limitations [45].
The effective delivery of circRNA-based therapeutics remains a significant challenge. Current delivery systems, including lipid nanoparticles and viral vectors, face limitations in specificity and efficiency, particularly in targeting metastatic or deep-seated tumors [18]. For example, ensuring the stability of circRNAs in circulation and achieving precise tumor tissue targeting without affecting healthy tissues require advanced engineering of delivery platforms [96]. Moreover, the lack of universal and scalable delivery strategies for encapsulating and transporting circRNAs hinders their widespread use in clinical settings [18]. The biological effects of circRNAs often vary depending on the cellular and microenvironmental contexts. A circRNA that acts as a tumor suppressor in one cancer subtype might function as an oncogene in another, complicating the design of therapeutic strategies [97, 98]. For example, circFOXO3 inhibits angiogenesis in breast cancer and may exert proapoptotic effects in other tissue contexts. Such functionality begs a context-dependent understanding of circRNA regulatory networks and demands caution when interventions are designed.
The possibility of off-target effects raises considerable safety concerns for circRNA-based therapies. RNA-based therapies, including circRNAs, can unintentionally interact with unintended molecular targets. Such interactions may lead to unpredictable side effects. For example, the widespread binding sites for microRNAs or proteins on circRNAs increase their likelihood of off-target interactions [99]. Moreover, the immune system may recognize circRNA therapies as foreign, hence leading to inflammatory responses or autoimmune reactions [100]. These safety concerns call for detailed preclinical assessments and the establishment of more precise mechanisms of targeting.
Another key area for future research is the integration of circRNA studies with multiomics technologies, such as single-cell RNA sequencing and spatial transcriptomics [101]. These approaches can uncover the dynamic regulation of circRNAs within the tumor microenvironment, revealing their interactions with immune cells, endothelial cells, and stromal components, which may contribute to drug resistance in breast cancer chemotherapy, targeted therapy, and endocrine therapy [102]. Such resistance poses significant challenges to cancer treatment, but the integration of advanced computational modeling and machine learning could aid in identifying novel circRNA signatures associated with metastatic progression, treatment outcomes, and potential strategies to overcome resistance [103].
In conclusion, while circRNAs provide valuable insights into the mechanisms of breast cancer metastasis, further research is needed to address the challenges associated with their dual roles and clinical applications. By focusing on the specific roles of circRNAs in different breast cancer subtypes and leveraging emerging technologies, researchers can unlock their full potential as biomarkers and therapeutic targets, paving the way for more effective strategies to combat breast cancer metastasis.
Acknowledgements
Funding
This research was funded by the Guangdong Basic and Applied Basic Research Foundation (2021B1515120053, Ziyun Guan). This study was supported by the National Natural Science Foundation of China (Nos. 82473036, Hailin Tang).
Author contributions
Qing Bao: Writing - original draft. Huan Zhang: Writing - original draft. Pangzhou chen: Writing - original draft. Song Wu: Data curation, Writing - original draft. Huan Wang: Data curation. Jingna Cao: Data curation. Wen Zhou: Data curation, supervision. Ziyun Guan: Funding acquisition, supervision, Writing - review & editing. Hailin Tang: Conceptualization, supervision, writing - review & editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Laversanne M, Sung H. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2024;74:229-63
2. Saranya I, Preetha D, Nivruthi S. et al. A comprehensive bioinformatic analysis of the role of TGF-β1-stimulated activating transcription factor 3 by non-coding RNAs during breast cancer progression. Comput Biol Chem. 2024;113:108208
3. Song W, Jibu L, Hongbo Z. et al. A novel axis of circKIF4A-miR-637-STAT3 promotes brain metastasis in triple-negative breast cancer. Cancer Letters. 2024;581:216508
4. Garrido-Castro AC, Lin NU, Polyak K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer discovery. 2019;9:176-98
5. Emma N, Geoffrey JL, Jane EV. Deciphering breast cancer: from biology to the clinic. Cell. 2023;186:1708-28
6. Park M, Kim D, Ko S. et al. Breast Cancer Metastasis: Mechanisms and Therapeutic Implications. International journal of molecular sciences. 2022;23(12):6806
7. Sousa B, Ribeiro AS, Paredes J. Heterogeneity and Plasticity of Breast Cancer Stem Cells. Adv Exp Med Biol. 2019;1139:83-103
8. Xu X, Wang X, Zheng Z. et al. Neutrophil Extracellular Traps in Breast Cancer: Roles in Metastasis and Beyond. J Cancer. 2024;15:3272-83
9. Bai X, Ni J, Beretov J. et al. Triple-negative breast cancer therapeutic resistance: Where is the Achilles' heel? Cancer Lett. 2021;497:100-11
10. Yin Y, Yan Y, Fan B. et al. Novel Combination Therapy for Triple-Negative Breast Cancer based on an Intelligent Hollow Carbon Sphere. Research (Wash D C). 2023;6:0098
11. Pang L, Gan C, Xu J. et al. Bone Metastasis of Breast Cancer: Molecular Mechanisms and Therapeutic Strategies. Cancers. 2022;14(23):5727
12. Rashid NS, Grible JM, Clevenger CV. et al. Breast cancer liver metastasis: current and future treatment approaches. Clin Exp Metastasis. 2021;38:263-77
13. Hosonaga M, Saya H, Arima Y. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev. 2020;39:711-20
14. Luo Y, Tang W, Xiang S. et al. Non-coding RNAs in breast cancer: Implications for programmed cell death. Cancer Lett. 2022;550:215929
15. Kristensen LS, Andersen MS, Stagsted LVW. et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675-91
16. Guo X, Gao C, Yang DH. et al. Exosomal circular RNAs: A chief culprit in cancer chemotherapy resistance. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2023;67:100937
17. Wu J, Zhu MX, Li KS. et al. Circular RNA drives resistance to anti-PD-1 immunotherapy by regulating the miR-30a-5p/SOX4 axis in non-small cell lung cancer. Cancer Drug Resist. 2022;5:261-70
18. Loan Young T, Chang Wang K, James Varley A. et al. Clinical delivery of circular RNA: Lessons learned from RNA drug development. Advanced drug delivery reviews. 2023;197:114826
19. Okholm TLH, Kamstrup AB, Nielsen MM. et al. circHIPK3 nucleates IGF2BP2 and functions as a competing endogenous RNA. bioRxiv. 2024;13:RP91783
20. Afzali F, Salimi M. Unearthing Regulatory Axes of Breast Cancer circRNAs Networks to Find Novel Targets and Fathom Pivotal Mechanisms. Interdiscip Sci. 2019;11:711-22
21. Lei P, Guo Q, Hao J. et al. Exploring the evolving roles and clinical significance of circRNAs in head and neck squamous cell carcinoma. J Cancer. 2024;15:3984-94
22. Han X, Tian R, Wang C. et al. CircRNAs: Roles in regulating head and neck squamous cell carcinoma. Front Oncol. 2022;12:1026073
23. Tang L, Jiang B, Zhu H. et al. The Biogenesis and Functions of circRNAs and Their Roles in Breast Cancer. Front Oncol. 2021;11:605988
24. Zeng Y, Zou Y, Gao G. et al. The biogenesis, function and clinical significance of circular RNAs in breast cancer. Cancer biology & medicine. 2021;19:14-29
25. Huang X, Song C, Zhang J. et al. Circular RNAs in breast cancer diagnosis, treatment and prognosis. Oncology research. 2023;32:241-9
26. Xie J, Ye F, Deng X. et al. Circular RNA: A promising new star of vaccine. Journal of translational internal medicine. 2023;11:372-81
27. Zhang F, Li L, Fan Z. circRNAs and their relationship with breast cancer: a review. World J Surg Oncol. 2022;20:373
28. Lyu L, Zhang S, Deng Y. et al. Regulatory mechanisms, functions, and clinical significance of CircRNAs in triple-negative breast cancer. J Hematol Oncol. 2021;14:41
29. Gong L, Zhou X, Sun J. Circular RNAs Interaction with MiRNAs: Emerging Roles in Breast Cancer. Int J Med Sci. 2021;18:3182-96
30. Yi J, Wang L, Hu GS. et al. CircPVT1 promotes ER-positive breast tumorigenesis and drug resistance by targeting ESR1 and MAVS. The EMBO journal. 2023;42:e112408
31. Li Z, Huang C, Bao C. et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nature structural & molecular biology. 2015;22:256-64
32. Du WW, Yang W, Liu E. et al. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic acids research. 2016;44:2846-58
33. Zhang Y, Nguyen TM, Zhang XO. et al. Optimized RNA-targeting CRISPR/Cas13d technology outperforms shRNA in identifying functional circRNAs. Genome biology. 2021;22:41
34. Liu P, Wang Z, Ou X. et al. The FUS/circEZH2/KLF5/ feedback loop contributes to CXCR4-induced liver metastasis of breast cancer by enhancing epithelial-mesenchymal transition. Molecular cancer. 2022;21:198
35. Wang Z, Yang L, Wu P. et al. The circROBO1/KLF5/FUS feedback loop regulates the liver metastasis of breast cancer by inhibiting the selective autophagy of afadin. Molecular cancer. 2022;21:29
36. Xu A, Li X, Cai Q. et al. CircXPO6 promotes breast cancer progression through competitively inhibiting the ubiquitination degradation of c-Myc. Mol Cell Biochem. 2025;480:1731-45
37. Zou Y, Zheng S, Xiao W. et al. circRAD18 sponges miR-208a/3164 to promote triple-negative breast cancer progression through regulating IGF1 and FGF2 expression. Carcinogenesis. 2019;40:1469-79
38. Chen B, Wei W, Huang X. et al. circEPSTI1 as a Prognostic Marker and Mediator of Triple-Negative Breast Cancer Progression. Theranostics. 2018;8:4003-15
39. Kong Y, Yang L, Wei W. et al. CircPLK1 sponges miR-296-5p to facilitate triple-negative breast cancer progression. Epigenomics. 2019;11:1163-76
40. He R, Liu P, Xie X. et al. circGFRA1 and GFRA1 act as ceRNAs in triple negative breast cancer by regulating miR-34a. J Exp Clin Cancer Res. 2017;36:145
41. Hao R, Zhang L, Si Y. et al. A novel feedback regulated loop of circRRM2-IGF2BP1-MYC promotes breast cancer metastasis. Cancer Cell Int. 2023;23:54
42. Li Y, Jiang B, Zeng L. et al. Adipocyte-derived exosomes promote the progression of triple-negative breast cancer through circCRIM1-dependent OGA activation. Environ Res. 2023;239:117266
43. Jie L, Xiufang G, Zhanqiang Z. et al. CircCD44 plays oncogenic roles in triple-negative breast cancer by modulating the miR-502-5p/KRAS and IGF2BP2/Myc axes. Molecular Cancer. 2021;20:138
44. Aiqi X, Lewei Z, Chengcai Y. et al. The therapeutic potential of circular RNA in triple-negative breast cancer. Cancer Drug Resistance. 2024;7:13
45. Xia W, Chen W, Ni C. et al. Chemotherapy-induced exosomal circBACH1 promotes breast cancer resistance and stemness via miR-217/G3BP2 signaling pathway. Breast Cancer Res. 2023;25:85
46. Chen D, Zeng S, Qiu H. et al. Circ-FOXO3 inhibits triple-negative breast cancer growth and metastasis via regulating WHSC1-H3K36me2-Zeb2 axis. Cellular signalling. 2024;117:111079
47. He Q, Hao Q, Wu Y. et al. CircRAD54L2 promotes triple-negative breast cancer progression by regulating the miR-888 family/PDK1 axis. Life Sci. 2023;312:121128
48. Chen H, Wang X, Cheng H. et al. CircRNA circRREB1 promotes tumorigenesis and progression of breast cancer by activating Erk1/2 signaling through interacting with GNB4. Heliyon. 2024;10:e28785
49. Wang Z, Li Y, Yang J. et al. CircCFL1 Promotes TNBC Stemness and Immunoescape via Deacetylation-Mediated c-Myc Deubiquitylation to Facilitate Mutant TP53 Transcription. Adv Sci (Weinh). 2024;11:e2404628
50. Pan G, Mao A, Liu J. et al. Circular RNA hsa_circ_0061825 (circ-TFF1) contributes to breast cancer progression through targeting miR-326/TFF1 signalling. Cell Prolif. 2020;53:e12720
51. Tang H, Huang X, Wang J. et al. circKIF4A acts as a prognostic factor and mediator to regulate the progression of triple-negative breast cancer. Molecular cancer. 2019;18:23
52. Sun Z, Jiang Q, Gao B. et al. AKT Blocks SIK1-Mediated Repression of STAT3 to Promote Breast Tumorigenesis. Cancer research. 2023;83:1264-79
53. Heerboth S, Housman G, Leary M. et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:6
54. Zhang B, Li Y, Wu Q. et al. Acetylation of KLF5 maintains EMT and tumorigenicity to cause chemoresistant bone metastasis in prostate cancer. Nat Commun. 2021;12:1714
55. Guo H, Ge Y, Li X. et al. Targeting the CXCR4/CXCL12 axis with the peptide antagonist E5 to inhibit breast tumor progression. Signal Transduct Target Ther. 2017;2:17033
56. Liu D, Guo P, McCarthy C. et al. Peptide density targets and impedes triple negative breast cancer metastasis. Nat Commun. 2018;9:2612
57. Yang F, Takagaki Y, Yoshitomi Y. et al. Inhibition of Dipeptidyl Peptidase-4 Accelerates Epithelial-Mesenchymal Transition and Breast Cancer Metastasis via the CXCL12/CXCR4/mTOR Axis. Cancer research. 2019;79:735-46
58. Zeng Y, Du W, Huang Z. et al. Hsa_circ_0060467 promotes breast cancer liver metastasis by complexing with eIF4A3 and sponging miR-1205. Cell Death Discov. 2023;9:153
59. Xu Y, Li X, Zhang S. et al. CircMMP2(6,7) Cooperates with β-Catenin and PRMT5 to Disrupt Bone Homeostasis and Promote Breast Cancer Bone Metastasis. Cancer Res. 2024;84:328-43
60. Lan J, Wang L, Cao J. et al. circBRAF promotes the progression of triple-negative breast cancer through modulating methylation by recruiting KDM4B to histone H3K9me3 and IGF2BP3 to mRNA. Am J Cancer Res. 2024;14:2020-36
61. Zhong S, Xu H, Wang D. et al. circNFIB decreases synthesis of arachidonic acid and inhibits breast tumor growth and metastasis. European journal of pharmacology. 2024;963:176221
62. Huang S, Xie J, Lei S. et al. CircDUSP1 regulates tumor growth, metastasis, and paclitaxel sensitivity in triple-negative breast cancer by targeting miR-761/DACT2 signaling axis. Mol Carcinog. 2023;62:450-63
63. Wei D, Zhang F, Li M. et al. CircDUSP16 mediates the effect of triple-negative breast cancer in pirarubicin via the miR-1224-3p/TFDP2 axis. Biochem Pharmacol. 2024;232:116719
64. Yu Y, Fang L. CircRPAP2 regulates the alternative splicing of PTK2 by binding to SRSF1 in breast cancer. Cell Death Discov. 2022;8:152
65. Guo XY, Liu TT, Zhu WJ. et al. CircKDM4B suppresses breast cancer progression via the miR-675/NEDD4L axis. Oncogene. 2022;41:1895-906
66. Xiao W, Zheng S, Zou Y. et al. CircAHNAK1 inhibits proliferation and metastasis of triple-negative breast cancer by modulating miR-421 and RASA1. Aging (Albany NY). 2019;11:12043-56
67. Ye F, Gao G, Zou Y. et al. circFBXW7 Inhibits Malignant Progression by Sponging miR-197-3p and Encoding a 185-aa Protein in Triple-Negative Breast Cancer. Mol Ther Nucleic Acids. 2019;18:88-98
68. Wang X, Jian W, Luo Q. et al. CircSEMA4B inhibits the progression of breast cancer by encoding a novel protein SEMA4B-211aa and regulating AKT phosphorylation. Cell death & disease. 2022;13:794
69. Yang R, Xing L, Zheng X. et al. The circRNA circAGFG1 acts as a sponge of miR-195-5p to promote triple-negative breast cancer progression through regulating CCNE1 expression. Mol Cancer. 2019;18:4
70. Qi L, Sun B, Yang B. et al. circRNA RPPH1 Facilitates the Aggravation of Breast Cancer Development by Regulating miR-542-3p/ARHGAP1 Pathway. Cancer Biother Radiopharm. 2022;37:708-19
71. Shu C, Wang S, Hu J. et al. CircNDST1 promotes papillary thyroid cancer progression via its interaction with CSNK2A1 to activate the PI3K-Akt pathway and epithelial-mesenchymal transition. J Endocrinol Invest. 2023;46:545-57
72. Zhang Q, Wang Z, Cai H. et al. CircPLK1 Acts as a Carcinogenic Driver to Promote the Development of Malignant Pleural Mesothelioma by Governing the miR-1294/HMGA1 Pathway. Biochem Genet. 2022;60:1527-46
73. Song X, Chen B, Liang Y. et al. CircEIF3H-IGF2BP2-HuR scaffold complex promotes TNBC progression via stabilizing HSPD1/RBM8A/G3BP1 mRNA. Cell death discovery. 2022;8:261
74. Zhang Y, Tan Y, Yuan J. et al. circLIFR-007 reduces liver metastasis via promoting hnRNPA1 nuclear export and YAP phosphorylation in breast cancer. Cancer Lett. 2024;592:216907
75. Luo J, Zhong H, Guo M. et al. CircAGFG1 Promotes Ovarian Cancer Progression Through the miR-409-3 p/ZEB1 Axis. Technol Cancer Res Treat. 2024;23:15330338241252423
76. Zhou X, LeBleu VS, Fletcher-Sananikone E. et al. Vascular heterogeneity of tight junction Claudins guides organotropic metastasis. Nature cancer. 2024;5:1371-89
77. Sun L, Chen S, Wang T. et al. Hsa_circ_0008673 Promotes Breast Cancer Progression by MiR-578/GINS4 Axis. Clinical breast cancer. 2023;23:281-90
78. Shi P, Liu Y, Yang H. et al. Breast cancer derived exosomes promoted angiogenesis of endothelial cells in microenvironment via circHIPK3/miR-124-3p/MTDH axis. Cellular signalling. 2022;95:110338
79. Darbeheshti F, Mahdiannasser M, Noroozi Z. et al. Circular RNA-associated ceRNA network involved in HIF-1 signalling in triple-negative breast cancer: circ_0047303 as a potential key regulator. Journal of cellular and molecular medicine. 2021;25:11322-32
80. Guo XQ, Hua YM. Circular RNAs: novel regulators of resistance to systemic treatments in breast cancer. Neoplasma. 2022;69:1019-28
81. Ghazimoradi MH, Babashah S. The role of CircRNA/miRNA/mRNA axis in breast cancer drug resistance. Front Oncol. 2022;12:966083
82. Xie H, Zheng R. Circ_0085495 knockdown reduces adriamycin resistance in breast cancer through miR-873-5p/integrin β1 axis. Anticancer Drugs. 2022;33:e166-e77
83. Zheng Y, Ren S, Zhang Y. et al. Circular RNA circWWC3 augments breast cancer progression through promoting M2 macrophage polarization and tumor immune escape via regulating the expression and secretion of IL-4. Cancer cell international. 2022;22:264
84. Tian T, Zhao Y, Zheng J. et al. Circular RNA: A potential diagnostic, prognostic, and therapeutic biomarker for human triple-negative breast cancer. Molecular therapy Nucleic acids. 2021;26:63-80
85. Tang Q, Hann SS. Biological Roles and Mechanisms of Circular RNA in Human Cancers. OncoTargets and therapy. 2020;13:2067-92
86. Kristensen LS, Jakobsen T, Hager H. et al. The emerging roles of circRNAs in cancer and oncology. Nature reviews Clinical oncology. 2022;19:188-206
87. Pilotto Heming C, Aran V. The potential of circulating cell-free RNA in CNS tumor diagnosis and monitoring: A liquid biopsy approach. Critical reviews in oncology/hematology. 2024;204:104504
88. Jiang W, Yu Y, Ou J. et al. Exosomal circRNA RHOT1 promotes breast cancer progression by targeting miR-204-5p/ PRMT5 axis. Cancer cell international. 2023;23:260
89. Deng Y, Xia J, Xu YE. Circular RNA circTP63 enhances estrogen receptor-positive breast cancer progression and malignant behaviors through the miR-873-3p/FOXM1 axis. Anti-cancer drugs. 2021;32:44-52
90. Tan C, Xie G, Wu S. et al. Simultaneous detection of breast cancer biomarkers circROBO1 and BRCA1 based on a CRISPR-Cas13a/Cas12a system. Biosensors & bioelectronics. 2024;258:116373
91. Liu X, Song J, Kang Y. et al. CircPDSS1 promotes the proliferation, invasion, migration, and EMT of breast cancer cell via regulating miR-320c/CKAP5 axis. Cancer cell international. 2022;22:238
92. Tomar D, Yadav AS, Kumar D. et al. Non-coding RNAs as potential therapeutic targets in breast cancer. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194378
93. Yang S, Wang Z, Wang C. et al. Comparative Evaluation of Machine Learning Models for Subtyping Triple-Negative Breast Cancer: A Deep Learning-Based Multi-Omics Data Integration Approach. J Cancer. 2024;15:3943-57
94. Lei K, Liang R, Liang J. et al. CircPDE5A-encoded novel regulator of the PI3K/AKT pathway inhibits esophageal squamous cell carcinoma progression by promoting USP14-mediated de-ubiquitination of PIK3IP1. J Exp Clin Cancer Res. 2024;43:124
95. Ganesan K, Xu C, Xie C. et al. Cryoprotective isoliquiritigenin-zein phosphatidylcholine nanoparticles inhibits breast cancer-bone metastasis by targeting JAK-STAT signaling pathways. Chem Biol Interact. 2024;396:111037
96. Kristensen LS, Hansen TB, Venø MT. et al. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37:555-65
97. Ghafouri-Fard S, Hussen BM, Taheri M. et al. Emerging role of circular RNAs in breast cancer. Pathol Res Pract. 2021;223:153496
98. Xu A, Zhu L, Yao C. et al. The therapeutic potential of circular RNA in triple-negative breast cancer. Cancer drug resistance (Alhambra, Calif). 2024;7:13
99. Gopikrishnan M, R HC, R G. et al. Therapeutic and diagnostic applications of exosomal circRNAs in breast cancer. Funct Integr Genomics. 2023;23:184
100. Wang Z, Deng H, Jin Y. et al. Circular RNAs: biology and clinical significance of breast cancer. RNA Biol. 2023;20:859-74
101. Shaikh M, Doshi G. Unraveling non-coding RNAs in breast cancer: mechanistic insights and therapeutic potential. Medical oncology (Northwood, London, England). 2024;42:37
102. Krisztian H, Zaman K, Athina S. 2278P Endothelial cell heterogeneity defined by single-cell spatial transcriptomic analysis of breast cancers. Annals of Oncology. 2023;09:1306
103. Wang Y, Zhang X, Wang T. et al. A machine learning framework for accurately recognizing circular RNAs for clinical decision-supporting. BMC medical informatics and decision making. 2020;20:137
Author contact
![]() Corresponding authors: Hailin Tang: tanghlorg.cn; Ziyun Guan: lyguanzyedu.cn; Wen Zhou: lyzhouwedu.cn.
Corresponding authors: Hailin Tang: tanghlorg.cn; Ziyun Guan: lyguanzyedu.cn; Wen Zhou: lyzhouwedu.cn.

 Global reach, higher impact
Global reach, higher impact