Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(10):3065-3079. doi:10.7150/jca.111362 This issue Cite
Research Paper
Impact of PIK3CA Mutations on the Clinical Benefits of CDK 4/6 Inhibitors in HR+/HER2- Advanced Breast Cancer: An Updated Pairwise and Network Meta-Analysis
1. Department of Pharmacy, Taipei Veterans General Hospital, No. 201, Sec. 2, Shipai Rd., Beitou, Taipei 112, Taiwan.
2. Department of Clinical Pharmacy, School of Pharmacy, Taipei Medical University, No. 250, Wuxing St., Xinyi, Taipei 110, Taiwan.
3. Department and Institute of Pharmacology, College of Medicine, National Yang-Ming Chiao-Tung University, No. 155, Sec. 2, Linong St., Beitou, Taipei 112, Taiwan.
4. Institute of Biopharmaceutical Sciences, College of Pharmaceutical Sciences, National Yang-Ming Chiao-Tung University, Taipei, Taiwan, No. 155, Sec. 2, Linong St., Beitou, Taipei 112, Taiwan.
5. Department of Pharmacy, College of Pharmaceutical Sciences, National Yang-Ming Chiao-Tung University, No. 155, Sec. 2, Li-Nong St., Beitou, Taipei 112, Taiwan.
6. Center for Evidence-based Medicine, Taipei Veterans General Hospital, No. 201, Sec. 2, Shipai Rd., Beitou, Taipei 112, Taiwan.
7. Department of Medical Education, Taipei Veterans General Hospital, No. 201, Sec. 2, Shipai Rd., Beitou, Taipei 112, Taiwan.
8. School of Medicine, College of Medicine, National Yang-Ming Chiao-Tung University, No. 155, Sec. 2, Linong St., Beitou, Taipei 112, Taiwan.
9. General Clinical Research Center, Taipei Veterans General Hospital, No. 201, Sec. 2, Shipai Rd., Beitou, Taipei 112, Taiwan.
10. Institute of Public Health and Community Medicine Research Center, National Yang-Ming Chiao-Tung University School of Medicine, No. 155, Sec. 2, Linong St., Beitou, Taipei 112, Taiwan.
11. Department of Medical Research, Taipei Veterans General Hospital, No. 201, Sec. 2, Shipai Rd., Beitou, Taipei 112, Taiwan.
12. Comprehensive Breast Health Center, Department of Surgery, Taipei Veterans General Hospital, No. 201, Sec. 2, Shipai Rd., Beitou, Taipei 112, Taiwan.
13. Division of Medical Oncology, Department of Oncology, Taipei Veterans General Hospital, No. 201, Sec. 2, Shipai Rd., Beitou, Taipei 112, Taiwan.
# equal contribution
Received 2025-1-31; Accepted 2025-6-19; Published 2025-7-4
Abstract
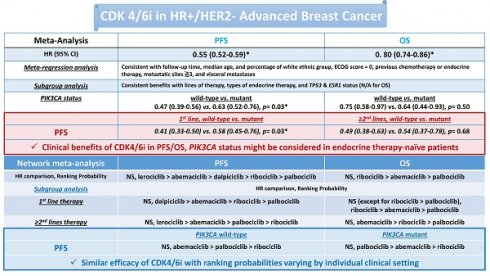
Background: Cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) have revolutionized the treatment of hormone receptor-positive (HR+) and human epidermal growth factor receptor-2 negative (HER2-) advanced breast cancer. However, identifying reliable biomarkers and determining overall survival (OS) outcomes for CDK4/6i remains challenging.
Methods: We conducted a systematic review and updated pairwise meta-analysis of randomized controlled trials to evaluate the clinical benefits and biomarker interactions of CDK4/6i in HR+ and HER2- advanced breast cancer. Hazard ratio (HR) and 95% confidence interval (CI) were calculated for progression-free survival (PFS) and OS across different clinical settings. Additionally, a network meta-analysis was performed to assess the comparative efficacy of different CDK4/6i in specific populations using ranking probabilities.
Results: CDK4/6i significantly improved PFS (HR 0.55, 95% CI 0.52-0.59) and OS (HR 0.80, 95% CI 0.74-0.86) in patients with HR+/HER2- advanced breast cancer. Sensitivity analyses confirmed the robustness of these findings. Subgroup and meta-regression analyses demonstrated consistent clinical benefits across different lines of therapy, endocrine therapy categories, patient characteristics, and follow-up durations. However, PIK3CA mutation status emerged as a potential CDK4/6i efficacy modifier, particularly among patients who were endocrine therapy-naïve for advanced disease (First-line treatment: p for interaction = 0.03; received prior treatment, p = 0.68). The network meta-analysis suggested comparable overall efficacy among CDK4/6i. However, ribociclib may offer a slight OS advantage over palbociclib in first-line treatment, with ranking probabilities varying by specific clinical settings.
Conclusions: This updated meta-analysis further validates the OS benefit of CDK4/6i in HR+/HER2- advanced breast cancer. The influence of PIK3CA mutation status on CDK4/6i efficacy appears more pronounced in endocrine therapy-naïve patients rather than those receiving later-line therapy. While currently approved CDK4/6 inhibitors exhibit similar efficacy overall, their ranking probabilities vary depending on individual clinical contexts. These findings highlight the need for further investigation into the modifying effects of PIK3CA status and specific CDK4/6i to optimize treatment strategies in HR+/HER2- advanced breast cancer.
Keywords: cyclin-dependent kinase 4/6, hormone receptor-positive, human epidermal growth factor receptor-2 negative, advanced breast cancer, PIK3CA
Introduction
Breast cancer is the most common cancer among women [1]. Gene expression profiling has identified distinct molecular subtypes of breast cancer into luminal A, luminal B, human epidermal growth factor receptor-2 (HER2)-enriched, and basal-like. Hormone receptor-positive (HR+)/HER2-negative (HER2-) breast cancers are the most common subtype [2].
Mitogenic responses, such as estrogen signaling, initiate events that activate genes necessary for the cell cycle process. Cyclin D, a protein that increases in response to signaling, plays a vital role in this process and binds to cyclin-dependent kinase (CDK) 4 or 6, forming a complex that phosphorylates Rb and activates the cell cycle. The cyclin D-CDK4/6-p16-Rb pathway is commonly dysregulated in cancer and is a promising target against cancer [3]. In breast cancer patients with HR+ tumors, the activation of the CDK4/6 pathway has been identified as a contributing factor to resistance against endocrine therapy [4]. Specific inhibitors against CDK4/6 were recently introduced in cancer therapy [5]. For patients with HR+ metastatic breast cancer, combining CDK 4/6 inhibitors with endocrine therapy is recommended [6]. However, the lack of direct comparative trials between CDK 4/6 inhibitors has led to ongoing controversy over which inhibitor should be prioritized.
Despite the proven efficacy of CDK4/6 inhibitors, it has been observed that a subset of patients may exhibit intrinsic or acquired resistance [7]. Although considerable effort has been made to assess potential resistance mechanisms, the available evidence is primarily derived from preclinical studies, with limited clinical evidence of acquired genomic alterations linked to resistance [8]. The phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) gene, which encodes the p110α isoform of phosphatidylinositol 3-kinase (PI3K), is frequently mutated (approximately 40%) in the HR+/HER2- subgroup [9]. Dysregulation of the PI3K pathway is associated with tumorigenesis and resistance, highlighting the prognostic relevance of PIK3CA mutations in breast cancer patients [10]. Recently, the aggregated data from multiple studies have demonstrated a correlation between PIK3CA mutation and shortened progression-free survival (PFS) and overall survival (OS) in patients with HR+/HER2-metastatic breast cancer, indicating that PIK3CA mutations possess negative prognostic value for these patients [11].
Pooled circulating tumor DNA analysis from the MONALEESA phase III advanced breast cancer trials showed that certain genomic alterations, such as Erb-B2 receptor tyrosine kinase 2 (HER2), might be associated with favorable PFS when treated with ribociclib compared to placebo [9]. In contrast, the clinical significance of PIK3CA alteration remains limited [9]. The PALOMA-2 trial found that palbociclib combined with fulvestrant provided a more extended period without cancer progression, regardless of PIK3CA status [12]. Similarly, the combination of abemaciclib and fulvestrant effectively treated breast cancer patients, irrespective of their PIK3CA status, as observed in the MONARCH-2 trial [13]. However, an interaction effect of PIK3CA alteration was noted in the MONARCH-3 trial [14].
Several lines of evidence have proposed the clinical benefits of CDK4/6 inhibitors in advanced breast cancer. However, the statistical significance of OS differences between CDK4/6 inhibitors remains controversial and uncertain [15]. Recently, several new CDK4/6 inhibitors, such as lerociclib and dalpiciclib, have been introduced [16, 17], and updated clinical benefits of CDK4/6 inhibitors have been released [9, 18, 19]. In this study, we further investigated the clinical benefits of CDK4/6 inhibitors on HR+ and HER2- advanced breast cancer using the latest reports to evaluate long-term clinical benefits and study the impact of biomarkers on the efficacy of CDK4/6 inhibitors, as well as compare the effectiveness of different CDK4/6 inhibitors in specific populations.
Materials and Methods
Search method and selection criteria
This study employed the PICO framework (Problem/population: HR+/HER2- advanced breast cancer; Intervention: CDK4/6 inhibitor; Comparison: Placebo; Outcome: PFS/OS). The research methodology included a systematic review, pairwise, and network meta-analysis to derive insightful results. We conducted searches in PubMed/PubMed Central, Embase, Cochrane Library, Web of Science, and the Google Scholar online source without language restrictions up to April 15, 2025. The search terms (field for clinical trial) used were modified according to the previous study [20]: """breast cancer"" OR ""breast cancers"" OR ""breast neoplasm"" OR ""breast tumor"" OR ""breast malignancy"" OR ""breast carcinoma"" OR ""breast adenocarcinoma"" AND ""CDK4/6 inhibitor"" OR ""CDK4/6 inhibitors"" OR ""abemaciclib"" OR ""palbociclib"" OR ""ribociclib"" OR ""CDK inhibitor"" OR ""CDK inhibitors"" AND ""survival""" (To ensure a comprehensive and rigorous search aligned with our research focus, we additionally included the terms “abemaciclib,” “ribociclib,” “CDK4/6 inhibitor(s),” and “CDK inhibitor(s),” while excluding other targeted therapies and chemotherapies). We compiled available data and selected the most recent studies if multiple publications reported on the same clinical trial. This study adhered to the Preferred Reporting of Systematic Reviews and Meta-Analyses (PRISMA) extension statement for reporting systematic reviews incorporating network meta-analyses of health care interventions [21] (Checklist for network meta-analysis provided in Supplementary Table 1). Studies were included if they met the following criteria: (1) Focused on patients with HR+/HER2- advanced breast cancer, (2) randomized controlled trial (RCT), (3) compared CDK4/6 inhibitors to placebo or other CDK4/6 inhibitors, (4) de novo CDK4/6 inhibitors intervention, and (5) reported survival outcomes. The following categories were excluded: non-CDK4/6 inhibitors, estrogen receptor (ER)-negative breast cancer, non-RCT, non-breast cancer, comparisons other than placebo, not de novo CDK4/6 inhibitor, early-stage breast cancer, HER2-positive breast cancer, lacking survival outcomes or non-retrievable.
Data collection and quality assessment
We used a standardized Microsoft Excel spreadsheet to document data from included studies. Extracted data included the following information: trial name, publication year, journal name, study design, number of patients, lines of therapy, category of endocrine therapy, biomarker status, follow-up time, median age, percentage of the white ethnic group, ECOG score = 0, previous chemotherapy or endocrine therapy, metastatic sites ≥ 3, and visceral metastases, PFS, and OS (Supplementary data).
The Cochrane tool (Risk of Bias 2) was employed to assess the risk of bias and quality in included RCTs [22, 23]. Each assessment was classified as having a high, medium, or low risk of bias. The systematic review, which included a meta-analysis, examined key aspects, such as attrition bias, detection bias, performance bias, reporting bias, selection bias, and others. The systematic review with network meta-analysis evaluated several domains, including deviations from intended interventions, missing outcome data, outcome measurement, randomization process, selection of the reported result, and overall bias. The eligibility of reference and risk of bias in included studies were independently evaluated by two authors, S.F. Wang and Y.W. Chao. Discrepancies were resolved by consulting a third author, H.M. Cheng. This study was registered with PROSPERO, registration number CRD42024531849.
Outcomes
The primary objective was to assess the clinical benefits of CDK4/6 inhibitors on PFS and OS. Subgroup analysis examined the impact of various factors, such as lines of therapy, category of endocrine therapy, and biomarker interaction, including PIK3CA, tumor protein p53 (TP53), and estrogen receptor 1 (ESR1) status. Meta-regression analysis evaluated the effects of follow-up time, median age, and percentage of white ethnic group, ECOG score = 0, previous chemotherapy or endocrine therapy, metastatic sites ≥3, and visceral metastases on PFS and OS. Sensitivity analysis for primary outcomes was conducted by excluding trials categorized as open-label RCT, such as PALOMA-1 RCT.
Statistical analyses
The study computed hazard ratio (HR), risk ratio (RR), and 95% confidence interval (CI). The random effects model was chosen as the primary approach for comparison due to varying follow-up duration, CDK4/6 inhibitors, and combination with different endocrine therapies in RCTs. A significant threshold of p < 0.05 was set. Meta-analysis was performed using RevMan software 5.4, while meta-regression was conducted using Comprehensive Meta-Analysis V3 software. Post-hoc power tests with dmetar: Companion R Package were used to calculate the power analysis of primary outcomes as in the previous study [24]. Statistical measures for heterogeneity included I², tau2, and Cochran's Q test, with significant heterogeneity defined as p < 0.10.
Confidence in Network Meta-Analysis (CINeMA) [25] was used to perform network meta-analysis. Ranking probability was determined by calculating P-scores based on network point estimates and standard errors using the netmeta package in R version 4.4.1. P-scores indicate the average level of certainty regarding the superior clinical benefit of treatments, rated on a scale from 1 (best) to 0 (worst). The netmeta package in R version 4.4.1 examined the assumptions of network meta-analysis, specifically homogeneity and consistency (inconsistency was not evaluated due to the lack of direct comparison between different CDK4/6 inhibitors). The similarity of baseline patient characteristics across various RCTs was assessed in Supplementary Fig. 1. The network diagram included nodes representing different CDK4/6 inhibitors and lines showing direct comparisons between pairs of interventions. Node magnitude in the network diagram represents the number of studies, the color indicates the level of bias, and the edge width represents the size of interventions. The absence of lines between nodes indicates a lack of studies comparing interventions between those nodes.
Results
Searching process, features of included studies, and design of analysis
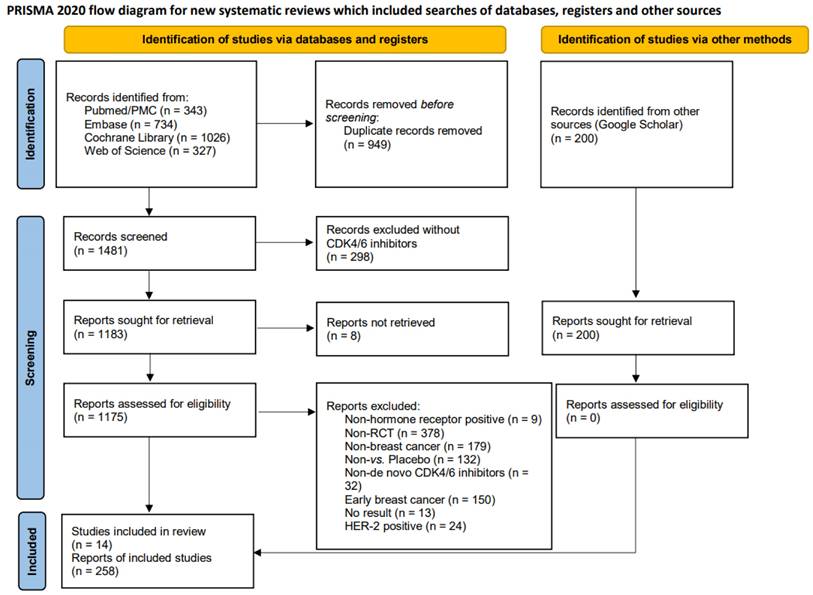
Throughout the search process, we identified a total of 2,430 publications from four databases: PubMed/PubMed Central (n = 343), Embase (n = 734), Cochrane Library (n = 1026), and the Web of Science (n = 327). In addition, 200 records were screened from Google Scholar. Fig. 1 illustrates the distribution of eligible publications across these databases. The preliminary step to exclude duplicate records (n = 949) was carried out using EndNote software. Subsequently, the remaining records underwent a rigorous screening process, which excluded any reports that did not involve CDK4/6 inhibitors (n = 298). Specifically, we excluded those records that were inaccessible (n = 8), unsuitable patient populations [i.e., non-HR+ breast cancer (n= 9), non-RCT (n = 378), non-breast cancer (n = 179), early breast cancer (n = 150), and HER2-positive breast cancer (n = 24)], lacked appropriate interventions [non-de novo CDK4/6 inhibitors (n = 32) and non vs. placebo (n = 132)], or lacked survival outcomes (n = 13). We also evaluated the Google Scholar online source. Finally, we included 258 reports, encompassing fourteen RCTs [16, 17, 19, 26-36], including DAWNA-2 [16], LEONARDA-1 [17], NCCH1607/PATHWAY [19], FLIPPER [26], MONALEESA-2 [27], MONALEESA-3 [28], MONALEESA-7 [29], MONARCH-2 [30], MONARCH-3 [31], MONARCHplus [32], PALOMA-1 [33], PALOMA-2 [34], PALOMA-3 [35], and PALOMA-4 [36] (Baseline information of included trials is provided in Supplementary Table 2).
Most RCTs were designed as Phase III, except for the FLIPPER and PALOMA-1 Phase II clinical trials. Most RCTs were at least double-blinded except for the PALOMA-1 open-label trial. Patients in the DAWNA-2, FLIPPER, MONALESSA-2, MONALEESA-7, MONARCH-3, PALOMA-1, PALOMA-2, and PALOMA-4 RCTs had received no prior treatment for advanced disease (1st line therapy for advanced disease). In contrast, patients in the LEONARDA-1, MONARCH-2, and PALOMA-3 RCTs had progressed after prior endocrine therapy (≥2nd line therapy for advanced disease). The MONALESSA-3, MONARCHplus (Cohort-A for 1st line and Cohort-B for ≥2nd line), and NCCH1607/PATHWAY trials were designed for mixed-line therapy for advanced disease. All CDK4/6 inhibitor interventions were combined with endocrine therapy. In the NCCH1607/PATHWAY trial, tamoxifen was incorporated, whereas the DAWNA-2, MONALEESA-2, MONARCH-3, PALOMA-1, PALOMA-2, and PALOMA-4 trials adapted aromatase inhibitors. Fulvestrant was used in the FLIPPER, LEONARDA-1, MONALEESA-3, MONARCH-2, and PALOMA-3 trials. Some RCTs involved mixed endocrine therapy, such as MONALEESA-7 (tamoxifen or aromatase inhibitors) and MONARCHplus (Cohort A: aromatase inhibitor; Cohort B: fulvestrant). Specific CDK4/6 inhibitors were used in individual RCTs: dalpiciclib for DAWNA-2, palbociclib for FLIPPER, NCCH1607/PATHWAY, PALOMA-1, PALOMA-2, PALOMA-3, and PALOMA-4, lerociclib for LEONARDA-1, ribociclib for MONALEESA-2, MONALEESA-3, and MONALEESA-7, and abemaciclib for MONARCH-2, MONARCH-3, and MONARCHplus.
To better understand the clinical benefits of CDK4/6 inhibitors in patients with advanced breast cancer who are HR+ and HER2-, we conducted a meta-analysis comparing pooled data on the use of CDK4/6 inhibitors versus placebo from the most recent reports. Subsequently, the studies were further sub-grouped according to different lines of therapy, types of endocrine therapy, and biomarker status. Additionally, we utilized network meta-analysis to indirectly compare the clinical benefits of various CDK4/6 inhibitors.
Meta-analysis for the effects of CDK4/6 inhibitors on PFS
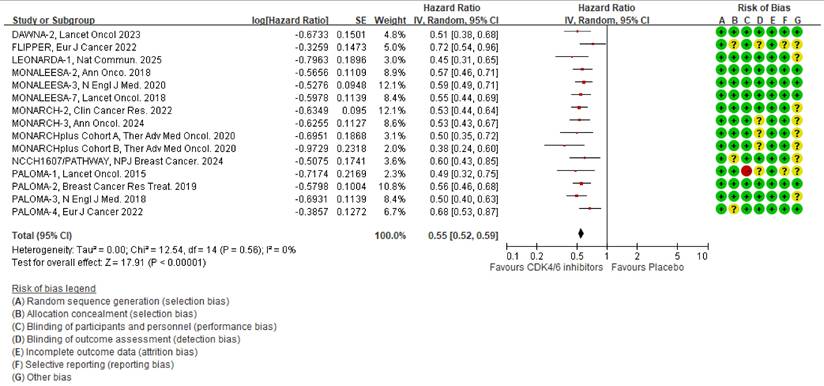
Based on the latest PFS reports [13, 16, 17, 19, 26, 29, 31-33, 36-40], we analyzed 14 RCTs that examined the impact of CDK4/6 inhibitors on PFS. The results showed that the CDK4/6 inhibitors significantly reduced the HR, as shown in Fig. 2 [HR 0.55 (95% CI 0.52-0.59), p < 0.00001]. The heterogeneity test for this outcome was insignificant (I2 = 0%, tau2 = 0, Q =12.54, p = 0.56), and the post-hoc power test showed 100 % effectiveness.
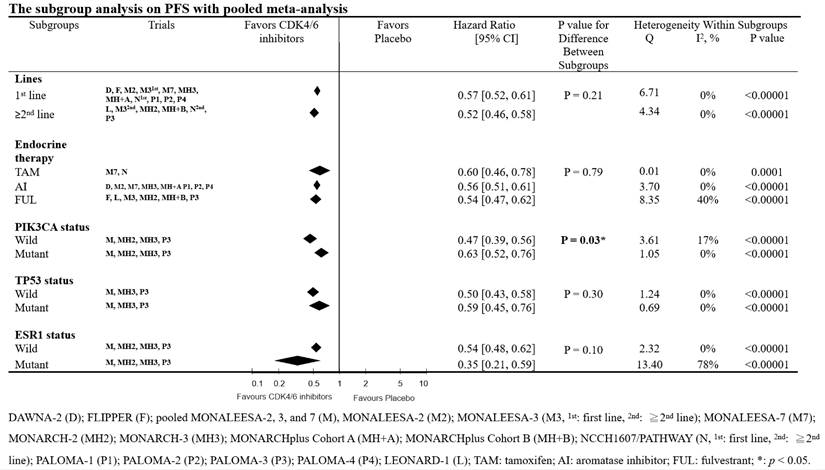
We conducted a subgroup analysis to examine the interactions of biomarkers (such as PIK3CA, TP53, and ESR1), different lines of therapy, and the category of combined endocrine therapy on the effectiveness of CDK4/6 inhibitors. For this purpose, we used the genomic analysis reports of the RCTs [9, 12-14]. The subgroup analyses consistently demonstrated a PFS clinical benefit of CDK4/6 inhibitors (Fig. 3). In the subgroup analysis, we found that the differences between lines of therapy, types of endocrine therapy, and TP53 & ESR1 gene interaction were not significant (lines of therapy, p = 0.21; types of endocrine therapy, p = 0.79; TP53 & ESR1 status interaction, p = 0.30 and 0.10, respectively). However, we observed a significant difference between PIK3CA wild-type and mutant breast cancer patients (p = 0.03).
To comprehensively understand the potential factors influencing the clinical benefit of CDK4/6 inhibitors on PFS, we conducted a meta-regression analysis with demographic and clinical characteristics, including follow-up time, median age, and percentage of white ethnic group, ECOG score = 0, previous chemotherapy or endocrine therapy, metastatic sites ≥ 3, and visceral metastases. Our analysis revealed that none of these factors statistically impacted PFS (Table 1).
The PRISMA 2020 flow diagram for this study. This figure illustrates the study identification, screening, and selection process of clinical trials included in this research.
Pooled meta-analysis of the effects of CDK4/6 inhibitors on PFS. The forest plot shows the hazard ratios of CDK4/6 inhibitors versus placebo on PFS and the risk of bias for the included clinical trials. Colors represent the risk of bias: red indicates high risk, yellow indicates unknown risk, and green indicates low risk.
The effect of PIK3CA status on the PFS clinical benefit of CDK4/6 inhibitors
Our study further investigated the potential impact of PIK3CA status on the PFS clinical benefit of CDK4/6 inhibitors in different lines of therapy. We conducted a subgroup analysis using the individual genomic reports [37, 41]. Consistently, we revealed a notable variation (p = 0.03) between different PIK3CA status patients who had not undergone previous endocrine therapy for advanced disease (first-line treatment) (Fig. 4A). Nevertheless, we noticed that the influence of PIK3CA status on the PFS of CDK4/6 inhibitors was nullified in patients who underwent second-line or subsequent treatments (p = 0.68) (Fig. 4B). Additionally, we further investigate the interaction of PIK3CA status in the patients within the endocrine therapy-control arm and found that PIK3CA status might slightly affect the PFS clinical benefit of the endocrine therapy in the patients receiving first-line treatment; however, the influence was not significant in patients who received prior therapy (Fig. 4C). These results suggest that PIK3CA status may significantly impact the clinical benefit of CDK4/6 inhibitors on PFS, especially in patients without prior treatment.
Meta-analysis for the effects of CDK4/6 inhibitors on OS
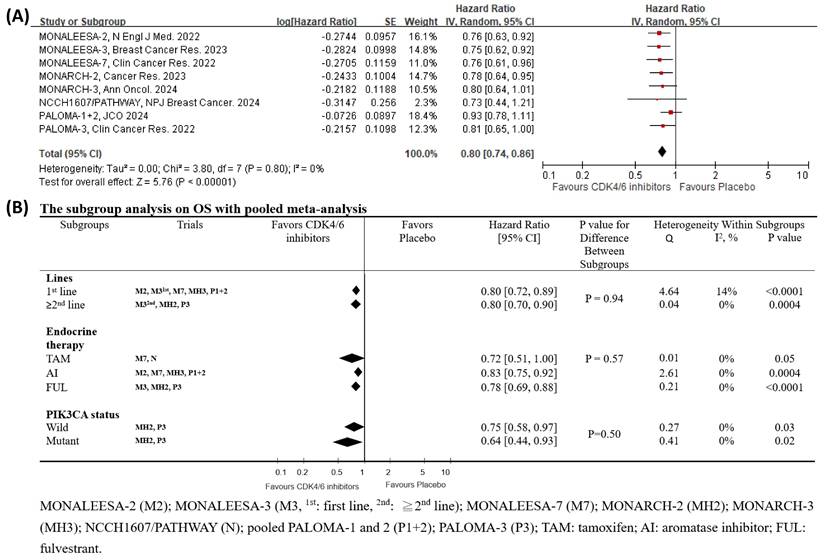
Drawing from the latest OS reports [12, 18, 19, 31, 42-45], we analyzed 9 RCTs that specifically examined the impact of CDK4/6 inhibitors on OS. The outcomes were encouraging, indicating a significant reduction in HR for CDK4/6 inhibitors, as depicted in Fig. 5A [HR 0.80 (95% CI 0.74-0.86), p < 0.00001]. The heterogeneity test for this outcome was insignificant (I2 = 0 %, tau2 = 0, Q =3.80, p = 0.80), and the post-hoc power test confirmed a remarkable 100% effectiveness.
Meta-regression analysis for potential factors in clinical benefit of CDK4/6 inhibitors on PFS and OS.
| PFS | Coefficient | SE | 95% | Z | 2-sided P-value |
|---|---|---|---|---|---|
| Median follow-up (months) | 0.0009 | 0.0016 | -0.0023-0.0040 | 0.54 | 0.5910 |
| Median age (yrs) | 0.0023 | 0.0061 | -0.0097-0.0143 | 0.38 | 0.7063 |
| Race (White, %) | 0.0026 | 0.0041 | -0.0054-0.0106 | 0.63 | 0.5286 |
| ECOG = 0 (%) | -0.0007 | 0.0044 | -0.0094-0.0080 | -0.16 | 0.8751 |
| Previous C/T (%) | -0.0001 | 0.0027 | -0.0055-0.0053 | -0.03 | 0.9767 |
| Previous ET (%) | -0.0003 | 0.0020 | -0.0042-0.0036 | -0.16 | 0.8714 |
| Metastatic sites ≥3 (%) | 0.0054 | 0.0073 | -0.0088-0.0197 | 0.75 | 0.4542 |
| Visceral metastases (%) | -0.0025 | 0.0076 | -0.0174-0.0125 | -0.32 | 0.7471 |
| OS | Coefficient | SE | 95% | Z | 2-sided P-value |
| Median follow-up (months) | 0.0028 | 0.0029 | -0.0029-0.0086 | 0.96 | 0.3355 |
| Median age (yrs) | 0.0032 | 0.0067 | -0.0100-0.0163 | 0.47 | 0.6369 |
| Race (White, %) | 0.0000 | 0.0041 | -0.0080-0.0081 | 0.01 | 0.9908 |
| ECOG = 0 (%) | -0.0101 | 0.0068 | -0.0234-0.0031 | -1.50 | 0.1344 |
| Previous C/T (%) | -0.0029 | 0.0053 | -0.0134-0.0075 | -0.55 | 0.5818 |
| Previous ET (%) | -0.0011 | 0.0031 | -0.0072-0.0051 | -0.34 | 0.7335 |
| Metastatic sites ≥3 (%) | 0.0128 | 0.0097 | -0.0062-0.0318 | 1.32 | 0.1859 |
| Visceral metastases (%) | -0.0149 | 0.0091 | -0.0328-0.0030 | -1.63 | 0.1025 |
C/T: chemotherapy; ET: endocrine therapy
Subgroup analysis of the effects of CDK4/6 inhibitors on PFS with pooled meta-analysis. This forest plot presents the hazard ratios of CDK4/6 inhibitors versus placebo on PFS across different subgroups.
We further conducted a subgroup analysis to examine the interactions of PIK3CA status, different lines of therapy, and the category of combined endocrine therapy on the OS effectiveness of CDK4/6 inhibitors [13, 46]. The interaction findings from the subgroup analysis, presented in Fig. 5B, were insignificant (lines of therapy, p = 0.94; types of endocrine therapy, p = 0.57; PIK3CA status interaction, p = 0.50). The subgroup analyses demonstrated a clinical benefit of CDK4/6 inhibitors except for the combination with tamoxifen (HR 0.72, p = 0.05, Fig. 5B). Additionally, the meta-regression consistently revealed that none of the potential factors had a statistically significant impact on OS (Table 1).
The sensitivity analysis for clinical benefits of CDK4/6 inhibitors in the pooled meta-analysis
In our pooled meta-analysis, we conducted a sensitivity analysis to evaluate the clinical benefits of CDK4/6 inhibitors in terms of OS and PFS. This process excluded RCTs categorized as open-label, such as PALOMA-1 RCT. The clinical benefits of CDK4/6 inhibitors remained significant in terms of PFS (HR 0.56, 95% CI 0.52-0.59, p < 0.00001) and OS (HR 0.80, 95% CI 0.74-0.86, p < 0.00001) as shown in Supplementary Fig. 2. The heterogeneity test for clinical benefits outcome was still insignificant for both PFS (I2 = 0 %, tau2 = 0, Q = 12.20, p = 0.51) and OS (I2 = 0 %, tau2 = 0, Q = 3.94, p = 0.79).
Network meta-analysis for the clinical outcomes of CDK4/6 inhibitors in HR+ and HER2- advanced breast cancer patients
Our study aimed to compare the clinical benefits of CDK4/6 inhibitors and highlight the significance of selecting the best option using network meta-analysis (Supplementary Figs. 3-5 show the diagram of network meta-analysis). There were no significant differences in PFS between CDK4/6 inhibitors (Fig. 6A). Clinical benefits of individual CDK4/6 inhibitors in comparison to placebo on PFS were observed (Fig. 6A). For ranking the probability of PFS, lerociclib was identified as having the highest clinical benefit (lerociclib>abemaciclib>dalpiciclib>ribociclib>palbociclib, Supplementary Table 3). The heterogeneity was insignificant (p = 0.5809).
We further dissect the effects of the lines of therapy and the interaction of PI3K status on PFS using individual network meta-analysis. There were no significant differences in PFS between CDK4/6 inhibitors in first-line and second- or subsequent-line therapy patients (Supplementary Fig. 6). (Ranking probability of PFS in first-line therapy: dalpiciclib > abemaciclib > ribociclib > palbociclib; in second line or beyond therapy: lerociclib > abemaciclib > palbociclib > ribociclib, Supplementary Table 4). The heterogeneity was insignificant (first-line therapy: p = 0.7290; second line or beyond therapy: p = 0.1952).
Consistently, we also found no significant differences in PFS between different CDK4/6 inhibitors in different PIK3CA statuses (Supplementary Fig. 7). Our data indicated that abemaciclib might deliver the highest PFS benefit in PIK3CA wild-type patients (abemaciclib>palbociclib>ribociclib, Supplementary Table 5), while palbociclib yield the most PFS benefit among PIK3CA mutant patients (palbociclib > abemaciclib > ribociclib, Supplementary Table 5). The heterogeneity was insignificant (PIK3CA wild-type: p = 0.1515; PIK3CA mutant: p = 0.3910).
Meta-analysis of the effects of merged lines of therapy and PIK3CA mutation status on PFS with CDK4/6 inhibitors. The forest plots show hazard ratios of CDK4/6 inhibitors versus placebo for patients receiving (A) first-line therapy and (B) second or subsequent lines of therapy. (C) Forest plots display PFS risk ratios for PIK3CA mutant versus wild-type patients within the placebo control arm (endocrine therapy alone).
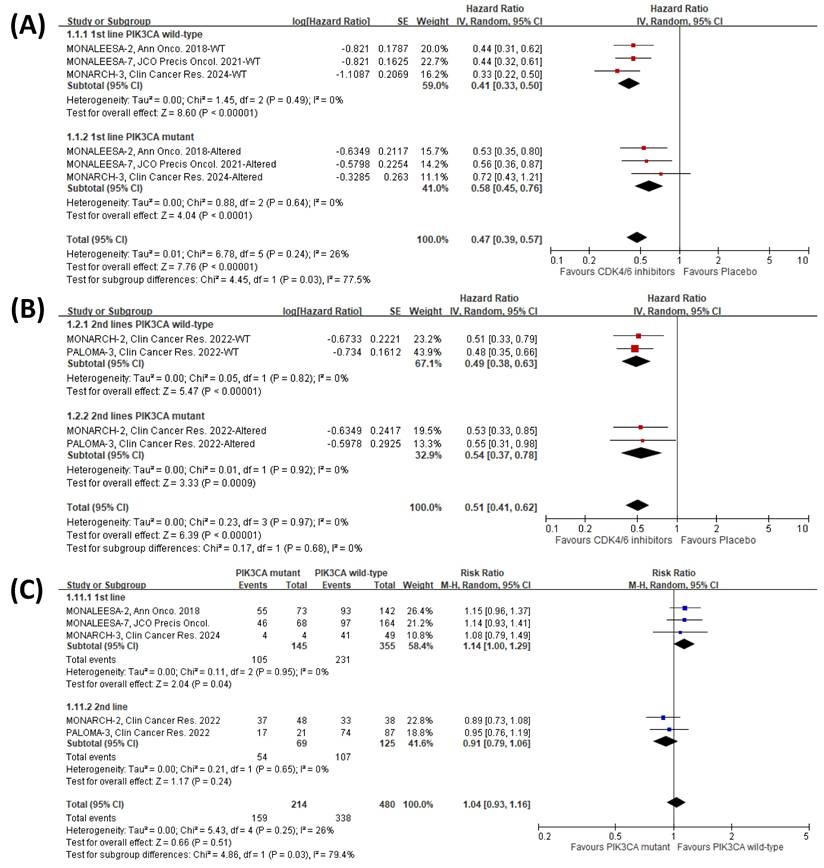
Pooled meta-analysis and subgroup analysis of the effects of CDK4/6 inhibitors on OS. (A) The forest plot shows the hazard ratios of CDK4/6 inhibitors versus placebo on OS for the included clinical trials. (B) Subgroup analysis compares the hazard ratios of CDK4/6 inhibitors versus placebo on OS across different subgroups.
Network meta-analysis of the effects of CDK4/6 inhibitors on PFS and OS. This network meta-analysis compares the effects of individual CDK4/6 inhibitors on (A) PFS and (B) OS, including hazard ratio (HR) and 95% confidence interval (CI). * indicates p < 0.05.
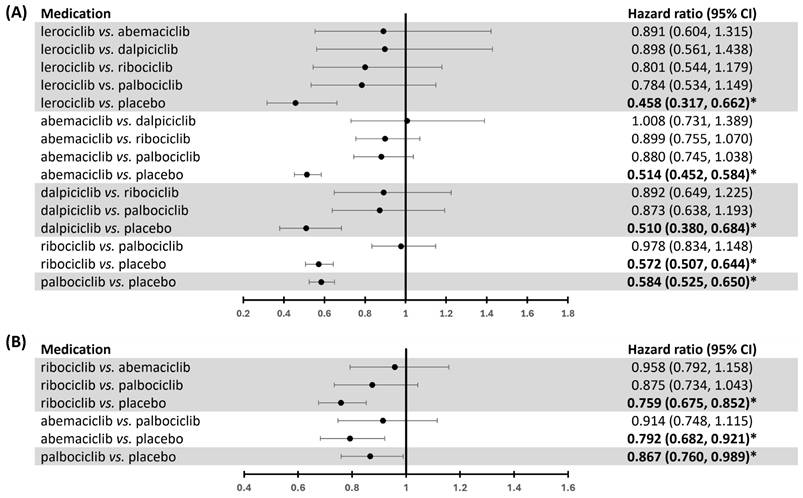
Our investigation demonstrated no significant differences in OS among the different CDK4/6 inhibitors (Fig. 6B). Nevertheless, each CDK4/6 inhibitor displayed a clinical OS benefit (Fig. 6B). We further conducted an individual network meta-analysis to analyze the effects of lines of therapy on OS. We found that ribociclib had a significant OS benefit compared to palbociclib in the first-line setting (HR 0.799, 95% CI 0.642-0.993) (Supplementary Fig. 8). In contrast, the effect was insignificant in patients who have received prior endocrine therapy (Supplementary Fig. 8).
In ranking probability, ribociclib might have the highest OS benefit (ribociclib>abemaciclib>palbociclib, Supplementary Table 6). The heterogeneity was insignificant (p = 0.9088). Regarding OS in the first-line therapy, ribociclib exhibited the highest likelihood of providing a significant clinical benefit (ribociclib>abemaciclib>palbociclib, Supplementary Table 7). In the second or later line in therapy, abemaciclib was identified as the treatment most likely to yield the highest clinical benefit for OS (abemaciclib>ribociclib>palbociclib, Supplementary Table 7). The heterogeneity was insignificant (first-line therapy: p = 0.7564; second line or beyond therapy: NA).
Discussion
The present study confirms that CDK4/6 inhibitors provide clinical benefits, including OS benefit consistent with PFS, to HR+/HER2- advanced breast cancer patients (Figs. 2 and 5A). Our findings indicate that the clinical benefits of CDK4/6 inhibitors are consistent across different lines of therapy, categories of combined endocrine therapy, follow-up duration, age, ethnicity, ECOG performance status, previous chemotherapy or endocrine therapy, and metastatic status (Table 1, Figs. 3 and 5B). Notably, we discovered that the PIK3CA status might influence the efficacy of CDK4/6 inhibitors in terms of PFS for the first line of therapy (Figs. 3-4).
Up to 40% of HR+ metastatic breast cancer patients may harbor PIK3CA mutations, and the PI3K/AKT/mammalian target of rapamycin pathway is implicated as a common escape route for endocrine therapy [9]. Active PI3K signaling (due to PIK3CA mutation) may interact significantly with the ER signaling pathway. Consequently, the negative prognostic impact of PIK3CA mutation is more pronounced in the first-line setting, where tumors are relatively naïve to endocrine therapy and CDK4/6 inhibitors. In contrast, the mechanisms of endocrine resistance are far more complex in the second line or beyond [47], potentially diluting the biological impact of PIK3CA mutation on CDK4/6 inhibitor combinations. Indeed, upon analyzing the interaction of PIK3CA status in patients undergoing only endocrine therapy (control arms of the CDK4/6 inhibitor clinical trials), it was observed that PIK3CA mutations may negatively impact the PFS clinical benefit for those receiving first-line treatment. However, this effect was not significant in patients who had previously undergone therapy (Fig. 4C). Moreover, an exploratory biomarker analysis from the postMONARCH trial—which evaluated abemaciclib with a modified endocrine therapy backbone after disease recurrence on prior CDK4/6 inhibitors and endocrine therapy—demonstrated consistent clinical benefits across genomic subgroups, including PIK3CA or ESR1 status, supporting the diminished impact of PIK3CA status in later-line settings [48]. In the first-line setting, combining inavolisib (a PI3K inhibitor) with palbociclib and fulvestrant recently demonstrated a clinically meaningful improvement in PFS in patients with PIK3CA-mutated, HR+, HER2-, endocrine-resistant, locally advanced or metastatic breast cancer [49]. However, it should be noted that there was a higher incidence of Grade 3/4 adverse events, including thrombocytopenia (14.2% vs. 4.3%), stomatitis or mucosal inflammation (5.6% vs. 0%), hyperglycemia (5.6% vs. 0%), and diarrhea (3.7% vs. 0%) [49]. Our findings have important implications for personalized treatment strategies in naïve-specific populations.
Despite the promising therapeutic efficacy of CDK4/6 inhibitors in breast cancer, intrinsic (de novo) and acquired resistance remain significant challenges to effective disease management [50, 51]. A systematic review of biomarkers predicting drug response, including intrinsic and acquired resistance to CDK4/6 inhibition in metastatic breast cancer, has been conducted [52]. These include genetic alterations among key players in CDK4/6 cell cycle regulation and cross-talk pathways. Accordingly, the retinoblastoma gene 1 (RB1), a key tumor suppressor gene, is one of the most extensively studied factors in drug resistance to CDK4/6 inhibitors [52]. Genetic mutations in the RB1 gene or other molecular mechanisms leading to RB1 function loss account for up to 9 % of patients who develop acquired resistance [52, 53]. Additionally, abnormal cyclin E1/E2 signaling and excessive CDK2 activity have been identified as alternative resistance mechanisms, particularly in patients with prior endocrine resistance [52]. Emerging evidence has also linked novel resistance mechanisms, such as FAT Atypical Cadherin 1 loss, which leads to increased CDK6 activity [52, 54]. However, given the genetic heterogeneity among samples in the analyzed RCTs, differences in testing methodologies, and the fact that not all genomic alterations were assessed, other driver mutations contributing to primary resistance to CDK4/6 inhibitors and/or endocrine therapy may also influence the impact of PIK3CA status in combination therapy, particularly in the first-line setting. In the FLIPPER trial, which evaluated first-line treatment with palbociclib and fulvestrant, ≥1 mutation in PIK3CA + TP53 was associated with early progression (≤ 12 months) regardless of the treatment arm [55]. Additionally, ≥ 2 mutations in PIK3CA + TP53 correlated with poorer PFS and OS outcomes [55]. Notably, patients with ≥ 2 mutations in PIK3CA + TP53 in the control arm exhibited early progression and worse OS, whereas this association was not observed in the palbociclib arm [55]. These findings suggest that, beyond PIK3CA status, other primary resistance mechanisms may collectively influence the therapeutic response to CDK4/6 inhibitor combination therapy in the first-line setting. A more comprehensive, well-designed study is warranted to further elucidate these interactions.
Apart from ER, no other reliable biomarkers are currently utilized for selecting combination therapy involving CDK4/6 inhibitors and endocrine therapy in breast cancer. ER loss, which occurs in only a minority of ER-positive breast cancer patients during treatment, is linked to resistance to endocrine therapy and remains a significant predictor of the effectiveness of CDK4/6 inhibitors combined with endocrine therapy [56]. In some cases, CDK4/6 inhibition has shown the potential to delay the onset of endocrine resistance, suggesting that resistance to combination therapy is primarily driven by resistance to the endocrine therapy backbone. This hypothesis may be supported by the circulating tumor DNA biomarker analysis from the MONARCH-3 trial, which found a lower incidence of ESR1 mutations under abemaciclib intervention [57].
Although the ESR1 mutation may play an important role in the progression of advanced HR+, HER2- breast cancer patients [58], its clinical role in CDK4/6 inhibitor resistance appears minimal [53]. Subgroup analysis from the Phase I/II TRINITI-1 trial suggests that the presence of ESR1 mutations is associated with a poorer prognosis in patients with advanced HR+/HER2- breast cancer treated with a combination of exemestane, ribociclib, and everolimus following progression on prior therapies [59]. However, recent real-world analyses further suggest that ESR1 status may not significantly affect the time-to-next-treatment of CDK4/6 inhibitor regimens and the choice of concomitant endocrine therapy for ESR1 mutations is more important than the efficacy of CDK4/6 inhibitors themselves, indicating that ESR1 variants might not be associated with CDK4/6 inhibitor resistance [60]. According to the PALOMA-3 study, ESR1 mutations are dynamic and reflect resistance to prior aromatase inhibitor therapy; however, they may have limited utility as predictive biomarkers for the efficacy of CDK4/6 inhibitors [61, 62]. Mechanistically, ESR1 mutations may predominantly contribute to resistance against endocrine therapy [63]. Indeed, the recent EMBER-3 trial demonstrated that imlunestrant, a novel oral selective estrogen receptor degrader, showed superior efficacy compared to standard endocrine therapy in endocrine therapy-pretreated/ER+/HER2- advanced breast cancer patients harboring ESR1 mutations [64]. Moreover, when combined with abemaciclib, imlunestrant significantly improved PFS compared to imlunestrant monotherapy, regardless of ESR1 mutation status [64]. A retrospective pharmacogenetic study further demonstrated that ESR1 mutations are independent predictors of resistance to adjuvant endocrine therapy; however, no difference in PFS was observed between patients with or without ESR1 mutations when treated with CDK4/6 inhibitors as first-line therapy [65]. The PADA-1 trial was specifically designed to address this issue by monitoring ESR1 mutation status in patients receiving combination therapy with an aromatase inhibitor and palbociclib [66]. Upon detection of rising ESR1 mutations, switching to fulvestrant plus palbociclib significantly improved PFS compared to continuing aromatase inhibitor plus palbociclib, highlighting the critical role of ESR1 mutations in mediating resistance to endocrine therapy [66].
Other cell cycle regulators, such as mouse double minute 2 homolog-TP53, might contribute to CDK4/6 inhibitor resistance [67], making them promising targets. Although patients with wild-type TP53 exhibited numerically longer PFS than those with TP53 alterations in the MONALEESA-2 study [37], the interaction between TP53 status and PFS was not statistically significant across the MONALEESA Phase III trials [9]. Despite a significant enrichment of TP53 mutations in tumor samples resistant to CDK4/6 inhibitors, in vitro studies have demonstrated comparable sensitivity to CDK4/6 inhibitors regardless of TP53 status [7]. However, a recent genomic cohort study demonstrated that TP53 mutations are associated with a lack of long-term disease control in patients with metastatic HR+/HER2- breast cancer treated with first-line CDK4/6 inhibitors and endocrine therapy [68]. Additionally, the study revealed that TP53 loss promotes CDK2 activation, thereby facilitating cell-cycle re-entry and tumor progression in an in vitro breast cancer model [68]. Moreover, TP53 mutations may contribute to primary resistance to endocrine therapy [69]. Given that TP53 might contribute to the reduced efficacy of both CDK4/6 inhibitors and endocrine therapy, as well as their combination, it might be challenging to discern a distinct impact of TP53 mutations on the HR of CDK4/6 inhibitor combination arms compared to those of endocrine therapy alone. We further conducted an exploratory analysis to evaluate the impact of TP53 and ESR1 mutations on PFS events within the CDK4/6 inhibitor plus endocrine therapy group and the endocrine therapy alone group, respectively. Our findings tentatively suggest that patients with TP53 wild-type status might experience a favorable PFS outcome, regardless of whether they received CDK4/6 inhibitor plus endocrine therapy or placebo plus endocrine therapy (Supplementary Figs. 9A and B). In contrast, ESR1 mutation status was not significantly associated with PFS outcomes (Supplementary Figs. 9C and D). However, these results should be interpreted carefully, as they were derived without multivariate analysis or randomization and may be influenced by potential confounding factors. Furthermore, the ESR1 mutations are acquired over time and tend to emerge with increasing frequency during the course of endocrine therapy, indicating that an initially wild-type ESR1 status may evolve during treatment [70, 71]. Given the uncertainties surrounding current clinical trials investigating the genomic impact on CDK4/6 inhibitor efficacy, further rigorous clinical-genomic studies with meticulous methods are warranted to enhance the translational relevance of these findings.
The updated genomic report of MONALEESA trials recently identified PI3KCA (40.6%) as the most prevalent baseline gene alteration, followed by TP53 (28.5%) [72]. Although RB1 alterations were relatively rare at baseline among patients treated with ribociclib (1.6%), their prevalence increased to 10% at the end-of-treatment (EOT) when comparing genomic alterations in paired baseline and EOT circulating tumor DNA samples from the ribociclib arm [72]. Additionally, RB1 gene alterations might interact with the PFS benefit of ribociclib in the MONALEESA trials [9]. Similar findings were reported in the PALOMA-3 trial, where RB1 mutation prevalence increased to 4.7% at EOT (predominantly in the palbociclib plus fulvestrant arm) [53], and the RB1 loss or loss of heterozygosity was associated with worse prognosis in this trial [73]. Moreover, the MONARCH-3 trial demonstrated a higher frequency of acquired RB1 alterations in the abemaciclib treatment arm compared to the placebo (5% vs. 0%) [14]. However, no significant interaction effect was observed between RB1 mRNA gene expression and treatment outcomes in the PALOMA-2 [56] and PALOMA-3 trials [74].
Acquired cyclin E1 (CCNE1) amplification has been identified in palbociclib- and abemaciclib-resistant preclinical models [75]. Although high CCNE1 mRNA expression [74] and CCNE1 copy number gain [73] were associated with poorer PFS, no patients exhibited acquired CCNE1 amplification at EOT in the PALOMA-3 trial [53]. Additionally, CCNE1 RNA expression did not show a significant interaction with the PFS benefit of CDK4/6 inhibitors in the PALOMA-2 trial [56]. Furthermore, alterations in cell cycle-related genes, including cyclin D1 (CCND1), CCND2, CDK4, CDK5, cyclin dependent kinase inhibitor 2A, CCNE1, RB1, and TP53, did not significantly influence PFS benefit in the MONARCH-3 trial [14]. CCND1 genomic alterations were observed in 8.5-13% of patients in the MONARCH-3 [14] and MONALEESA [9] trials. Our analysis further confirmed that CCND1 alterations had no significant interaction with the PFS benefit of CDK4/6 inhibitors (p for interaction: 0.71, Supplementary Fig. 10), consistent with findings on CCND1 RNA expression in the PALOMA-2 [56] and PALOMA-3 trials [74]. In the MONALEESA trials, BRCA1/2 gene alterations were identified in 4% of patients [9], while in the MONARCH-3 trial, acquired BRCA2 genomic alteration were more frequent in the abemaciclib arm than in the placebo (4% vs. 0%, P = 0.029) [14]. However, BRCA1/2 genomic alterations showed no significant interaction with PFS benefit in the MONALEESA trials [9]. Given that these biomarkers were present in less than 10% of patients, the statistical power of these findings may be limited by sample size. Moreover, we found that PIK3CA alterations might not exhibit significant mutual exclusivity or co-occurrence with TP53, ESR1, RB1, CCNE1, CCND1, and BRCA1/2 alterations in HR+/HER2- metastatic breast cancer patients by analysis of the established genomic dataset [76] (Supplementary Table 8). Further large-scale genomic evaluations are warranted to validate these observations.
When comparing the selectivity and potency of CDK4/6 inhibitors, it has been demonstrated that ribociclib preferentially inhibits CDK4 over CDK6. Conversely, palbociclib exhibits similar potency in targeting CDK4 and CDK6 [77]. Previous evidence indicated that abemaciclib displays the highest potency in inhibiting CDK4 among the current recommended CDK4/6 inhibitors for advanced breast cancer [77]. Additionally, abemaciclib has demonstrated some characteristics as a pan-CDK inhibitor [77], suggesting it may have additional targets beyond CDK4/6. However, similar to the other evidence [15], our study demonstrated no statistically significant difference in clinical benefits among the CDK4/6 inhibitors. Although our analysis suggests that ribociclib may exhibit greater clinical efficacy than palbociclib in terms of OS for first-line treatment (Supplementary Fig. 8) and reveals the ranking probability of CDK4/6 inhibitors in specific clinical outcomes of individual populations (Supplementary Tables 3-7), the limited evidence underscores the need for additional research to determine the most efficacious treatment option for clinical settings more conclusively. Moreover, it is worth noting that the effectiveness of lerociclib and dalpiciclib can only be demonstrated in the local region RCT. Further clinical trials and real-world analyses are imperative to address this knowledge gap.
CDK4/6 inhibitors consistently enhance PFS and OS in HR+/HER2- advanced breast cancer patients, regardless of treatment line, endocrine therapy type, ESR1 and TP53 status, follow-up duration, age, ethnicity, ECOG performance status, previous chemotherapy or endocrine therapy, and metastatic status. PIK3CA status may impact the PFS of CDK4/6 inhibitors in patients who are endocrine therapy-naïve for advanced disease, but not in those previously treated. While clinical benefits among CDK4/6 inhibitors are largely comparable, limited evidence suggests ribociclib may offer superior OS benefit over palbociclib in first-line clinical settings, with ranking probabilities varying by clinical context. These findings highlight the need to consider PI3KCA status when selecting first-line CDK4/6 inhibitors for HR+/HER2- advanced breast cancer. The efficacy of CDK4/6 inhibitors may differ based on the specific CDK4/6 inhibitor and clinical scenario. Further research and prospective validation in dedicated studies are essential to comprehensively assess the impact of PIK3CA status and different CDK4/6 inhibitors on clinical outcomes in HR+/HER2- advanced breast cancer.
Abbreviations
CCND1: cyclin D1; CCNE1: cyclin E1; CI: confidence interval; CDK: cyclin-dependent kinase; CDK4/6i: Cyclin-dependent kinase 4/6 inhibitors; CINeMA: Confidence in Network Meta-Analysis; EOT: end-of-treatment; ESR1: estrogen receptor 1; ER: estrogen receptor; HR: hazard ratio; HR+: hormone receptor-positive; HER2: human epidermal growth factor receptor-2; HER2-: human epidermal growth factor receptor-2 negative; OS: overall survival; PI3K: phosphatidylinositol 3-kinase; PIK3CA: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; PFS: progression-free survival; PRISMA: Preferred reporting of systematic reviews and meta-analyses; RB1: Retinoblastoma gene 1; RCT: randomized control trial; RR: risk ratio; TP53: tumor protein p53.
Supplementary Material
Supplementary figures and tables.
Supplementary data.
Acknowledgements
The authors express their gratitude for the technical support provided by the Center for Evidence-based Medicine at Taipei Veterans General Hospital.
Funding
The present work was supported by the Taiwan National Science and Technology Council (Grant Number: 112-2314-B-075-027-MY3 and 113-2320-B-075 -002), the Taipei Veterans General Hospital (Grant Numbers: V111B-007, V112C-047, V113C-030, V114C-232, and V113C-001), the Melissa Lee Cancer Foundation (Grant Numbers: MLCF_V112_B11201, MLCF_V112_A11206, MLCF_V113_A11301, MLCF_V114_A11408, and MLCF_V114_B11405), the Teh-Tzer Study Group for Human Medical Research Foundation, and the Szu-Yuan Research Foundation of Internal Medicine.
Author contributions
Conceptualization: SFW and CYL; Data curation: SFW, YWC, and TKC; Formal analysis: SFW, YWC, and TKC; Funding acquisition: SFW and CYL; Investigation: SFW, YWC, and TKC; Methodology: SFW, WYY, HMC, and CYL; Project administration: SFW, HMC, and CYL; Software: SFW, HMC, and CYL; Resources: SFW, CYC, WYY, YLC, CEC, CHC, LMT, HCL, HMC, and CYL; Supervision: CYC, YLC, CEC, CHC, LMT, HCL, HMC, CYL; Validation: SFW, YWC, TKC, HMC, and CYL, Visualization: SFW, YWC, TKC, HMC, and CYL; Writing - original draft: SFW, YWC, and TKC; Writing - review & editing: CYC, WYY, YLC, CEC, CHC, LMT, HCL, HMC, and CYL.
Data transparency
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63
2. Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A. et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-41
3. Caldon CE, Daly RJ, Sutherland RL, Musgrove EA. Cell cycle control in breast cancer cells. J Cell Biochem. 2006;97:261-74
4. Miller TW, Balko JM, Fox EM, Ghazoui Z, Dunbier A, Anderson H. et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1:338-51
5. Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130-46
6. Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, Agnese D. et al. NCCN guidelines(R) insights: Breast cancer, version 4.2023. J Natl Compr Canc Netw. 2023;21:594-608
7. Wander SA, Cohen O, Gong X, Johnson GN, Buendia-Buendia JE, Lloyd MR. et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 2020;10:1174-93
8. Alvarez-Fernandez M, Malumbres M. Mechanisms of sensitivity and resistance to CDK4/6 inhibition. Cancer Cell. 2020;37:514-29
9. Andre F, Su F, Solovieff N, Hortobagyi G, Chia S, Neven P. et al. Pooled ctDNA analysis of MONALEESA phase III advanced breast cancer trials. Ann Oncol. 2023;34:1003-14
10. Mukohara T. PI3K mutations in breast cancer: prognostic and therapeutic implications. Breast Cancer (Dove Med Press). 2015;7:111-23
11. Fillbrunn M, Signorovitch J, Andre F, Wang I, Lorenzo I, Ridolfi A. et al. PIK3CA mutation status, progression and survival in advanced HR + /HER2- breast cancer: A meta-analysis of published clinical trials. BMC Cancer. 2022;22:1002
12. Cristofanilli M, Rugo HS, Im SA, Slamon DJ, Harbeck N, Bondarenko I. et al. Overall survival with palbociclib and fulvestrant in women with HR+/HER2- ABC: Updated exploratory analyses of PALOMA-3, a double-blind, phase iii randomized study. Clin Cancer Res. 2022;28:3433-42
13. Tolaney SM, Toi M, Neven P, Sohn J, Grischke EM, Llombart-Cussac A. et al. Clinical significance of PIK3CA and ESR1 mutations in circulating tumor DNA: Analysis from the MONARCH 2 study of abemaciclib plus fulvestrant. Clin Cancer Res. 2022;28:1500-6
14. Goetz MP, Hamilton EP, Campone M, Hurvitz SA, Cortes J, Johnston S. et al. Landscape of baseline and acquired genomic alterations in circulating tumor DNA with abemaciclib alone or with endocrine therapy in advanced breast cancer. Clin Cancer Res. 2024;30:2233-44
15. Kappel C, Elliott MJ, Kumar V, Nadler MB, Desnoyers A, Amir E. Comparative overall survival of CDK4/6 inhibitors in combination with endocrine therapy in advanced breast cancer. Sci Rep. 2024;14:3129
16. Zhang P, Zhang Q, Tong Z, Sun T, Li W, Ouyang Q. et al. Dalpiciclib plus letrozole or anastrozole versus placebo plus letrozole or anastrozole as first-line treatment in patients with hormone receptor-positive, HER2-negative advanced breast cancer (DAWNA-2): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2023;24:646-57
17. Xu B, Zhang Q, Luo Y, Tong Z, Sun T, Shan C. et al. Lerociclib plus fulvestrant in patients with HR+/HER2- locally advanced or metastatic breast cancer who have progressed on prior endocrine therapy: LEONARDA-1 a phase III randomized trial. Nat Commun. 2025;16:716
18. Llombart-Cussac A, Sledge G, Toi M, Neven P, Sohn JH, Inoue K. et al. PD13-11 Final overall survival analysis of Monarch 2: A phase 3 trial of abemaciclib plus fulvestrant in patients with hormone receptor-positive, HER2-negative advanced breast cancer. Cancer Res. 2023;83(5_Supplement):PD13-11
19. Noguchi E, Yamanaka T, Mukai H, Yamamoto N, Chung CF, Lu YS. et al. A phase 3 study (PATHWAY) of palbociclib plus tamoxifen in patients with HR-positive/HER2-negative advanced breast cancer. NPJ Breast Cancer. 2024;10:76
20. Giuliano M, Schettini F, Rognoni C, Milani M, Jerusalem G, Bachelot T. et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20:1360-9
21. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-84
22. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD. et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928
23. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I. et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898
24. Wang SF, Lin YS, Yeh WY, Chang YL, Chiang CE, Chen CH. et al. The clinical benefits of antiresorptive agents in patients with primary breast cancer receiving adjuvant endocrine therapy: A systematic review with pairwise and network meta-analysis. J Clin Endocrinol Metab. 2023;108:e1433-e47
25. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M. et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082
26. Albanell J, Martínez MT, Ramos M, O'Connor M, de la Cruz-Merino L, Santaballa A. et al. Randomized phase II study of fulvestrant plus palbociclib or placebo in endocrine-sensitive, hormone receptor-positive/HER2-advanced breast cancer: GEICAM/2014-12 (FLIPPER). Eur J Cancer. 2022;161:26-37
27. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-48
28. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA. et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465-72
29. Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N. et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018;19:904-15
30. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X. et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875-84
31. Goetz MP, Toi M, Huober J, Sohn J, Tredan O, Park IH. et al. Abemaciclib plus a nonsteroidal aromatase inhibitor as initial therapy for HR+, HER2- advanced breast cancer: Final overall survival results of MONARCH 3. Ann Oncol. 2024;35:718-27
32. Zhang QY, Sun T, Yin YM, Li HP, Yan M, Tong ZS. et al. MONARCH plus: Abemaciclib plus endocrine therapy in women with HR+/HER2- advanced breast cancer: The multinational randomized phase III study. Ther Adv Med Oncol. 2020;12:1758835920963925
33. Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25-35
34. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K. et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925-36
35. Turner NC, Ro J, André F, Loi S, Verma S, Iwata H. et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209-19
36. Xu B, Hu X, Li W, Sun T, Shen K, Wang S. et al. Palbociclib plus letrozole versus placebo plus letrozole in Asian postmenopausal women with oestrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Primary results from PALOMA-4. Eur J Cancer. 2022;175:236-45
37. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S. et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29:1541-7
38. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA. et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382:514-24
39. Rugo HS, Finn RS, Diéras V, Ettl J, Lipatov O, Joy AA. et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174:719-29
40. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N. et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926-36
41. Bardia A, Su F, Solovieff N, Im SA, Sohn J, Lee KS. et al. Genomic profiling of premenopausal HR+ and HER2- metastatic breast cancer by circulating tumor DNA and association of genetic alterations with therapeutic response to endocrine therapy and ribociclib. JCO Precis Oncol. 2021;5:PO.20.00445
42. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L. et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386:942-50
43. Neven P, Fasching PA, Chia S, Jerusalem G, De Laurentiis M, Im SA. et al. Updated overall survival from the MONALEESA-3 trial in postmenopausal women with HR+/HER2- advanced breast cancer receiving first-line ribociclib plus fulvestrant. Breast Cancer Res. 2023;25:103
44. Lu YS, Im SA, Colleoni M, Franke F, Bardia A, Cardoso F. et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: A phase III randomized clinical trial. Clin Cancer Res. 2022;28:851-9
45. Slamon DJ, Diéras V, Rugo HS, Harbeck N, Im SA, Gelmon KA. et al. Overall survival with palbociclib plus letrozole in advanced breast cancer. J Clin Oncol. 2024;42:994-1000
46. Palbociclib plus fulvestrant maintains long-term overall survival benefit in HR+/HER2- advanced breast cancer. Oncologist. 2021; 26 Suppl 3: S5-S6.
47. Liu CY, Wu CY, Petrossian K, Huang TT, Tseng LM, Chen S. Treatment for the endocrine resistant breast cancer: Current options and future perspectives. J Steroid Biochem Mol Biol. 2017;172:166-75
48. Kalinsky K, Bianchini G, Hamilton E, Graff SL, Park KH, Jeselsohn R. et al. Abemaciclib plus fulvestrant in advanced breast cancer after progression on CDK4/6 inhibition: Results from the phase III postMONARCH trial. J Clin Oncol. 2025;43:1101-12
49. Turner NC, Im SA, Saura C, Juric D, Loibl S, Kalinsky K. et al. Inavolisib-based therapy in PIK3CA-mutated advanced breast cancer. N Engl J Med. 2024;391:1584-96
50. Portman N, Alexandrou S, Carson E, Wang S, Lim E, Caldon CE. Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer. Endocr Relat Cancer. 2019;26:R15-R30
51. Pandey K, An HJ, Kim SK, Lee SA, Kim S, Lim SM. et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int J Cancer. 2019;145:1179-88
52. Asghar US, Kanani R, Roylance R, Mittnacht S. Systematic review of molecular biomarkers predictive of resistance to CDK4/6 inhibition in metastatic breast cancer. JCO Precis Oncol. 2022;6:e2100002
53. O'Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K. et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 Trial. Cancer Discov. 2018;8:1390-403
54. Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C. et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell. 2018;34:893-905 e8
55. Rojo Todo FG, Meier L, Martínez MT, Esteban López M, Paris L, O'Connor M. et al. Plasma mutational burden in PIK3CA and TP53 independently predicts early progression in patients with HR+/HER2- metastatic breast cancer (MBC) enrolled in the GEICAM/2014-12 FLIPPER trial. J Clin Oncol. 2024;42:1029-1029
56. Finn RS, Liu Y, Zhu Z, Martin M, Rugo HS, Dieras V. et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naive metastatic breast cancer. Clin Cancer Res. 2020;26:110-21
57. Zhou FH, Downton T, Freelander A, Hurwitz J, Caldon CE, Lim E. CDK4/6 inhibitor resistance in estrogen receptor positive breast cancer, a 2023 perspective. Front Cell Dev Biol. 2023;11:1148792
58. Yang C, Li Z, Bhatt T, Dickler M, Giri D, Scaltriti M. et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36:2255-64
59. Bardia A, Hurvitz SA, DeMichele A, Clark AS, Zelnak A, Yardley DA. et al. Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR(+)/HER2(-) advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin Cancer Res. 2021;27:4177-85
60. Lloyd MR, Brett JO, Carmeli A, Weipert CM, Zhang N, Yu J. et al. CDK4/6 inhibitor efficacy in ESR1-mutant metastatic breast cancer. NEJM Evid. 2024;3:EVIDoa2300231
61. Fribbens C, O'Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M. et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016;34:2961-8
62. O'Leary B, Hrebien S, Morden JP, Beaney M, Fribbens C, Huang X. et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018;9:896
63. Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12:573-83
64. Jhaveri KL, Neven P, Casalnuovo ML, Kim SB, Tokunaga E, Aftimos P. et al. Imlunestrant with or without Abemaciclib in Advanced Breast Cancer. N Engl J Med. 2025;392:1189-202
65. Crucitta S, Ruglioni M, Lorenzini G, Bargagna I, Luculli GI, Albanese I. et al. CDK4/6 inhibitors overcome endocrine ESR1 mutation-related resistance in metastatic breast cancer patients. Cancers (Basel). 2023;15:1306
66. Bidard FC, Hardy-Bessard AC, Dalenc F, Bachelot T, Pierga JY, de la Motte Rouge T. et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23:1367-77
67. Portman N, Milioli HH, Alexandrou S, Coulson R, Yong A, Fernandez KJ. et al. MDM2 inhibition in combination with endocrine therapy and CDK4/6 inhibition for the treatment of ER-positive breast cancer. Breast Cancer Res. 2020;22:87
68. Kudo R, Safonov A, Jones C, Moiso E, Dry JR, Shao H. et al. Long-term breast cancer response to CDK4/6 inhibition defined by TP53-mediated geroconversion. Cancer Cell. 2024;42:1919-35 e9
69. Grote I, Bartels S, Kandt L, Bollmann L, Christgen H, Gronewold M. et al. TP53 mutations are associated with primary endocrine resistance in luminal early breast cancer. Cancer Med. 2021;10:8581-94
70. Burstein HJ, DeMichele A, Somerfield MR, Henry NL, Biomarker T. et al. Testing for ESR1 mutations to guide therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2023;41:3423-5
71. Brett JO, Spring LM, Bardia A, Wander SA. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23:85
72. Andre F, Solovieff N, Su F, Bardia A, Neven P, Yap YS. et al. Acquired gene alterations in patients treated with ribociclib plus endocrine therapy or endocrine therapy alone using baseline and end-of-treatment circulating tumor DNA samples in the MONALEESA-2, -3, and -7 trials. Ann Oncol. 2025;36:54-64
73. O'Leary B, Cutts RJ, Huang X, Hrebien S, Liu Y, Andre F. et al. Circulating tumor DNA markers for early progression on fulvestrant with or without palbociclib in ER+ advanced breast cancer. J Natl Cancer Inst. 2021;113:309-17
74. Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S. et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2019;37:1169-78
75. Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I. et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301-13
76. Li Q, Jiang B, Guo J, Shao H, Del Priore IS, Chang Q. et al. INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discov. 2022;12:356-71
77. Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 inhibitors: The mechanism of action may not be as simple as once thought. Cancer Cell. 2018;34:9-20
Author contact
![]() Corresponding authors: Chun-Yu Liu, MD, PhD, Division of Medical Oncology, Department of Oncology, Taipei Veterans General Hospital, Taipei, Taiwan, Address: No. 201, Sec. 2, Shih-Pai Rd., Beitou, Taipei 112, Taiwan, E-mail: cyliu3gov.tw, Tel.: +886-2-2875-7522, Fax: +886-2-2875-7695; Hao-Min Cheng, MD, PhD, ORCID: 0000-0002-3885-6600, Department of Medical Education, Taipei Veterans General Hospital, Taipei, Taiwan, Address: No. 201, Sec. 2, Shih-Pai Rd., Beitou, Taipei 112, Taiwan, E-mail: hmchenggov.tw, Tel.: +886-2-2875-3873, Fax: +886-2-2877-1746.
Corresponding authors: Chun-Yu Liu, MD, PhD, Division of Medical Oncology, Department of Oncology, Taipei Veterans General Hospital, Taipei, Taiwan, Address: No. 201, Sec. 2, Shih-Pai Rd., Beitou, Taipei 112, Taiwan, E-mail: cyliu3gov.tw, Tel.: +886-2-2875-7522, Fax: +886-2-2875-7695; Hao-Min Cheng, MD, PhD, ORCID: 0000-0002-3885-6600, Department of Medical Education, Taipei Veterans General Hospital, Taipei, Taiwan, Address: No. 201, Sec. 2, Shih-Pai Rd., Beitou, Taipei 112, Taiwan, E-mail: hmchenggov.tw, Tel.: +886-2-2875-3873, Fax: +886-2-2877-1746.

 Global reach, higher impact
Global reach, higher impact