Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(8):2680-2689. doi:10.7150/jca.112394 This issue Cite
Research Paper
Prognostic Value of Des-Gamma-Carboxy Prothrombin in AFP-Negative Hepatocellular Carcinoma Patients Following Liver Resection: A Multicenter Study
1. School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, China.
2. Department of Laboratory Medicine, The Fifth Affiliated Hospital of Wenzhou Medical University, Zhejiang Province, China.
3. Department of Gastroenterology, The Fifth Affiliated Hospital of Wenzhou Medical University, Zhejiang Province, China.
4. Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Navy Medical University), Shanghai, China.
5. General Surgery, Cancer Center, Department of Hepatobiliary & Pancreatic Surgery and Minimally Invasive Surgery, Zhejiang Provincial People's Hospital, Affiliated People's Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, China.
6. Department of Hepatobiliary Surgery, The Fifth Affiliated Hospital of Wenzhou Medical University, Zhejiang Province, China.
Received 2025-2-18; Accepted 2025-5-16; Published 2025-6-12
Abstract
Background: Hepatocellular carcinoma (HCC) is a major global health concern with high postoperative recurrence rates. Des-gamma-carboxy prothrombin (DCP) is a promising biomarker for HCC prognosis, but its optimal cutoff value remains unclear, especially in AFP-negative patients. This study aimed to determine the ideal cutoff value of DCP in AFP-negative HCC patients following liver resection and to investigate its impact on long-term outcomes.
Methods: This multicenter retrospective study included 661 patients who underwent curative HCC resection between 2015 and 2020 at three Chinese hospitals. Patients with AFP levels < 20 ng/mL were included. The primary endpoints were overall survival (OS) and time to recurrence (TTR). DCP levels were categorized as low (≤ 600 mAU/ml) and high (> 600 mAU/ml).
Results: Among the 661 patients (median age 56 years; 88.4% men), 477 had low DCP levels and 184 had high DCP levels. Patients with high DCP levels had more aggressive tumor characteristics, including larger tumor size, microvascular invasion, and macrovascular invasion. The 5-year OS rates were 76.3% in the low DCP group vs. 57.6% in the high DCP group (P < 0.001), and the 5-year recurrence rates were 44.9% vs. 61.0% (P < 0.001), respectively. Multivariable analysis showed that high DCP levels were an independent risk factor for decreased OS (HR 1.548, 95%CI 1.135-2.111; P = 0.006) and increased TTR (HR 1.390, 95%CI 1.081-1.787; P = 0.010).
Conclusion: A DCP cutoff value of 600 mAU/ml effectively stratifies AFP-negative HCC patients into high- and low-risk groups for survival and recurrence after liver resection. This cutoff value can guide clinical decision-making and improve prognostic accuracy.
Keywords: hepatectomy, des-gamma-carboxy prothrombin, recurrence, survival, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC), a type of liver cancer, ranks as the sixth most prevalent cancer and the third major contributor to cancer-related deaths globally[1]. In general, 40% of HCC occur in China, with approximately 85% of these cases being associated with hepatitis B virus (HBV)[2,3]. Surgical resection treatment is recognized as the preferred method for HCC patients who conform to the hepatectomy indications[4,5]. Unfortunately, long-term survival after liver resection for HCC remains less impressive, compromised by high rates of postoperative recurrence, which can range from 50% to 70% within 5 years after surgery. Therefore, a further recognition of tumor characteristics and recurrence-related risk factors for HCC patients are indispensable.
Traditional HCC staging, such as Barcelona clinic liver cancer (BCLC) stage and American Joint Committee on Cancer (AJCC) TNM stage, and serum alpha-fetoprotein (AFP) are currently being widely used to guide clinical practice[6,7]. AFP, a frequently employed biomarker for HCC, has a crucial role in the monitoring, diagnosis, and prognostic assessment of HCC patients. It is important to note that AFP, when used at a threshold level of 20 ng/mL, has limited efficacy in detecting HCC. Several studies have demonstrated that the diagnostic sensitivity and specificity of AFP to detect HCC are approximately 60% and 80%, respectively[8]. This means that a proportion of HCC patients have negative AFP levels at initial diagnosis, especially in patients with small HCC[9]. Furthermore, the global incidence of AFP-negative HCC is on the rise, especially among those of non-viral etiology[10].
Des-gamma-carboxy prothrombin (DCP), also known as prothrombin induced by vitamin K absence or antagonist-II (PIVKA-II), has been found to be another useful tumor marker for HCC[11]. Previous studies have shown that DCP is a more effective biomarker for the diagnosis of HCC compared to AFP. Although, DCP, using various cutoff values according to different studies, is also an independent risk factor for HCC patients who underwent curative resection, there is no widely accepted optimal cutoff value for DCP so far[12-15]. Many studies did not consider the effect of AFP when determining the cutoff value of DCP. DCP and AFP reportedly demonstrate that consensus appears to have been reached on them being independent HCC markers[16,17]. However, when selecting the optimal cutoff value of DCP, AFP and DCP will inevitably produce the so-called superimposed effect on the prognosis. For instance, when HCC patients had a very high AFP level but normal DCP levels, their long-term prognosis were poor, which may be influenced by the high AFP levels. As such, using a multicenter database from China, the aim of this study was to identify ideal cutoff value of DCP in AFP-negative HCC patients following liver resection and investigate clinical features, long-term oncologic prognosis of patients with low or high DCP levels.
Methods
Study design and patient cohort
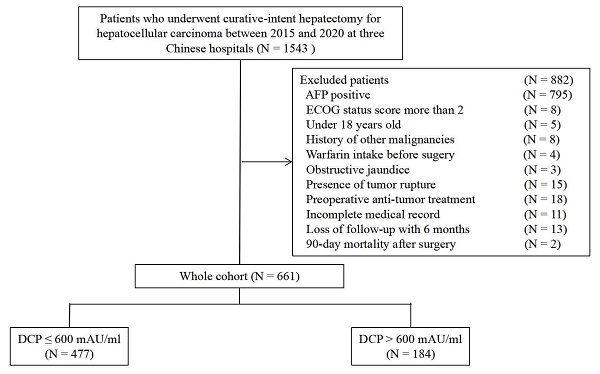
Patients who underwent curative HCC resection between 2015 and 2020 were identified from databases at three Chinese hospitals: the Fifth Affiliated Hospital of Wenzhou Medical University, Eastern Hepatobiliary Surgery Hospital, Zhejiang Provincial People's Hospital. Postoperative pathological examination confirmed the presence of HCC. Curative liver resection was defined as the complete removal of HCC with a microscopically negative margin, also known as R0 resection.
The inclusion criteria were: 1) R0 resection; 2) no history of previous anti-cancer therapy; 3) no history of other malignancies; 4) no warfarin intake before surgery; and 5) no obstructive jaundice. Exclusion criteria were as follows: 1) AFP positive; 2) Eastern Cooperative Oncology Group (ECOG) status score more than 2; 3) under 18 years old; 4) 90-day postoperative mortality; 5) presence of tumor rupture; and 6) incomplete preoperative or postoperative follow-up medical record. The study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies and was approved by the Institutional Review Boards at the participating hospitals.
Data collection and variable definitions
Risk factors of survival and recurrence were assessed in relation to the patient conditions, tumor characteristics, and treatment methods. The demographic and clinical laboratory test results included the age, gender, American Society of Anesthesiologists (ASA) score, body mass index (BMI), hepatitis B virus (HBV), hepatitis C virus (HCV), cirrhosis, portal hypertension, Child-Pugh grade, preoperative serum alanine aminotransferase (ALT), aspartate aminotransferase (AST). Portal hypertension was defined as the condition characterized by the presence of esophageal varices or splenomegaly accompanied by a platelet count ≤ 100×109/L. The tumor-related variables encompassed the maximum tumor diameter, tumor number (solitary or multiple), presence of microvascular invasion (MVI), macrovascular invasion, and satellite lesions, tumor differentiation grade, and tumor encapsulation status. Operative variables included operation duration, intraoperative blood loss, the necessity for intraoperative blood transfusion, extent of hepatectomy (minor or major), type of liver resection (anatomical or non-anatomical), resection margin status. Cirrhosis and tumor-related factors were identified through imaging modalities, including ultrasonography, contrast-enhanced computed tomography (CT), and magnetic resonance imaging (MRI) of the abdomen. By definition, major hepatectomy refers to the surgical removal of three or more Couinaud segments via partial hepatectomy, whereas minor hepatectomy involves removing fewer than three segments. Specifically, anatomical liver resection was categorized in accordance with the Brisbane 2000 anatomical classification system, whereas non-anatomical resection comprised wedge resections and limited resections[18]. The serum concentrations of AFP and DCP were determined by the commercially available AR CHITECT immunoassay in accordance with the defined protocol (Abbott Diagnostics). Both AFP and DCP were detected in HCC patients during the week before surgery. AFP positivity was determined as a serum level ≥ 20 ng/ml, and DCP positivity was defined as a serum level ≥ 40 mAU/ml according to the clinically adopted cutoff values.
Follow-up and primary outcomes
Patients underwent follow-up at each participating hospital, following a tailored surveillance strategy implemented by the institutions. After discharge, patients were monitored using serum AFP or DCP levels, ultrasonography, CT scans, or abdominal MRIs. Surveillance was conducted at intervals of 2-3 months for the first 6 months, 3-4 months for the next 18 months, and subsequently every 3-6 months. Suspected HCC recurrence prompted the performance of clinically indicated tests, including contrast-enhanced CT, MRI, bone scans, or positron emission tomography. Tumor recurrence was defined as the appearance of intrahepatic or extrahepatic tumor nodules, potentially accompanied by an increase in serum AFP or DCP levels. These intrahepatic nodules exhibited imaging features consistent with HCC on CT or MRI scans. Patients with tumor recurrence received active treatments, such as re-resection, transplantation, transcatheter arterial chemoembolization (TACE), percutaneous ablation, radiotherapy, oral sorafenib/lenvatinib, or conservative management, either alone or in combination. Primary study endpoints included survival and recurrence. Overall survival (OS) was measured from the date of liver resection to either the date of death or the last follow-up, while time to recurrence (TTR) was defined as the time from hepatectomy to diagnosis of tumor recurrence or the last follow-up. Recurrence-free survival (RFS) was defined as the time period from the date of surgery to the first occurrence of HCC recurrence or death from any cause.
Statistical analysis
Continuous variables were reported as either mean ± standard deviation (SD) or median with interquartile range (IQR), depending on whether the data met the normal distribution. Meanwhile, categorical variables were expressed as frequencies and percentages. To account for both survival and recurrence, we set the outcome variable to RFS and the DCP upper limit to 1000 mAU/ml. Based on R package “CatPredi”, the optimal cutoff value for the was determined to be 600 mAU/ml. Baseline characteristics between the two groups (low DCP vs. high DCP) were compared using either a Student's t test or a Mann-Whitney U test for continuous variables, and the Chi-square or Fisher's exact tests for categorical variables, based on the suitability of each test. Time-dependent receiver operating characteristic (ROC) curves were employed to assess various cutoff values for DCP discrimination and clinical utility. The area under the ROC curve (AUC) was calculated for each DCP cutoff value, where higher AUC values suggest better discrimination. Using the Kaplan-Meier method examined by the log rank test, OS and TTR rates were compared between the low DCP and high DCP groups. Multivariable Cox regression analyses were performed to determine the independent risk factors related to worse OS and TTR after liver resection. Variable with a P < 0.05 in the univariable analysis were included in the multivariable Cox regression model. Subgroup analyses were conducted to assessed the prognostic value serum DCP levels in various groups. Interaction tests were performed to identify whether the impact of preoperative serum DCP levels on OS and TTR differed significantly among the subgroups. Statistical analyses were conducted using R 4.3.1 and SPSS software (version 25.0, SPSS, Inc.). A two-sided P < 0.05 was considered statistically significant.
Results
Patient characteristics
Among 661 patients identified, there were 584 men (88.4%) and 77 women (11.6%) with a median age of 56 (range 23-86) years. Using this cutoff value, patients were divided into low DCP levels (≤ 600 mAU/ml, n = 477) and high DCP levels (> 600 mAU/ml, n = 184) cohorts (Figure 1). Baseline characteristics and operative variables differed among patients with and without high DCP level (Table 1). Significant differences were observed between the two groups in terms of age, BMI, presence of HBV infection, preoperative AST level, tumor size, tumor number, presence of MVI, macrovascular invasion, and satellites, operation time, intraoperative blood loss, presence of blood transfusion, extent of liver resection, type of hepatectomy (all P < 0.05). Patients with high DCP levels were more likely to be elderly, have advanced tumor burden, including tumor size, MVI, macrovascular invasion, and satellites.
Baseline characteristics of the patients in the two cohorts.
| N (%) | Total (N = 661) | DCP ≤ 600 mAU/ml (N = 477) | DCP > 600 mAU/ml (N = 184) | P value |
|---|---|---|---|---|
| Clinicalpathologic Characteristics | ||||
| Age > 60 years | 253 (38.3) | 170 (35.6) | 83 (45.1) | 0.025 |
| Male | 584 (88.4) | 425 (89.1) | 159 (86.4) | 0.335 |
| ASA score > 2 | 115 (17.4) | 81 (17.0) | 34 (18.5) | 0.649 |
| Body mass index > 24 Kg/m2 | 350 (53.0) | 269 (56.4) | 81 (44.0) | 0.004 |
| HBV (+) | 566 (85.6) | 419 (87.8) | 147 (79.9) | 0.009 |
| HCV (+) | 16 (2.4) | 10 (2.1) | 6 (3.3) | 0.555 |
| Cirrhosis | 419 (63.4) | 309 (64.8) | 110 (59.8) | 0.232 |
| Portal hypertension | 99 (15.0) | 70 (14.7) | 29 (15.8) | 0.726 |
| Child-Pugh grade B | 25 (3.8) | 21 (4.4) | 4 (2.2) | 0.178 |
| Preoperative ALT level > 40 U/L | 147 (22.2) | 102 (21.4) | 45 (24.5) | 0.395 |
| Preoperative AST level > 40 U/L | 101 (15.3) | 57 (11.9) | 44 (23.9) | <0.001 |
| Largest tumor diameter > 5 cm | 223 (33.7) | 108 (22.6) | 115 (62.5) | <0.001 |
| Multiple Tumors | 74 (11.2) | 61 (12.8) | 13 (7.1) | 0.036 |
| Microvascular invasion | 216 (32.7) | 129 (27.0) | 87 (47.3) | <0.001 |
| Macrovascular invasion | 9 (1.4) | 3 (0.6) | 6 (3.3) | 0.025 |
| Satellites | 38 (5.7) | 22 (4.6) | 16 (8.7) | 0.043 |
| Poor tumor differentiation | 483 (73.1) | 351 (73.6) | 132 (71.7) | 0.632 |
| Incomplete tumor encapsulation | 456 (69.0) | 335 (70.2) | 121 (65.8) | 0.265 |
| Operative Variables | ||||
| Operation time | 125 (100-158) | 120 (95-150) | 145 (120-180) | <0.001 |
| Intraoperative blood loss > 400 ml | 53 (8.0) | 28 (5.9) | 25 (13.6) | 0.001 |
| Intraoperative blood transfusion | 34 (5.1) | 16 (3.4) | 18 (9.8) | 0.001 |
| Major liver resection | 82 (12.4) | 39 (8.2) | 43 (23.4) | <0.001 |
| Non-anatomical liver resection | 577 (87.3) | 429 (89.9) | 148 (80.4) | 0.001 |
| Resection margin < 1cm | 416 (62.9) | 291 (61.0) | 125 (67.9) | 0.098 |
| Perioperative Outcomes | ||||
| Postoperative 30-day morbidity | 195 (29.5) | 130 (27.3) | 65 (35.3) | 0.041 |
| Minor morbidity | 165 (25.0) | 110 (23.1) | 55 (29.9) | 0.069 |
| Major morbidity | 30 (4.5) | 20 (4.2) | 10 (5.4) | 0.414 |
| Postoperative hospital stays, days | 8.3±3.7 | 8.4±4.1 | 8.2±2.3 | 0.687 |
Abbreviations: DCP, des-gamma-carboxy prothrombin; ASA, American Society of Anesthesiologists; HBV, hepatitis B virus; HCV, hepatitis C virus; AST, aspartate transaminase; ALT, alanine aminotransferase.
Long-term outcomes
The long-term prognostic outcomes of the 661 patients treated with curative hepatectomy for HCC were stratified according to the DCP levels (Table 2). Time-dependent ROC curves analysis confirmed the superior discrimination of 600 mAU/ml as cutoff value for DCP to predict survival and recurrence (Figure 2). The mortality rates were 25.6% among patients with low DCP levels and 45.7% in those with high DCP levels (P < 0.05). The recurrence rates 45.7% and 61.4% respectively. Compared with low DCP group patients, patients in the high DCP group tended to experience intrahepatic recurrence and the extent of recurrent HCC were more likely to be at an intermediate stage. The 1-, 3-, and 5-year OS and TTR rates among patients with high DCP levels compared to those among patients with low DCP levels are shown in Figure 3A and Figure 3B.
Selection of the study population.
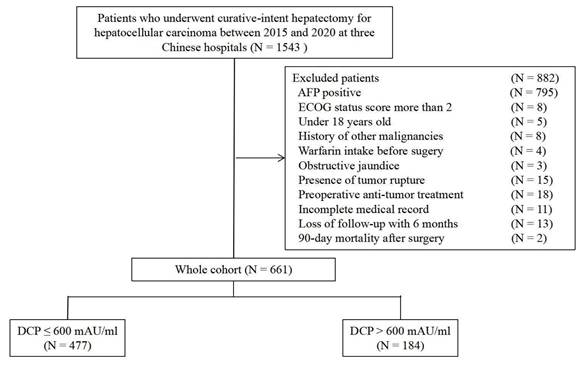
Time-dependent receiver operating characteristic curves comparing the predictive performance of the different cutoff values of DCP for overall survival (A) and time to recurrence (B).
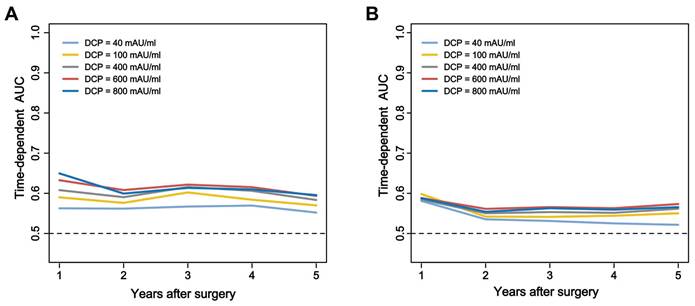
Curves comparisons of overall survival (A) and time to recurrence (B) between two groups (calculated by log-rank test).
Long-term outcomes after excluding postoperative early deaths.
| N (%) | Total (N = 661) | DCP ≤ 600 mAU/ml (N = 477) | DCP > 600 mAU/ml (N = 184) | P value |
|---|---|---|---|---|
| Period of follow-up, months | 56.5±20.3 | 58.9±19.4 | 50.4±21.5 | <0.001 |
| Recurrence during the follow-up | 331 (50.1) | 218 (45.7) | 113 (61.4) | <0.001 |
| Intrahepatic recurrence | 310 (46.9) | 204 (42.8) | 106 (57.6) | 0.001 |
| Extrahepatic recurrence | 4 (0.6) | 4 (0.8) | 0 (0.0) | 0.580 |
| Intra- & extrahepatic recurrence | 17 (2.6) | 10 (2.1) | 7 (3.8) | 0.217 |
| BCLC stage of initial recurrence | 331 (50.1) | 218 (45.7) | 113 (61.4) | <0.001 |
| BCLC A stage | 216 (32.7) | 153 (32.1) | 63 (34.2) | 0.595 |
| BCLC B stage | 84 (12.7) | 47 (9.9) | 37 (20.1) | <0.001 |
| BCLC C stage | 31 (4.7) | 18 (3.8) | 13 (7.1) | 0.073 |
| Mortality during the follow-up | 206 (31.2) | 122 (25.6) | 84 (45.7) | <0.001 |
| Median overall survival (OS), 95% CI | 61.5 (44.9-68.4) | 61.7 (51.7-69.7) | 58.5 (32.5-65.7) | <0.001 |
| 1-year OS rate, % | 97.9% | 98.5% | 95.1% | |
| 3-year OS rate, % | 83.4% | 88.2% | 71.1% | |
| 5-year OS rate, % | 71.1% | 76.3% | 57.6% | |
| Median time-to-recurrence (TTR), 95% CI | 46.3 (17.3-63.9) | 54.0 (20.6-65.1) | 58.5 (10.5-61.4) | <0.001 |
| 1-year TTR rate, % | 18.0% | 16.8% | 28.8% | |
| 3-year TTR rate, % | 40.6% | 36.2% | 52.0% | |
| 5-year TTR rate, % | 49.3% | 44.9% | 61.0% |
Prognostic analyses for overall survival and recurrence
Table 3 and Table 4 describe the independent risk factors associated with long-term OS and TTR after curative hepatectomy for HCC confirmed by univariable and multivariable Cox regression analyses. Multivariable analyses demonstrated that high DCP levels were risk factor for decreased OS (HR 1.548, 95%CI 1.135-2.111; P = 0.006). Other independent prognostic factors for OS included cirrhosis, tumor size > 5 cm, microvascular invasion, macrovascular invasion, satellites, incomplete tumor encapsulation, narrow resection margin (all P < 0.05). Similarly, high DCP levels were risk factor for increased TTR (HR 1.390, 95%CI 1.081-1.787; P = 0.010). Other independent prognostic factors for TTR included male, cirrhosis, tumor size > 5 cm, multiple tumors, MVI, macrovascular invasion, satellites, intraoperative blood loss > 400ml, non-anatomical liver resection (all P < 0.05).
Subgroup analyses
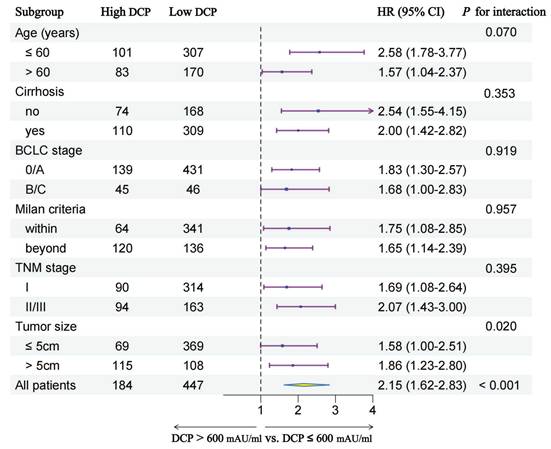
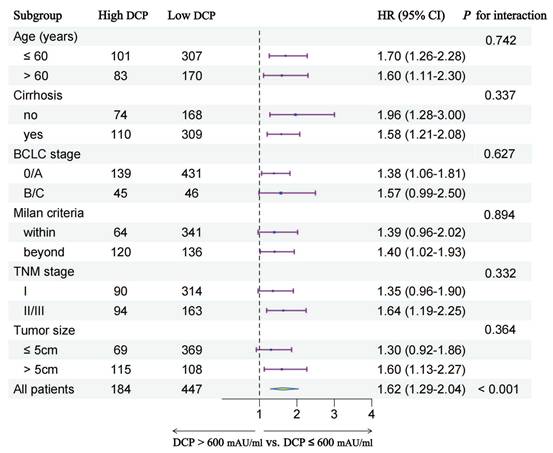
In order to further explore the prognostic value of preoperative serum DCP levels in different patient subgroups, we conducted subgroup analyses stratified by multiple clinical characteristics. Figure 4 and Figure 5 show the outcomes of univariable Cox regression analysis for OS and TTR based on the different DCP levels, with high DCP levels linked to poorer OS and TTR in various subgroups, such as age, cirrhosis, tumor size and different tumor staging.
Univariable and multivariable Cox regression analyses of risk factors for survival.
| Variables | HR comparison | UV HR (95% CI) | UV P | MV HR (95% CI) | MV P |
|---|---|---|---|---|---|
| Age | > 60 vs. ≤ 60 years | 1.397 (1.062-1.839) | 0.017 | NA | 0.062 |
| Sex | Male vs. Female | 0.890 (0.594-1.335) | 0.574 | ||
| ASA score | > 2 vs. ≤ 2 | 1.269 (0.904-1.781) | 0.169 | ||
| Body mass index | > 24 vs. ≤ 24 Kg/m2 | 0.739 (0.562-0.972) | 0.030 | NA | 0.053 |
| HBV (+) | Negative vs. Negative | 0.752 (0.525-1.078) | 0.121 | ||
| Cirrhosis | Presence vs. Absence | 1.334 (0.993-1.792) | 0.056 | 1.362 (1.008-1.842) | 0.045 |
| Portal hypertension | Presence vs. Absence | 0.992 (0.677-1.454) | 0.968 | ||
| Child-Pugh grade | B vs. A | 1.416 (0.771-2.601) | 0.262 | ||
| Preoperative ALT level | > 40 vs. ≤ 40 U/L | 1.171 (0.854-1.607) | 0.327 | ||
| Preoperative AST level | > 40 vs. ≤ 40 U/L | 1.595 (1.140-2.233) | 0.006 | NA | 0.564 |
| Preoperative DCP level | > 600 vs. ≤ 600 mAU/ml | 2.146 (1.624-2.835) | < 0.001 | 1.548 (1.135-2.111) | 0.006 |
| Largest tumor diameter | > 5 vs. ≤ 5 cm | 2.077 (1.580-2.731) | < 0.001 | 1.811 (1.324-2.477) | < 0.001 |
| Tumor number | Multiple vs. Solitary | 1.323 (0.893-1.959) | 0.163 | ||
| Microvascular invasion | Yes vs. no | 2.452 (1.864-3.225) | < 0.001 | 2.096 (1.574-2.792) | < 0.001 |
| Macrovascular invasion | Yes vs. no | 7.314 (3.585-14.920) | < 0.001 | 2.498 (1.189-5.251) | 0.016 |
| Satellites | Yes vs. no | 3.504 (2.305-5.327) | < 0.001 | 2.048 (1.317-3.187) | 0.001 |
| Poor tumor differentiation | Yes vs. no | 1.069 (0.783-1.458) | 0.675 | ||
| Incomplete tumor encapsulation | Yes vs. no | 1.573 (1.145-2.160) | 0.005 | 1.757 (1.269-2.431) | 0.001 |
| Intraoperative blood loss | > 400 vs. ≤ 400 ml | 1.969 (1.295-2.993) | 0.002 | NA | 0.081 |
| Intraoperative transfusion | Yes vs. no | 2.033 (1.237-3.342) | 0.005 | NA | 0.190 |
| Extent of liver resection | Major vs. ≤ Minor | 1.726 (1.199-2.484) | 0.003 | NA | 0.631 |
| Non-anatomical liver resection | Yes vs. no | 0.935 (0.624-1.401) | 0.743 | ||
| Resection margin | < 1 vs. ≥ 1 cm | 1.888 (1.375-2.592) | < 0.001 | 1.665 (1.207-2.297) | 0.002 |
Abbreviations: DCP, des-gamma-carboxy prothrombin; ASA, American Society of Anesthesiologists; HBV, hepatitis B virus; HCV, hepatitis C virus; AST, aspartate transaminase; ALT, alanine aminotransferase; CI, Confidence interval; HR, Hazard ratio; MV, multivariable; UV, univariable.
Univariable and multivariable Cox regression analyses of risk factors for recurrence.
| Variables | HR comparison | UV HR (95% CI) | UV P | MV HR (95% CI) | MV P |
|---|---|---|---|---|---|
| Age | > 60 vs. ≤ 60 years | 0.935 (0.748-1.169) | 0.556 | ||
| Sex | Male vs. Female | 1.419 (0.975-2.066) | 0.067 | 1.645 (1.123-2.409) | 0.011 |
| ASA score | > 2 vs. ≤ 2 | 0.771 (0.569-1.045) | 0.094 | NA | 0.073 |
| Body mass index | > 24 vs. ≤ 24 Kg/m2 | 0.934 (0.752-1.159) | 0.535 | ||
| HBV (+) | Negative vs. Negative | 1.363 (0.968-1.918) | 0.076 | NA | 0.120 |
| Cirrhosis | Presence vs. Absence | 1.811 (1.418-2.313) | < 0.001 | 1.876 (1.462-2.407) | < 0.001 |
| Portal hypertension | Presence vs. Absence | 1.123 (0.841-1.501) | 0.431 | ||
| Child-Pugh grade | B vs. A | 1.289 (0.780-2.132) | 0.322 | ||
| Preoperative ALT level | > 40 vs. ≤ 40 U/L | 1.271 (0.992-1.629) | 0.057 | NA | 0.325 |
| Preoperative AST level | > 40 vs. ≤ 40 U/L | 1.394 (1.054-1.844) | 0.020 | NA | 0.414 |
| Preoperative DCP level | > 600 vs. ≤ 600 mAU/ml | 1.625 (1.294-2.041) | < 0.001 | 1.390 (1.081-1.787) | 0.010 |
| Largest tumor diameter | > 5 vs. ≤ 5 cm | 1.545 (1.239-1.927) | < 0.001 | 1.550 (1.200-2.002) | 0.001 |
| Tumor number | Multiple vs. Solitary | 1.602 (1.177-2.180) | 0.003 | 1.552 (1.134-2.124) | 0.006 |
| Microvascular invasion | Yes vs. no | 1.612 (1.291-2.013) | < 0.001 | 1.439 (1.141-1.815) | 0.002 |
| Macrovascular invasion | Yes vs. no | 4.428 (2.190-8.954) | < 0.001 | 2.823 (1.356-5.876) | 0.006 |
| Satellites | Yes vs. no | 3.165 (2.182-4.590) | < 0.001 | 2.089 (1.406-3.103) | < 0.001 |
| Poor tumor differentiation | Yes vs. no | 1.193 (0.930-1.529) | 0.165 | ||
| Incomplete tumor encapsulation | Yes vs. no | 1.251 (0.985-1.589) | 0.066 | NA | 0.051 |
| Intraoperative blood loss | > 400 vs. ≤ 400 ml | 1.726 (1.215-2.451) | 0.002 | 1.615 (1.125-2.320) | 0.009 |
| Intraoperative transfusion | Yes vs. no | 1.077 (0.652-1.781) | 0.771 | ||
| Extent of liver resection | Major vs. ≤ Minor | 1.225 (0.889-1.688) | 0.215 | ||
| Non-anatomical liver resection | Yes vs. no | 1.406 (0.981-2.014) | 0.064 | 1.679 (1.156-2.347) | 0.006 |
| Resection margin | < 1 vs. ≥ 1 cm | 1.551 (1.227-1.962) | < 0.001 | NA | 0.088 |
Abbreviations: DCP, des-gamma-carboxy prothrombin; ASA, American Society of Anesthesiologists; HBV, hepatitis B virus; HCV, hepatitis C virus; AST, aspartate transaminase; ALT, alanine aminotransferase; CI, Confidence interval; HR, Hazard ratio; MV, multivariable; UV, univariable.
Subgroup analysis of overall survival between two groups.
Subgroup analysis of time-to-recurrence between two groups.
Discussion
DCP, which was first identified in 1984, not only showed superior sensitivity in the diagnosis of HCC, but also its preoperative levels were associated with the prognosis of HCC patients following liver resection[19]. However, studies on the cutoff values of preoperative DCP as an independent factor for survival and recurrence in patients with HCC were various, especially in liver resection[12-14,20-26]. In order to determine the optimal prognostic cutoff value of preoperative DCP level for HCC patients who underwent liver resection, we selected AFP-negative patients for this study, thus eliminating the combined effect of DCP and AFP on long-term oncologic prognosis. To the best of our knowledge, this is the first study having largest number of HCC patients with AFP-negative after surgery so far regarding DCP. In this large study from a prospectively and retrospectively collected multicenter database, the risk factors, recurrent patterns, and long-term outcomes regarding OS and TTR after curative liver resection for HCC patients with high or low DCP levels (> 600 or ≤ 600 mAU/ml) were evaluated. Among the patients in current study, HBV infection remained the primary etiology, accounting for more than 85%. The results of the cohort of patients undergoing HCC resection revealed that patients with high DCP levels tended to have more aggressive tumor pathological characteristics than the low DCP group patients. Compared with low DCP group patients, patients with high DCP levels were more likely to experience postoperative morbidity.
When we explored the relationship between pathological features and serum DCP level in the cohort, MVI was found in 87 (47.3%) of 184 patients with DCP > 600 mAU/ml, but in only 129 (27.0%) of 477 patients with DCP ≤ 600 mAU/ml, which was similar to the results of previous studies[27,28]. Furthermore, HCC patients with high levels of DCP have greater chances of macrovascular invasion and Satellites than those patients with lower levels of DCP[29]. As far as long-term oncologic prognosis is concerned, patients in the high DCP group had worse 5-year survival rate (57.6% vs. 76.3%, P < 0.001) and 5-year recurrence rate (61.0 vs. 44.9%, P < 0.001) than those in the low DCP group. Meanwhile, multivariable Cox regression identified that high DCP levels was an independent risk factor for decreased OS and elevated TTR, a finding also consistently reported in other studies[20,30]. In addition to some confirmed factors, such as cirrhosis, tumor size, tumor number, vascular invasion, tumor encapsulation, and resection margin, are associated with long-term prognosis for HCC. Interestingly, we observed that male were an independent risk factor for postoperative recurrence in AFP-negative HCC patients, which may be due to differences in sex hormones[31,32] and the greater propensity of men to drink alcohol, leading to more severe cirrhosis[33,34]. Furthermore, patients with elevated DCP levels were at an increased risk for intrahepatic recurrence[21,35], often presenting at BCLC B/C stages, potentially owing to the high occurrence of MVI. Several traditional HCC staging systems based on tumor burden and vascular invasion are widely used to guide clinical decision-making, such as the BCLC stage[6], AJCC TNM stage[7], and the Milan criteria[36]. However, these tumor stagings did not take into account indicators reflecting tumor biological characteristics. In this study, we further stratified AFP-negative HCC patients after surgery according to preoperative DCP levels in different subgroup. In general, the prognostic of preoperative DCP, as a HCC-specific tumor marker, was consistent across the different tumor stagings, which proved that different DCP levels are a good supplement for clinical tumor staging.
Apart from indicating poor long-term prognosis of patients, positive preoperative DCP levels can be identified by timely postoperative follow-up. Dynamic observation of DCP changes to evaluate the efficacy of therapeutic interventions and the surveillance of recurrences[37]. For patients with unresectable HCC, the use of DCP as a prognostic marker exhibits dual characteristics. The primary treatment modalities for these patients include interventional therapy and molecular targeted drug therapy. Therefore, in some cases, DCP levels may increase in response to anti-VEGF therapy and TACE[38,39]. This elevation is attributed to the hypoxic environment induced by anti-VEGF agents and local arterial embolization within the tumor, which results in vitamin K deficiency. This means that elevated levels of DCP may indicate two possibilities: either that systemic therapy is effective or that it is ineffective and the tumor is progressing. For HCC patients who underwent hepatectomy with positive preoperative levels of DCP, when the first postoperative follow-up levels did not decrease to normal or to a specific percentage due to extremely high preoperative DCP levels, we need to be alert to the possibility that residual microlesions or circulating tumor cells may exist despite the removal of the neoplasm and surgeons should consider aggressive postoperative adjuvant treatment options[1,40].
Our study has some limitations. First, the study was retrospective in nature, which could lead to inherent biases. In addition, chronic HBV infection, accounting for 85.6% of the whole cohort, is the primary cause of HCC in this study, and differs from the pattern of disease observed in the United States and other Western countries. Furthermore, The variability in test results for DCP across different hospitals poses challenges for conducting large-scale studies. Last, This study focused on AFP-negative HCC patients, and further research is needed in the general patients.
Conclusion
A DCP cutoff value of 600 mAU/ml effectively stratifies AFP-negative HCC patients into high- and low-risk groups and high DCP level is an independent risk factor for OS and TTR after liver resection. Both preoperative DCP value and changes when postoperative follow-up can guide clinical decision-making.
Acknowledgements
We appreciate the trust and cooperation of the patients and their families.
Funding
This study was supported by the Public Welfare Technology Application Research Projects from the Science and Technology Bureau of Lishui (No. 2023GYX42, No. 2022GYX50), the Natural Science Foundation of Zhejiang Province (No. LQ23H160049).
Author contributions
Si-Yu Liu and Bin Ye had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conception: Si-Yu Liu, Jun-Wei Liu, and Bin Ye. Study design: Lei Liang, and Si-Yu Liu. Administrative support: Bin Ye, and Jun-Wei Liu. Data collection and acquisition: Lei Liang, Jun-Wei Liu, Si-Yu Liu and Bin Ye. Data analysis: Mu-Gen Dai, and Lei Liang. Manuscript preparation: Critical revision: Si-Yu Liu, Jun-Wei Liu, and Bin Ye. Final approval of manuscript: All authors.
Ethics approval and consent to participate
This retrospective study meeting the ethical standards of the Helsinki Declarations was approved by the Fifth Affiliated Hospital of Wenzhou Medical University Medical Ethics Committee. This study did not breach personal privacy or commercial interests and retrospectively obtained patient diagnosis and treatment data from hospitals' medical system. Following review by the the Fifth Affiliated Hospital of Wenzhou Medical University Medical Ethics Committee, our study was granted an exemption from obtaining signed informed consent.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Llovet JM, Pinyol R, Yarchoan M. et al. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat Rev Clin Oncol. 2024;21(4):294-311
2. Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R. et al. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer. 2022;161:108-118
3. Suarez AAR, Zoulim F. Opportunities and challenges for hepatitis B cure. eGastroenterology. 2023;1:e100021
4. Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491
5. Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA. et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-1965
6. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á. et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693
7. Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25:845-847
8. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314
9. Kim DY, Toan BN, Tan CK, Hasan I, Setiawan L, Yu ML. et al. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin Mol Hepatol. 2023;29:277-292
10. Kudo M. Urgent Global Need for PIVKA-II and AFP-L3 Measurements for Surveillance and Management of Hepatocellular Carcinoma. Liver Cancer. 2024;13:113-118
11. Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD. et al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427-1431
12. Qiu ZC, Wu YW, Qi WL, Li C. PIVKA-II combined with tumor burden score to predict long-term outcomes of AFP-negative hepatocellular carcinoma patients after liver resection. Cancer Med. 2023;13:e6835
13. Kim JM, Hyuck C, Kwon D, Joh JW, Lee JH, Paik SW. et al. Protein induced by vitamin K antagonist-II (PIVKA-II) is a reliable prognostic factor in small hepatocellular carcinoma. World J Surg. 2013;37:1371-1378
14. Nanashima A, Morino S, Yamaguchi H, Tanaka K, Shibasaki S, Tsuji T. et al. Modified CLIP using PIVKA-II for evaluating prognosis after hepatectomy for hepatocellular carcinoma. Eur J Surg Oncol. 2003;29:735-742
15. Wang MD, Sun LY, Qian GJ, Li C, Gu LH, Yao LQ. et al. Prothrombin induced by vitamin K Absence-II versus alpha-fetoprotein in detection of both resectable hepatocellular carcinoma and early recurrence after curative liver resection: A retrospective cohort study. Int J Surg. 2022;105:106843
16. Tsai SL, Huang GT, Yang PM, Sheu JC, Sung JL, Chen DS. Plasma des-gamma-carboxyprothrombin in the early stage of hepatocellular carcinoma. Hepatology. 1990;11:481-488
17. Cui R, He J, Zhang F, Wang B, Ding H, Shen H. et al. Diagnostic value of protein induced by vitamin K absence (PIVKAII) and hepatoma-specific band of serum gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma markers complementary to alpha-fetoprotein. Br J Cancer. 2003;88:1878-1882
18. Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford). 2002;4:99 99-100
19. Yang Y, Li G, Lu Z, Liu Y, Kong J, Liu J. Progression of Prothrombin Induced by Vitamin K Absence-II in Hepatocellular Carcinoma. Front Oncol. 2021;11:726213
20. Kim HS, Park JW, Jang JS, Kim HJ, Shin WG, Kim KH. et al. Prognostic values of alpha-fetoprotein and protein induced by vitamin K absence or antagonist-II in hepatitis B virus-related hepatocellular carcinoma: a prospective study. J Clin Gastroenterol. 2009;43:482-488
21. Suehiro T, Sugimachi K, Matsumata T, Itasaka H, Taketomi A, Maeda T. Protein induced by vitamin K absence or antagonist II as a prognostic marker in hepatocellular carcinoma. Comparison with alpha-fetoprotein. Cancer. 1994;73:2464-2471
22. Liu C, Yang H, Feng Y, Liu C, Rui F, Cao Y. et al. A K-nearest Neighbor Model to Predict Early Recurrence of Hepatocellular Carcinoma After Resection. J Clin Transl Hepatol. 2022;10:600-607
23. Ota M, Komeda K, Iida H, Ueno M, Kosaka H, Nomi T. et al. The Prognostic Value of Preoperative Serum Markers and Risk Classification in Patients with Hepatocellular Carcinoma. Ann Surg Oncol. 2023;30:2807-2815
24. Devillers M, Pluimers J, van Hooff MC, Doukas M, Polak WG, de Man RA. et al. The Role of PIVKA-II as a Predictor of Early Hepatocellular Carcinoma Recurrence-Free Survival after Liver Transplantation in a Low Alpha-Fetoprotein Population. Cancers (Basel). 2023;16:4
25. Yao CC, Wang JH, Chen CH, Hung CH, Yen YH, Kee KM. et al. Short Half-Life of Des-γ-Carboxy Prothrombin Is a Superior Factor for Early Prediction of Outcomes of Hepatocellular Carcinoma Treated with Radiofrequency Ablation. Diagnostics (Basel). 2023;13:696
26. Nagaoka S, Yatsuhashi H, Hamada H, Yano K, Matsumoto T, Daikoku M. et al. The des-gamma-carboxy prothrombin index is a new prognostic indicator for hepatocellular carcinoma. Cancer. 2003;98:2671-2677
27. Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L. et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854
28. Si YQ, Wang XQ, Fan G, Wang CY, Zheng YW, Song X. et al. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vascular invasion and tumor differentiation. Infect Agent Cancer. 2020;15:70
29. Li T, Yu Y, Liu J, Tian X, Kong M, Wu L. et al. PIVKA-II level is correlated to development of portal vein tumor thrombus in patients with HBV-related hepatocellular carcinoma. Infect Agent Cancer. 2019;14:13
30. Tsukamoto M, Nitta H, Imai K, Higashi T, Nakagawa S, Okabe H. et al. Clinical significance of half-lives of tumor markers α-fetoprotein and des-γ-carboxy prothrombin after hepatectomy for hepatocellular carcinoma. Hepatol Res. 2018;48:E183-183E193
31. Villa E, Camellini L, Dugani A, Zucchi F, Grottola A, Merighi A. et al. Variant estrogen receptor messenger RNA species detected in human primary hepatocellular carcinoma. Cancer Res. 1995;55:498-500
32. Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM. et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124
33. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-50
34. Cho EJ, Chung GE, Yoo JJ, Cho Y, Shin DW, Kim YJ. et al. The association between alcohol consumption and the risk of hepatocellular carcinoma according to glycemic status in Korea: A nationwide population-based study. PLoS Med. 2023;20:e1004244
35. Imamura H, Matsuyama Y, Miyagawa Y, Ishida K, Shimada R, Miyagawa S. et al. Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg. 1999;86:1032-1038
36. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699
37. Wang M, Qian G, Xiao H, Liu X, Sun L, Chen Z. et al. Prognostic significance of postoperative serological incomplete conversion of AFP and PIVKA-II after hepatic resection for hepatocellular carcinoma: a multicenter analysis of 1755 patients. Oncologist. 2024;29:e1723-1723e1733
38. Murata K, Suzuki H, Okano H, Oyamada T, Yasuda Y, Sakamoto A. Cytoskeletal changes during epithelial-to-fibroblastoid conversion as a crucial mechanism of des-gamma-carboxy prothrombin production in hepatocellular carcinoma. Int J Oncol. 2009;35:1005-1014
39. Kodama K, Kawaoka T, Namba M, Uchikawa S, Ohya K, Morio K. et al. Correlation between Early Tumor Marker Response and Imaging Response in Patients with Advanced Hepatocellular Carcinoma Treated with Lenvatinib. Oncology. 2019;97:75-81
40. Chen W, Li C, Liu Z, Kong Q, Sun L, Zeng Y. et al. Novel online calculator to predict reduced risk of early recurrence from adjuvant transarterial chemoembolisation for patients with hepatocellular carcinoma. eGastroenterology. 2023;1:e100008
Author contact
![]() Corresponding authors: Dr. Zhuo-Kai Li, Department of Hepatobiliary Surgery, The Fifth Affiliated Hospital of Wenzhou Medical University, Zhejiang Province, China; E-mail: lizhuokaicom. Dr. Bin Ye, Department of Gastroenterology, The Fifth Affiliated Hospital of Wenzhou Medical University, Zhejiang Province, China; E-mail: 408252097com.
Corresponding authors: Dr. Zhuo-Kai Li, Department of Hepatobiliary Surgery, The Fifth Affiliated Hospital of Wenzhou Medical University, Zhejiang Province, China; E-mail: lizhuokaicom. Dr. Bin Ye, Department of Gastroenterology, The Fifth Affiliated Hospital of Wenzhou Medical University, Zhejiang Province, China; E-mail: 408252097com.

 Global reach, higher impact
Global reach, higher impact