Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(8):2595-2612. doi:10.7150/jca.109420 This issue Cite
Review
Development of Traditional Chinese Medicine in combination with EGFR Inhibitors against Cancer
1. Department of Oncology, School of Medicine, Nantong University, Nantong, China.
2. Institute of Energy Metabolism and Health, Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai, China.
3. Department of Respiratory Medicine, Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai, China.
4. Department of Pulmonology, Sixth People's Hospital Affiliated to Shanghai Jiao Tong University, China.
Received 2024-12-25; Accepted 2025-5-6; Published 2025-6-5
Abstract
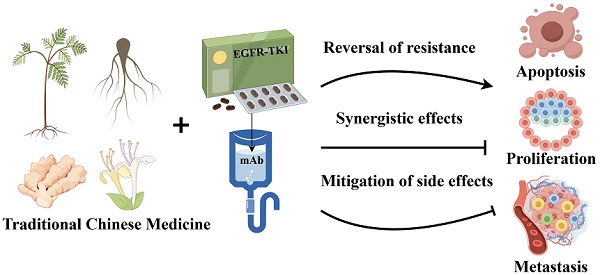
Epidermal growth factor receptor (EGFR) is one of the most important and therapeutically significant targets in most cancer treatments, and EGFR-targeted therapy is widely performed to treat various tumors, such as non-small cell lung cancer. Although EGFR-targeted therapy has fewer side effects than conventional chemotherapy, they have limited applications and are prone to drug resistance. Traditional Chinese medicine (TCM), are promising solutions to address these challenges because of their biological activities, such as inhibition of EGFR-related signaling pathways, reversal of drug resistance, and mitigation of side effects of targeted therapy. Moreover, TCM is characterized by multiple targets, few side effects and good therapeutic effect Here, we summarized several typical traditional Chinese medicine monomers derived from traditional Chinese medicine that can be used along with EGFR inhibitors, as well as herbal plants with potential for natural product development and TCM. We focused on their mechanisms of action underlying the reversal of drug resistance, enhancement of drug efficacy, and mitigation of side effects; we also assessed relevant clinical studies that are available. However, the potential adverse effects (e.g., drug-drug interactions, hepatotoxicity, and immune-related side effects) of TCMs along with EGFR inhibitors need to be further investigated. This article provided new perspectives on the use of TCMs in EGFR-targeted therapy and emphasized the importance of safety assessment in future studies.
Keywords: EGFR, EGFR-TKI, EGFR monoclonal antibody, traditional Chinese medicine monomers, traditional Chinese medicine, cancer
Introduction
Cancer is a major social, public health, and economic issue of the 21st century, with nearly one-sixth (16.8%) of global deaths and a quarter (22.8%) of deaths due to noncommunicable diseases (NCDs). Studies suggest that about one-fifth of men or women will suffer from cancer in their lifetime. By 2022, about one-ninth of men and one-twelfth of women were predicted to have died due to cancer, and nearly 20 million new cases of cancer developed worldwide. The estimated number of deaths due to cancer worldwide is 9.7 million [1]. These data suggest that cancer is a major global problem affecting humans. In China and most developed countries, cancer is becoming the leading cause of death, and deaths due to cancer have surpassed those due to cardiovascular disease [2]. By 2022, about 4,824,700 new cancer cases and 2,574,200 new cancer-related deaths were expected in China [3]. Cancer is also a major medical and health problem in China, as it hinders the development of the economy and the health of people.
Many patients with cancer display strong EGFR expression levels, abnormal posttranslational modifications, and even sequence mutations [4, 5]. Therefore, EGFR is one of the most promising and challenging targets for addressing cancer [6]. Although there are fewer side effects of EGFR-targeted therapy than traditional chemotherapy, it is suitable for fewer people and is prone to developing drug resistance [7]. Hence, EGFR-targeted therapy faces many challenges.
Traditional Chinese medicine monomers have a high diversity, and the majority of them originate from herbal plants that have been used in Traditional Chinese Medicine for a long time. TCM application involves using natural products with different pharmacological effects to prevent and treat diseases. The application of TCM is ubiquitous [8]. Since the development of medicine, many natural products including traditional Chinese medicine monomers or their analogs, such as paclitaxel, have been used in clinical practice because of their anticancer properties [8, 9]. Many types of TCMs, such as the Chinese herbal Fuzheng Yiai Decoction, are used as supplements, conditioning methods, or adjuvant treatments in clinical antitumor treatment plans. [10, 11]. Therefore, they can be used as drugs or supplemental adjuncts to address the challenges associated with EGFR treatment techniques. Based on the current research on EGFR-targeted therapy, we summarized several traditional Chinese medicine monomers and compound prescriptions that produce positive effects in combination with EGFR inhibitors (EGFRIs).
Epidermal growth factor receptor signal transduction pathway
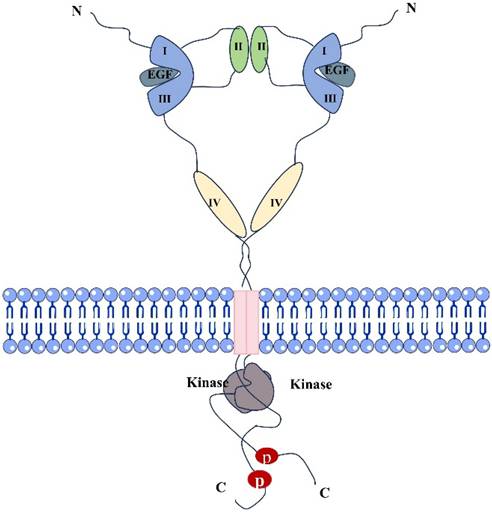
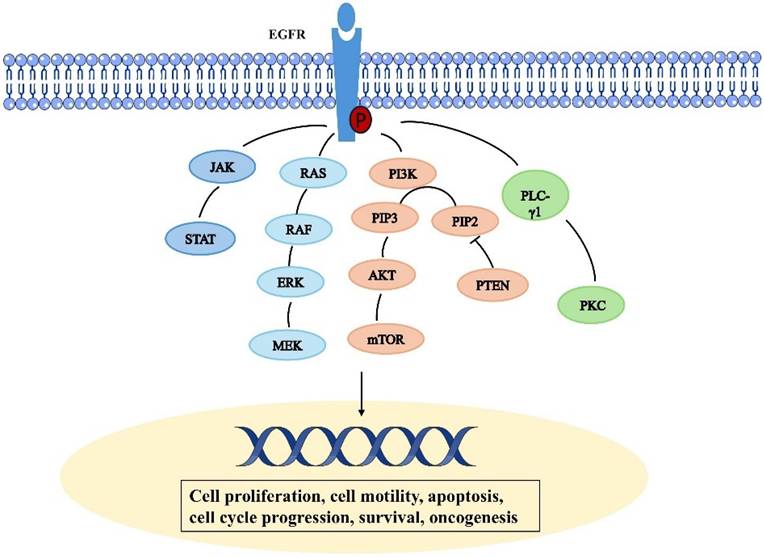
Epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor in the ErbB family [12]. It is a transmembrane glycosylated protein, that includes an extracellular domain, a transmembrane domain, and an intracellular domain (Fig. 1). Its extracellular domain consists of subregions, I, II, III, and IV; the II domain is involved in the formation of homodimers and heterodimers. The transmembrane domain is a hydrophobic region anchored to the cell membrane, and consists of 19-25 amino acid residues. The intracellular region is the carboxy-terminal region of the cytoplasm with a protein kinase domain, which consists of about 550 amino acid residues and contains three subregions, including the tyrosine kinase region (TK), the near membrane region (JM), and the C-terminal region (CTD). When EGFR and its ligands dimerize and are activated, ATP binds to the tyrosine kinase region and activates downstream signaling pathways [13, 14]. The near-membrane region can regulate kinase dimerization and has a moderation effect on downstream signaling pathways. The C-terminal domain facilitates self-phosphorylation when EGFR is activated, and phosphorylated residues activate intracellular signaling pathways [15]. It participates in the occurrence and metastasis of related cancers, demonstrating a moderation role in these pathways [6]. The relevant pathways are summarized in Figure 2. Human EGFR is encoded by two transcripts of 10.5 kb and 5.8 kb (isoform A) [16]. Except for full-length EGFR, the other isoforms are generated by alternative RNA splicing. Along with these two transcripts that generate the full-length EGFR isoform, the EGFR gene also produces three alternative transcripts that are 1.8, 2.4, and 3.0 kb in size and encode isoforms C, B, and D, respectively [17]. The 3.0 kb isoform D is associated with cell membranes and can be detected in human serum [18]. Studies and methodological tools for the application of these subtypes to the treatment and diagnosis of diseases have progressed modestly but need to be further investigated [19].
RAS/RAF/mitogen-activated protein kinase pathway
EGF stimulates the activation of the downstream RAS and increases its GTP-binding state (Ras-GTP), thereby linking the RAS to upstream receptor tyrosine kinase (RTK) signaling [20]. Adaptor proteins such as Grb2 recognize the sequence homology 2 (SH2) domain and thus recruit guanine nucleotide exchange factors (GEF) such as SOS-1 or CDC25 to the cell membrane. GEF facilitates conformational changes and the exchange of GDP and GTP via interacting with Ras proteins at the cell membrane. Raf is drawn to the cell membrane by binding to the Ras switch I domain and lipid binding when Ras is activated [21]. When RAF protein kinase activity is activated by activated Ras, RAF kinase is phosphorylated, which in turn activates MEK (serine/tyrosine/threonine kinase). The mitogen activated protein kinase ERK is activated by MEK phosphorylation, and when ERK is phosphorylated, its kinase activity is triggered as well as several downstream targets involved in the control of cell proliferation are phosphorylated. Resistance to EGFR treatment is linked to abnormal RAS protein activity [22].
Phosphatidylinositol triphosphate (PI3K) and serine-threonine protein kinase (AKT) pathways
PI3K/Akt/mTOR signaling is one of the important signaling pathways regulating cellular function, and PI3K/Akt/mTOR hyperactivation is seen in most solid tumors and is one of the most common dysregulated signals found in cancer patients [23]. PI3K/Akt/mTOR signaling pathway is regulated by phosphatase and tensin homologs (PTEN) that are missing on chromosome 10 and human epidermal growth factor receptor (EGFR) [24]. When cytosolic EGFR binds to ligands, cytoplasmic tyrosine kinases are activated, which leads to autophosphorylation and downstream conduction patency. This results in the PI3K/Akt chain reaction, which is the most important known EGFR-regulated transduction pathway to date [25]. There are three types of PI3K, but class I PI3K is the most extensively researched. When PI3K is activated, a second messenger, PIP3, is produced on the plasma membrane. This messenger binds to the signal proteins AKT and PDK1 (phosphoinositide dependent kinase-1), which have PH domains in cells. This encourages PDK1 to phosphorylate Ser308 of the AKT protein, which in turn activates AKT. By phosphorylating downstream components such as a variety of enzymes, kinases, and transcription factors, activated AKT modulates cellular activity. mTOR is a mammalian target of rapamycin (mTOR), which is an important serine-threonine protein kinase downstream of PI3K/Akt [26]. It regulates the growth, survival, and invasion and metastasis of tumor cells by activating ribosomal kinases.
Structural diagram of dimers formed by EGFR and EGF ligand binding.
Signal transducer and activator of transcription (STAT) pathway
STAT is a nuclear transcription factor. However, STAT is found in the cytoplasm of cells that are at rest. Following activation, STAT molecules dimerize and go into the cell's nucleus. STAT proteins exist as inactive monomers. Through their SH2 domain, signal transducers and transcriptional activators (STAT-1, 3, and 5) interact with EGFR; nevertheless, their transcriptional activity remains non-functional until EGFR phosphorylation occurs [27]. EGFR can regulate the STAT signaling pathway through two different mechanisms: JAK dependent and JAK independent. EGFR stimulation induces Tyr 701 phosphorylation of STAT 1, which further initiates the formation of complexes between STAT 1 and STAT 3 and JAK 1 and JAK 2 [28]. STAT3 plays a critical role in cancer development and abnormal activation is associated with several types of cancer, including brain, lung, pancreas, endometrium, colorectal, kidney, breast. It also plays an important role in resistance to tyrosine kinase inhibitors [29].
PLC-γ1 pathway
The binding of ligands to EGFR leads to phosphorylation of PLC - γ 1 tyrosine residues and activation of lipase. Phosphatidylinositol-specific phospholipase C (PLC) plays an important role in transmembrane signaling [30]. In response to extracellular stimuli such as hormones, growth factors, and neurotransmitters, PLC hydrolyzes phosphatidylinositol-4,5 bisphosphate (PIP2) and produces the second messenger's inositol 1,4,5 triphosphate (IP3) and diglycerides (DAG) [31]. In addition, DAG activates protein kinase C (PKC) and IP 3, which induce the release of calcium from intracellular storage. These secondary messengers trigger a series of molecular interactions and alter the physiological state of cells [32].
Epidermal growth factor receptor inhibitors approved for clinical practice
The main epidermal growth factor receptor inhibitors (EGFRIs) used in clinical practice are EGFR-TKIs and EGFR monoclonal antibodies. Specific monoclonal antibodies can bind to the extracellular domains II and III of EGFR. This type of antibody has several advantages. First, owing to its strong binding ability, it blocks the binding of ligands to EGFR, thereby inhibiting the activation of EGFR [33]; Second, owing to its binding site in the extracellular region, EGFR can effectively target mutations in both wild-type and intracellular tyrosine kinase activity regions [34].
Schematic representation depicting the epidermal growth factor receptor signal transduction pathway.
Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI)
The most commonly used drugs in clinical practice are tyrosine kinase inhibitors (TKIs) that target the EGFR activation domain. Moreover, many EGFR-TKIs are undergoing clinical trials [35]. Its main mechanism of action involves binding to the tyrosine kinase domain of EGFR and competitively inhibiting ATP binding, thereby blocking the phosphorylation of tyrosine residues and inhibiting signal transduction [36]. There are four generations of EGFR-TKIs approved for clinical use. Erlotinib and gefitinib are the first-generation small molecule EGFR-TKIs used to treat non-small cell lung cancer [37, 38]. EGFR inhibition is reversible, and when EGFR T790M mutates, resistance to first generation drugs occurs [39]. The second-generation EGFR-TKI is an irreversible small molecule drug developed to address the issue of acquired resistance in the first generation of drugs, its clinical efficacy is not satisfactory [40]. The emergence of third-generation drugs can effectively solve the above problems and have acceptable clinical efficacy, but drug resistance quickly emerges [5]. Fourth-generation EGFR-TKIs have good therapeutic effects on various types of tumors and cancers with multiple mutations [41]. Researchers from many countries are intensively developing methods to address the unpredictable and changing situation of cancer. However, the efficacy of EGFR-TKIs is limited by drug resistance and the adverse effects of treatment.
Monoclonal antibodies targeting EGFR receptors
Monoclonal antibodies targeting EGFR receptors have been approved for clinical use. They have become an important part of drug therapy for early cancer patients [42]. Multiple monoclonal antibody drugs have been approved for clinical practice, making EGFR targeted therapy more selective [43]. Cetuximab was the first approved EGFR mAb by the FDA in the US. After preliminary clinical trials, cetuximab was approved for treating advanced cancer in 2004, and two years later it was approved for treating head and neck squamous cell carcinoma that did not meet surgical indications [44, 45]. However, cetuximab has almost no therapeutic effect on advanced colorectal cancer patients with KRAS and other RAS family gene activation mutations [46, 47]. After the emergence of cetuximab, panitumumab was approved for use by the FDA via fast track. It is the first fully humanized antibody that binds to the extracellular domain of EGFR with high affinity [48]. Additionally, panitumumab is ineffective in patients with other RAS mutations, along with those with KRAS mutations in exon 2 [49]. Other monoclonal antibodies approved for clinical use include necitumumab, nimotuzumab, and amivantamab [50-52]. Many researchers in the field of medicine are trying to develop new monoclonal antibodies to address the complex cancer situation. However, monoclonal antibodies can be ineffective due to KRAS mutations and other RAS factor mutations or because of excessive accumulation of hyaluronic acid (HA) in the tumor microenvironment [53].
Combination with approved EGFRIs
Two types of drugs used clinically to target EGFR for cancer treatment include monoclonal antibodies and EGFR-TKIs. The former binds to the extracellular domains II and III of EGFR, whereas EGFR-TKIs bind to the tyrosine kinase domain of EGFR [13]. The latter is widely used. Owing to their low toxicity and minimal side effects, traditional herbs and their natural compounds have been studied and extracted by several researchers and have been applied in clinical practice to treat various diseases [54, 55]. EGFR-TKIs or mAbs have been combines with other medications, such as various natural ingredients and formulations from TCM, in clinical practice and in several studies to produce a significantly greater effect than that achieved by its components when acting alone [56, 57]. The biochemical and molecular properties of the traditional Chinese medicine monomers herein are summarized in Table 1.
Combination with EGFR-TKIs to reverse resistance
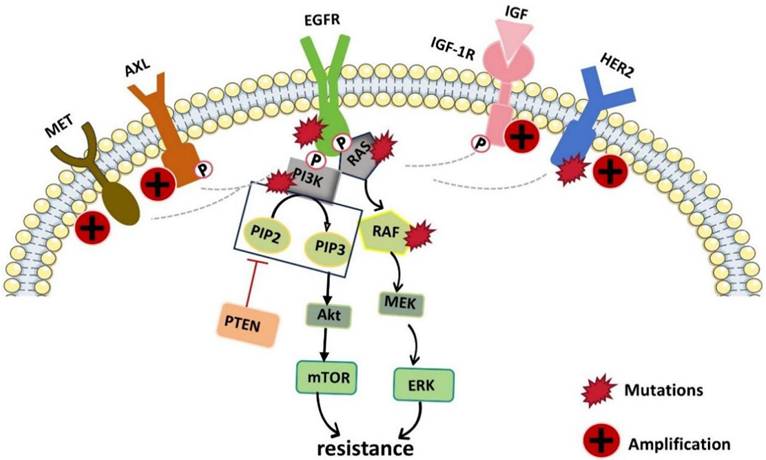
Although EGFR-TKIs have negligible side effects and satisfactory therapeutic efficacy, their treatment plans are hindered by almost inevitable drug resistance. When patients are administered EGFR-targeted therapy with significant early effects, drug resistance-related problems occur within only 9-12 months, which greatly hinders the subsequent treatment of tumors and the efficacy of drugs [76]. Resistance to EGFR-TKIs is caused mainly by acquired EGFR mutations or EGFR-independent pathways, including gene mutations, bypass activation, histological transformation, overexpression of efflux pumps, increased metabolic inactivation, etc. (Fig. 3.) [77]. Therefore, many researchers have investigated ways to reverse or delay this resistance. The use of traditional Chinese medicine monomers and traditional Chinese herbs as supplements is a promising strategy for reversing drug resistance. In this section, we described some of the common and more important TCMs that can reverse or prevent the development of tumor resistance to EGFRIs. The results are summarized in Table 2.
Schematic diagram of the molecular pathways related to EGFR-TKI resistance. EGFR kinase domain mutations and the upregulation or activation of other RTKs (such as AXL, MET, HER2 and IGF-1R which can cross the suppressed EGFR signaling pathway and activate downstream pathways) lead to resistance to EGFR-TKIs.
Biochemical and molecular characterization of traditional Chinese medicine monomers.
| Name | Chemical class | PKDM | solubility | Toxicity | Mechanism of Action | Bioactivity | Ref |
|---|---|---|---|---|---|---|---|
| Bufalin | steroid | Low bioavailability; Excreted mainly through both hepatic pathway and renal pathway. | Insolubility in water; Good solubility in organic solvent | Endogenously expressed in humans and should not cause toxicity if used at physiologic doses | Acting on Na/K-ATPase pump; Promotion of cell cycle arrest and apoptosis. | harbouring cardiotonic, anti-inflammatory, and diuretic properties, anti-cancer. | [58, 59] |
| Berberine | alkaloid | Low bioavailability (0.37 ± 0.11%); Excreted mainly via fecal and renal excretion route. | Low solubility and poor permeability. Berberine sulfate is usually more water-soluble and stable | High doses or prolonged use may cause toxic reactions. | Inhibition of DNA topoisomerase, Induction of reactive oxygen species production | antioxidant, anti-microbial, antiinflammatory, anti-cancer, anti-diabetes, anti-dyslipidemia, and anti-obesity | [60, 61] |
| Luteolin | flavonoid | Low bioavailability; The majority of luteolin possibly be metabolized to other compounds, and these metabolites could then absorb into the systemic circulation or return to the small intestine through the enterohepatic circulation. | Low solubility in water | Nontoxic side effects as a dietary supplement. | Inhibition of ROS-induced DNA, lipid, and protein damage, induction of apoptosis and cell cycle arrest, inhibition of metastasis and angiogenesis. | Anti-microbial, anti-inflammatory, anti-allergy, anti-oxidant, and anti-cancer. | [62, 63] |
| Polyphyllin I | Saponins | Low oral bioavailability (about 0.62%); | Low solubility in water | Activation of the JNK signaling pathway, inhibition of PDK1/Akt/mTOR signaling, induction of autophagy, cell cycle arrest and apoptosis | Anti-fungal, anti-cancer, anti-inflammatory, anti-oxidant | [64, 65] | |
| Shikonin | naphthoquinone | Low bioavailability; metabolized primarily in the liver, involving the CYP450 and UGT enzyme systems, and excreted in urine and bile. | Insolubility in water; Good solubility in alkaline substances and organic solvent | May cause toxicity, especially in drug interactions based on effective inhibition of CYP enzymes. | Inhibition of PKM2 TNF-α and NF-κB pathway | Anti-inflammatory, anti-cancer, cardiovascular protective, antimicrobial, | [66, 67] |
| Curcumin | phenolic | Low bioavailability; Absorption in the small intestine is low and rapidly eliminated in the liver | Poor solubility in water at physiological pH | prescribed doses of curcumin have no side effects in the body | Inhibition of NF-κB, p300/CREB-binding protein-specific | Antioxidant, anti-inflammatory, anti-infective, antiangiogenic and anti-cancer | [68, 69] |
| Rhein | lipophilic anthraquinone | Low bioavailability The Rhine absorbed into the body is first metabolized into forms of glucuronide and sulfate, and then excreted into the hepatoenteric circulation through bile. | Insolubility in water; Good solubility in alkaline substances | Mainly related to the concentration and duration of administration. Low-dose short-term treatment has a protective effect on the liver and kidneys | Regulation of PI3K/AKT/ERK pathways, interference with NF-κB signaling pathway. | Hypoglycemic, lipid-lowering, anti-cancer, anti-inflammatory, anti-fibrotic, craniocerebral protection, antibacterial, and antiviral | [70] |
| epigallocatechin-3-gallate (EGCG) | polyphenol | Low bioavailability Poor intestinal stability and Low intestinal absorption. Excreted mainly via fecal, renal and air excretion route | Medium solubility in water, high solubility in acidic conditions | High doses or prolonged use may pose risks of liver toxicity, gastrointestinal discomfort, and drug interactions | Inhibition of cell proliferation and glutamate dehydrogenase 1/2 (GDH1/2, GLUD1/2) activity, induction of apoptosis, | Antioxidative, anti-inflammatory, anti-viral, anticancer, anti-microbial, anti-obesity and neuroprotective. | [71] |
| Platycodin D | Triterpenoid saponin | Low bioavailability (1.89%); Following intravenous and oral administration, small amounts of unchanged PD are excreted via the urinary and biliary (or fecal) routes | Good solubility in water. | Intravenous saponins may cause hemolysis. | Induction of apoptosis and autophagy in tumor cells, activation of AMPKα, inhibition of NF-κB pathway | Anti-viral, anti-oxidation, anti-tumor, anticoagulant, spermicidal, anti-inflammatory. | [72, 73] |
| ginsenoside Rg3 | saponins | Low bioavailability easily transformed to active ingredients by gut microbiota | Poor solubility in water | Lower toxicity and higher safety | Targeting hypoxia-induced multiple signaling pathways, inhibition of NF-κB and MAPK pathway | Antioxidant, anti-inflammatory, antiangiogenic and anti-cancer | [74, 75] |
Traditional Chinese medicine monomers or compound prescriptions used in combination with anti-EGFR agents to reverse resistance.
| Name | Origins | Structure | EGFRIs | Research Object | Effects (Target) | Ref | |
|---|---|---|---|---|---|---|---|
| In vitro | In vivo | ||||||
| Bufalin | Chan Su |  | Osimertinib | NSCLC cells (PC-9/OR and HCC827/OR) | PC-9/OR xenograft mouse | Targeting the Ku70/MCL-1 signaling axis | [83] |
| Berberine | Coptis chinensis plant |  | Osimertinib | EGFRm NSCLC cells (HCC827/AR) | HCC827/AR xenograft mouse | MET | [85] |
| Luteolin | Honeysuckl celery |  | Osimertinib | NSCLC cells (H1975/OR) | MET | [88] | |
| Polyphyllin I | Paris polyphylla |  | Osimertinib | NSCLC cells (PC-9/OR and H1975/OR) | H1975/OR xenograft mouse | PI3K/Akt | [93] |
| Polyphyllin I | Paris polyphylla |  | Gefitinib | NSCLC cells (PC9 and PC9/GR) | PC9/GR xenograft mouse | HIF-1a | [92] |
| Rhein | herbs such as rhubarb |  | erlotinib | Human pancreatic cancer cells (BxPC-3, PANC-1, Patu8988T and AsPC-1) | BxPC-3 and PANC-1 xenograft mouse | STAT3 | [97] |
| Sijunzi Tang (SJZ) | Gefitinib | NSCLC cells (PC-9/GR) | PC-9/GR xenograft mouse | SLC1A5, GLS and GS | [102] | ||
Osimertinib is the first approved third-generation EGFR-TKI that can effectively address the issue of acquired resistance associated with first-generation EGFR-TKIs, such as those that target acquired T790M mutations and other common EGFR activating mutations [78]. Therefore, it is widely used in clinical practice. However, after about 10-19 months, drug resistance emerges which leads to uncontrolled cancer and further disease progression [79]. Therefore, corresponding measures need to be developed to overcome acquired resistance to Osimertinib. Bufalin is a types of bufadienolides, and it is an effective ingredient in secretions of toads and in certain plants [80]. Bufalin can induce apoptosis in various types of cancer cells [59]. Prior research has demonstrated that bufalin can inhibit MCL-1 [81]. One important mechanism controlling the effectiveness of Osimertinib is U70-mediated overexpression of MCL-1 [82]. Cao et al., reported that bufalin and osimertinib together significantly increased the expression of cleaved-caspase-3 and PARP and induced apoptosis in osimertinib-resistant lung cancer cells compared to either medication alone. Therefore, bufalin can increase the sensitivity of Osimertinib-resistant lung cancer cells. The results of this study demonstrated a novel therapeutic combination that targets the Ku70/MCL-1 signaling axis to overcome osimertinib resistance [83]. The main limitations include their low solubility, potential cardiotoxicity and rapid distribution and metabolism in vivo, which make their use in the clinic difficult.
Berberine is an isoquinoline alkaloid compound isolated from Coptis chinensis plants. Due to its antibacterial, anti-inflammatory, and metabolism-regulating qualities, berberine is used as a traditional Chinese medicine monomer. It has been studied as the main and complementary therapeutic technique for treating various diseases [61]. It exhibits antitumor effects in many types of cancer, including breast cancer, lung cancer, and liver cancer. [84]. Chen et al. reported that berberine, an inhibitor of MET, shows therapeutic potential when it is administered along with osimertinib or other third-generation EGFR-TKIs to overcome acquired resistance caused by MET amplification. In vivo, the growth of MET-amplified osimertinib-resistant tumors is more effectively inhibited when berberine and osimertinib are used together [85].
Luteolin, also known as 3',4',5,7-tetrahydroxylflavone, is a component of flavonoids. It is widely distributed in various fruits, vegetables, and herbs, such as honeysuckle and celery [62]. It has anti-inflammatory, antitumor, and antioxidant effects on the intestinal flora and neuroprotective effects [62]. Luteolin prevents the progression of various types of cancer [86]. Owing to the synergistic effect of luteolin and PD-1 inhibitors, it can be used as an adjuvant therapy [87]. Huang et al. reported that luteolin, in combination with osimertinib overcomes acquired resistance to osimertinib induced by MET amplification and overactivation by inhibiting the EGF-MET-Akt pathway [88]. To prevent or reverse the resistance to EGFR-TKIs caused by MET amplification, whether it can be appropriately combined with berberine or luteolin should be considered. However, further validaion by many subsequent studies is necessary.
Polyphyllin I is isolated from the natural herb Paris polyphylla. PPI has anticancer potential [65]. It can inhibit cisplatin-resistant NSCLC growth by regulating p53 and CIP2A/AKT/mTOR signal transduction [89]. HIF-1 accelerates tumor growth and metastasis by regulating the metabolism and immune escape of tumor cells, and the level of this protein is elevated in gefitinib resistant lung cancer cells [90, 91]. Zhang et al. reported that PPI can improve gefitinib resistance and inhibit the VEGF/VEGFR2/p38 pathway by targeting HIF-1α in lung adenocarcinoma [92]. Lai et al. reported that PPI reversed resistance in osimertinib-resistant NSCLC cell lines and xenografts by regulating PI3K/AKT signaling and controlling the expression of apoptosis-related proteins. These findings suggested that PPI is a promising therapeutic agent for reversing osimertinib resistance [93]. Thus, PPI can reverse gefitinib resistance and osimertinib resistance, and it might serve as a promising treatment for reversing resistance to EGFR-TKIs.
Rhein is an anthraquinone compound widely found in herbs such as rhubarb and is popular to for its anti-inflammatory and antitumor properties [70]. Rhein is structurally similar to several STAT3 inhibitors [94]. Many studies have reported that cancer drug resistance is closely related to the activation of STAT3 [95, 96]. Yang et al. conducted experiments and also tentatively confirmed our speculations. The results of in vitro experiments revealed that rhein and erlotinib, when used in combination, inhibited the phosphorylation of STAT3 and EGFR in pancreatic cancer cell lines, thereby inhibiting the proliferation of pancreatic cancer cells. Similarly, in vivo experiments confirmed this finding [97]. Studies on colorectal cancer reached similar conclusions [98]. The combination of rhein and EGFRIs may, therefore, be a novel antitumor strategy that can overcome resistance to EGFRIs and have a stronger antitumor effect.
TCM has played a significant role in reducing the toxicity of chemotherapy, enhancing immune function, and even directly treating and slowing the progression of cancer in recent years [99]. A combination of TCM compounds can reverse resistance to EGFR-targeted drugs [99]. Si Jun Zi Tang (SJZT) is a classic formula that has Qi tonifying properties and can manage the underlying causes of lung cancer, colon cancer, and stomach cancer [100, 101]. SJZT combined with gefitinib has an antitumor effect on gefitinib-resistant (PC9-GR) cells. SJZT may affect glutamine metabolism by modulating key targets involved in glutamine metabolism (SLC1A5, GLS and GS) and regulating the levels of related metabolic markers, ultimately reversing gefitinib resistance [102]. Therefore, SJZT may be used as an adjunct to gefitinib therapy to prevent or reverse resistance.
Synergistic effects through other pathways
Owing to their biodiversity and structural complexity, TCMs can serve as drugs for multitarget therapy [103, 104]. The efficacy of EGFR-targeted therapy, however, is often limited by the singularity of their targets. Therefore, multitargeted therapy for cancer to improve its efficacy has gained the attention of the medical community [105]. The research, extraction, translation and clinical application of traditional Chinese medicine monomers or compound prescriptions for single-target treatment of cancer have not been solved. Therefore, its use as a complementary or adjuvant therapy in combination with EGFR-targeted treatment of cancer provides a new way to address these problems [106, 107]. The following section describes several representative traditional Chinese medicine monomers or compound prescriptions that, when used along with EGFRIs for treating cancer, can have other synergistic effects, such as delaying or preventing metastasis and multitargeting effects to improve the efficacy and response to chemoresistant advanced tumors (Table 3).
Cancer treatment modalities have been updated and optimized, and the use of combinations of two or more compounds has gained greater attention. Epigallocatechin-3-gallate (EGCG) is a common active ingredient extracted from green tea and has anti-inflammatory, antibacterial and anticancer activities [71]. EGCG has anticancer activities against various types of cancer, such as colorectal cancer, squamous cell carcinoma of the head and neck (SCCHN), and thyroid cancer [108]. Abedul Haque et al. reported that the combination of EGCG and erlotinib induced the expression of the cell cycle regulatory proteins p21 and p27 and the apoptosis-regulating protein Bim and inhibited the expression of Bcl-2 [109]. Therapeutic techniques targeting EGFR remain the mainstay of treatment for advanced cancers, and this preclinical study may influence future treatment options for SCCHN.
Curcumin is a TCM monomers isolated from the rhizome of turmeric. It has anti-inflammatory, antioxidant, and anticancer effects [110]. It is safe for pharmacological applications and has negligible toxicity with high efficacy, which can promote cancer cell apoptosis, antitumor proliferation, antitumor metastasis, etc. [111, 112]. Curcumin can impede the EGFR signaling pathway in cancer cells by directly or indirectly inhibiting the enzymatic activity of the EGFR intracellular domains, inhibiting EGFR phosphorylation and inducing EGFR ubiquitination [113]. It can also be used along with different chemotherapeutic drugs to cause apoptosis and block the proliferation of various tumor cells [114]. Curcumin can also be used in combination with EGFRIs. Combination treatment increases the inhibitory effect of curcumin and cetuximab on survival and the induction of apoptosis in cisplatin-resistant oral cancer. Kuroda et al. found a close relationship between cisplatin resistance and the EGFR signaling pathway in lung cancer cells. These findings suggest that the use of EGFRIs may be a promising strategy for treating cisplatin resistance [115]. Curcumin is, therefore, a possible adjuvant therapy, and the combination of curcumin and cetuximab may result in a novel treatment for oral cancer and even cisplatin resistance.
Shikonin is the main component of the Chinese herb Lithospermum erythrorhizon (zicao) [116]. This pkm2 inhibitor has antitumor effects on different types of cancer [117]. Tang et al. reported that gefitinib inhibits EGFR wild-type lung cancer cells more strongly when PKM2 is knocked down. Therefore, shikonin, a natural PKM2 inhibitor, significantly promoted apoptosis and inhibited the proliferation of EGFR wild-type lung cancer cells when combined with gefitinib. The results of in vivo and in vitro experiments confirmed this conclusion [118]. EGFR TKIs were originally only targeted at a subset of the population, and such studies may help improve the efficacy of EGFR TKIs against EGFR wild-type tumors.
Platycodin D (PD) is the main triterpenoid saponin extracted from the roots of Platycodon grandiflorum [72]. PD suppresses the lung metastasis of breast cancer by downregulating PD-L1 expression and enhances the anticancer effects of sorafenib in AKT-positive and PTEN-negative prostate cancer cells through the ubiquitination of p-Akt [119, 120]. PD and cetuximab (CTX) can prevent colorectal cancer (CRC) cells from proliferating [121]. When the two drugs are used in combination, the metastasis inhibition effect is the best, as both relatively unrelated pathways are inhibited [122]. When individuals are clinically susceptible to the metastasis of CRC, we can pay attention to these TCM natural products, such as PD.
Modern Western medicine and TCMs have been combined in recent years to increase clinical efficacy and decrease painful responses. Additionally, EGFR-TKIs and the components of TCM can prolong patient survival. TCM drugs in conjunction with EGFR-TKIs can prolong patient survival and postpone drug resistance; they can be administered along with chemotherapy, targeted treatment, and prompt replacement of a new generation of TKI preparations [123].
TCM monomers or compound prescriptions used in combination with anti-EGFR agents to produce synergistic effects through other pathways.
| Name | Origins | Structure | EGFRIs | Research Object | Effects (Target) | Ref. | |
|---|---|---|---|---|---|---|---|
| In vitro | In vivo | ||||||
| epigallocatechin-3-gallate (EGCG) | green tea | 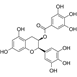 | erlotinib | SCCHN cells (Tu686, Tu212) | Bim, Bcl-2 | [109] | |
| Curcumin | Curcuma longa |  | cetuximab | oral cancer cells (CAR) | EGFR | [115] | |
| Shikonin | Lithospermum erythrorhizon (zicao) | 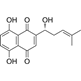 | gefitinib | NSCLC cells (A549, H1299, H1975, HCC827) | A549 xenograft mouse | PKM2 | [118] |
| Platycodin D | platycodon grandiflorum |  | Cetuximab | CRC cells (HT29 and CaCo2) | HT29 and CaCo2 xenograft mouse | β-catenin | [122] |
Traditional Chinese medicine monomers and compounds can serve as potential drugs for multitarget therapy because of their biodiversity and structural complexity. The efficacy of EGFR-targeted therapeutic techniques, however, is often limited by the singularity of their targets. Therefore, multitargeted therapy for cancer to improve its efficacy has gained the attention of the medical community. The research, extraction, translation and clinical application of traditional Chinese medicine monomers and compounds for single-target treatment of cancer have not been solved; therefore, their use in complementary or adjuvant therapy in combination with EGFR-targeted treatment of cancer provides a new way to address these problems.
Mitigation of related side effects
Although EGFRIs have relatively few side effects, many individuals experience symptoms, including diarrhea, alopecia, and skin toxicity [124]. In addition to the application of antibiotics and topical ointments, Chinese herbal medicines can be used to alleviate severe skin diseases caused by EGFRI treatment [125]. The following section describes several representative TCM monomers or compound prescriptions used clinically to alleviate the adverse effects induced by EGFR-targeted therapies (Table 4).
Honeysuckle is a traditional herb used to treat rashes and is safe for humans [126]. A clinical study demonstrated that honeysuckle can effectively reduce the frequency and intensity of acne-like rash caused by EGFRIs, especially in preventive treatment. [127]. An acne-like rash usually appears within the first 1-2 weeks after treatment with EGFRIs. The preventive treatment with honeysuckle showed promising efficacy in reducing the incidence and severity of EGFRI-induced acne-like rashes. The application of EGFRIs for treatment may improve preventive therapy [128].
Qi Yin San Liang San Decoction (QYSLS) (30 g of raw Astragalus membranaceus, 30 g of honeysuckle, 30 g of angelica, 10 g of raw liquor, and 1 g of centipede) has been used to treat immune-related disorders and adverse effects associated with tumor therapy [129]. To construct a mouse model of adverse skin reactions, gefitinib was used to induce adverse skin responses in mice in vivo. Besides having a preventive effect, treatment with different doses of QYSLS can repair the immunological damage induced by gefitinib and reduce the incidence of cutaneous side effects. HaCaT cells were used as an in vitro adverse reaction model to validate the relevant active chemicals and targets. These findings showed that in HaCaT cells treated with gefitinib, the active components luteolin and quercetin, which were identified through network pharmacology and molecular docking techniques, increased the expression of PTGS2 and MMP9 and decreased the production of CCL2 [130].
Pien Tze Huang is a TCM developed by ancient Chinese imperial physicians. Its main ingredients include musk, bezoar, snake gall, and Sanqi [131]. Many studies have shown that this TCM can treat diseases such as tumors and inflammation [132, 133]. Additionally, Pien Tze Huang can promote skin healing and alleviate many problems related to skin damage [134]. An external ointment based on the traditional formula of Pien Tze Huang combined with oil-based ingredients has been developed. In clinical practice, PZH Unguentum Compositum was used to cure skin damage caused by erlotinib treatment. One patient developed a grade 2 rash after treatment with erlotinib and was clinically treated with PZH Unguentum Compositum. After two weeks, the symptoms were significantly relieved and had almost disappeared without any related side effects. [135]. However, the relevant mechanism needs to be elucidated. In China, Pien Tze Huang, especially PZH Unguentum Compositum, is widely used and is anticipated to play a significant role in the treatment of EGFR-TKI-related rashes. However, further studies are needed on the therapeutic effects, related mechanisms, and clinical application plans.
TCM monomers or compound prescriptions used in combination with anti-EGFR agents to alleviate related side effects.
| Name | structure | EGFRIs | Research Object | Side effects | Effects (Target) | Ref. | |
|---|---|---|---|---|---|---|---|
| In vitro | In vivo | ||||||
| Honeysuckle | Gefitinib | Clinical research | acneiform rash | [128] | |||
| Qi Yin San Liang San Decoction | 30 g of raw Astragalus membranaceus, 30 g of honeysuckle, 30 g of angelica, 10 g of raw liquor, and 1 g of centipede | Gefitinib | HaCaT cell | a mouse model of adverse skin reactions | Skin adverse reactions and immune damage | PTGS2, MMP9 and CCL2 | [130] |
| Pien Tze Huang | musk, bezoar, snake gall, and Sanqi | erlotinib | a case report | skin damage caused by erlotinib | [135] | ||
| Modified Shenling Baizhu powder (SBP) | Baibiandou, Lianzi, Dangshen, Fuling, Yiyiren, Chenpi, Baizhu, Jiegeng, Zhigancao, Sharen and Shanyao | EGFR-TKIs | Clinical research | diarrhea | [139] | ||
Shenling Baizhu powder (SBP) is a classic Chinese medicinal compound. In vivo studies have shown that SBP can alleviate diarrhea caused by pyrrolitinib treatment [136]. Although targeted therapy can achieve high efficacy, the dose needs to be reduced or treatment may even need to be stopped for some patients due to the occurrence of adverse reactions such as diarrhea. This has a strong negative effect on the therapeutic effect of cancer [137]. On the other hand, SBP is safe and has strong therapeutic effects because of its long history. Therefore, some researchers speculate that SBP can alleviate diarrhea caused by targeted therapy [138]. A prospective randomized controlled study using modified SBP confirmed this finding. The results suggested that modified SBP was better than imodium in improving the symptoms of diarrhea, inhibiting the recurrence of diarrhea, and improving the physical condition of patients.[139]. However, further clinical and basic studies are needed to elucidate the mechanism of action of modified SBP and to guide the clinical use of this drug.
To summarize, there is some evidence that TCMs, as a complementary or adjuvant therapy, can alleviate or even eliminate the adverse effects induced during EGFRI-based cancer treatment. However, only a few studies on this topic have been published, and the methodological rigor and precision of the studies are not adequate to draw definitive conclusions.
Clinical evidence of combination therapy
Many researchers have stated the clinical importance of TCMs as stand-alone options for treating cancer. However, in most cases, TCMs are used as part of combination therapy owing to their bioavailability, metabolic instability, potential toxicity, and poor targeting ability. Many studies have emphasized its potential for use in combination with targeted therapy for treating cancer. Moreover, TCM is a patient-specific personalized treatment plan consisting of natural products with different pharmacological effects. Here, we summarized some findings of clinical trials demonstrating the clinical potential of combination therapy. Moreover, by analyzing the results of these clinical trials, we aimed to determine the opportunities provided by and challenges associated with natural products and personalized TCM treatments to further utilize their strengths and avoid their weaknesses for better treatment of patients with lung cancer.
The main active ingredient of ginseng known as Rg3 ginsenoside has antiangiogenic and antitumor activity [75]. A clinical trial at Xinqiao Hospital of the Third Military Medical University investigated the clinical benefits of the combination of ginsenoside and an EGFR-TKI in the treatment of advanced non-small cell carcinoma in patients. The results revealed that the median PFS for patients treated with the combination therapy was 12.4 months (95% CI: 9.2-15.6), whereas it was 9.9 months (95% CI: 8.4-12.6) for patients treated with EGFR-TKIs alone. Their OS values were different but not statistically significant. [140]. These results suggest that the addition of ginsenoside Rg3 to EGFR-TKI reduces drug resistance in the treatment of NSCLC patients with EGFR-active mutations, and that ginsenoside Rg3 could be an important agent for improving median PFS and delaying the acquisition of resistance to EGFR-TKI in patients.
In China, TCM is the most widely used complementary therapy. Fu Zheng Kang Ai Formula has been used as a complementary and alternative therapy for cancer treatment for several years [141]. In a randomized controlled clinical trial, patients who met the inclusion criteria were randomly divided into the gefitinib + FZKA (GF) group and the gefitinib (G) group. The PFS of the GF group was 12.5 months (95% CI: 3.30-21.69), whereas that of the G group was 8.4 months (95% CI: 6.30-10.50). Additionally, the MST of the GF group was 21.5 months (95% CI: 17.28-25.73), whereas that of the G group was 18.3 months (95% CI: 17.97-18.63). These findings suggested that FZKA can prolong the survival of patients with advanced NSCLC treated with gefitinib [142]. Some experimental results have shown that FZKA enhances the apoptosis of lung cancer cells induced by gefitinib through the mitochondrial pathway. These findings suggested a new molecular mechanism by which FZKA delays drug resistance in the treatment of patients with lung cancer [143].
A cohort study found that mPFS was 12.3 months for patients treated with TCM and EGFR-TKI, compared to just 8.9 months for patients treated with EGFR-TKI monotherapy. The results also showed that patients in the combination therapy group had an mOS of 28.2 months compared to 24.2 months in the monotherapy group. The TCM treatments administered are tailored to each patient's specific symptoms, ensuring a personalized approach to care [144]. EGFR-TKIs combined with TCM as first-line therapy resulted in longer mPFS than EGFR-TKIs alone and significantly prolonged patient survival.
The three studies examined the efficacy of combining EGFR-TKI with TCM in patients with cancer, with PFS as the primary endpoint. Due to the small sample size of the experiments, it was not sufficient for OS analysis. And the existing results showed that there was no statistically significant difference in OS. All three studies demonstrated a significant improvement in PFS with EGFR-TKI + TCM compared to EGFR-TKI alone. Despite clinical heterogeneity (TCM composition, study design), all studies consistently showed PFS benefit. The HRs < 1 and P-values < 0.05 indicate a statistically significant reduction in progression/death risk with combination therapy.
Safety Considerations and Adverse Effects
While the combination of TCM monomers and compound prescriptions with EGFRIs offers promising therapeutic benefits, the adverse effects of these combined regimens need to be considered. Many natural products and TCM compounds can interact with EGFRIs, altering their pharmacokinetics or pharmacodynamics. For example, curcumin, an anti-inflammatory and anticancer agent, inhibits cytochrome P450 enzymes, which may increase the plasma concentrations of EGFR-TKIs and exacerbate their side effects [145]. Similarly, berberine, which can effectively overcome MET-induced resistance, may cause gastrointestinal discomfort, potentially worsening the diarrhea commonly associated with EGFRIs [146].
Some TCM monomers, such as bufalin and PPI, have intrinsic toxicity that may cause problems when combined with EGFR-TKIs. Bufalin, derived from toad venom, has a narrow therapeutic window and may cause cardiotoxicity at high doses [147]. Rhein, while effective in reversing resistance, may also cause hepatotoxicity or nephrotoxicity, especially when combined with EGFR-TKIs that already have hepatotoxic effects [70].
Certain TCM formulae, such as the Fu Zheng Kang Ai Formula, are designed to enhance immune function, which may increase the risk of immune-related adverse events when combined with EGFRIs. Additionally, while TCM formulae such as modified SBP can alleviate EGFRI-induced diarrhea, their long-term safety and potential for additive gastrointestinal toxicity require further investigation.
In conclusion, while the combination of TCM monomers and compound prescriptions with EGFRIs holds great promise, the potential adverse effects need to be considered. Future studies should focus on dose optimization, patient-specific factors, and rigorous safety evaluations to ensure the safe and effective use of these combined regimens in clinical practice.
Cost-effectiveness
Evaluating the economic viability of combining TCM monomers and compound prescriptions with EGFRIs will be critical for widespread adoption, especially in resource-limited settings.
One of the primary advantages of combining TCM monomers and compound prescriptions with EGFRIs is the ability to reduce drug resistance and mitigate side effects, both of which contribute to significant cost savings. Natural products such as bufalin and berberine can reverse resistance to EGFR-TKIs, such as osimertinib, thus extending the duration of effective treatment [83, 85]. This reduces the need for costly second-line therapy or experimental drugs, which are often associated with higher prices and limited efficacy. Moreover, by reducing the severity of these side effects, these therapeutic methods can decrease the need for additional medications, hospital visits, and supportive care, reducing overall treatment costs.
TCM monomers or compound prescriptions often exhibit multitarget effects, which can increase the efficacy of EGFRIs while reducing the need for additional targeted therapy. This synergistic effect may reduce the need for combination therapy with other expensive targeted agents, thereby making the treatment more cost-effective.
Although TCM monomers and compound prescriptions are generally perceived as inexpensive because of their relatively low production costs, several factors must be considered. The production of high-quality, standardized TCM natural products or compound prescriptions requires rigorous quality control measures, which can increase costs. Most natural products have poor oral bioavailability, poor intestinal permeability, and high toxicity, necessitating the use of higher doses or advanced delivery systems to achieve therapeutic effects. These modifications can increase production costs but may still be more economical than targeted therapy.
The combination of TCMs with EGFRIs is a promising strategy to increase therapeutic efficacy, reduce drug resistance, and mitigate side effects. While these treatment techniques can decrease costs through reduced treatment durations, fewer side effects, and improved patient outcomes, their economic viability depends on factors such as production costs, bioavailability, and long-term clinical benefits.
Discussion
This review summarizes some TCM monomers and compound prescriptions that can be used in combination with EGFR inhibitors. It is found that the enhancement of the therapeutic effect on cancer mainly occurs through three aspects: reversing drug resistance, generating synergistic effects, and alleviating adverse reactions. Moreover, some clinical trials have verified that the combination has produced good effects on patients. At the same time, we have also summarized the biochemical and molecular characteristics of these TCM monomers and found that they all have poor bioavailability and certain toxic effects and other features. Therefore, several challenges must be addressed to translate these preclinical findings into clinical practice. How to improve bioavailability and the cost brought by the process of improving its utilization are also issues that need to be considered and solved urgently. At the same time, we have to consider whether more serious toxic and side effects will occur when combined, which brings great difficulties to clinical application.
Mechanistic Insights and Therapeutic Potential
TCM monomers and compound prescriptions exhibit diverse mechanisms of action, including inhibition of EGFR-related signaling pathways, reversal of drug resistance, and mitigation of side effects. For instance, bufalin and berberine have shown efficacy in overcoming osimertinib resistance by targeting the Ku70/MCL-1 axis and MET amplification, respectively. Similarly, luteolin and polyphyllin I synergize with EGFR-TKIs to inhibit cancer cell proliferation and metastasis. These findings underscore the multitargeted nature of TCMs, which could complement the singular focus of EGFRIs and improve therapeutic outcomes.
Clinical Evidence and Practical Applications
Clinical studies support the adjunctive use of TCM monomers or compound prescriptions with EGFRIs. For example, ginsenoside Rg3 and Fu Zheng Kang Ai Formula have demonstrated the ability to prolong progression-free survival (PFS) and overall survival (OS) in patients with non-small cell lung cancer (NSCLC). Additionally, TCM formulations like Qi Yin San Liang San Decoction and Pien Tze Huang have been effective in alleviating EGFRI-induced skin toxicity and diarrhea. These results highlight the potential of TCMs to enhance patient quality of life and treatment adherence.
Challenges and Limitations
Bioavailability and Toxicity
The poor bioavailability of many TCM monomers represents a major translational barrier. Most bioactive compounds exhibit limited aqueous solubility, rapid metabolic clearance, and inefficient systemic absorption [148]. For example, curcumin's extensive first-pass metabolism and rapid conjugation in the liver and intestinal wall result in negligible systemic availability [149]. Similarly, berberine's high affinity for P-glycoprotein efflux pumps and extensive phase II metabolism severely limit its oral bioavailability [150]. These pharmacokinetic shortcomings often necessitate the administration of supraphysiological doses to achieve therapeutic effects, which in turn increases the risk of adverse reactions.
Addressing these challenges requires innovative formulation strategies and careful clinical monitoring. Advanced drug delivery systems, including nanoparticle encapsulation and lipid-based formulations, have shown promise in improving the bioavailability of problematic compounds while potentially mitigating their toxicity [151]. Furthermore, the development of structurally modified analogs with optimized pharmacokinetic profiles may offer a path forward. However, these approaches must be balanced against the potential loss of therapeutic efficacy that sometimes accompanies structural modification of TCM monomer scaffolds.
Standardization and Quality Control
The clinical integration of Traditional Chinese Medicine (TCM) and natural product formulations faces significant challenges due to their inherent complexity and variability. Unlike single-compound pharmaceuticals, TCM preparations typically contain hundreds of bioactive constituents that interact in complex, often poorly understood ways.
Modern standardization efforts must therefore develop innovative strategies that respect the integrity of traditional formulations while meeting contemporary pharmaceutical standards. This requires moving beyond simple chemical fingerprinting to develop more sophisticated systems of characterization that can capture the essential therapeutic qualities of these complex mixtures. The challenge lies in identifying which aspects of this complexity are therapeutically essential and which can be standardized without compromising efficacy.
Drug Interactions
While the combination of TCM monomers and compound prescriptions with EGFRIs holds great promise, the potential adverse effects need to be considered. Many natural products and TCM compounds can interact with EGFRIs, altering their pharmacokinetics or pharmacodynamics. Future studies should focus on dose optimization, patient-specific factors, and rigorous safety evaluations to ensure the safe and effective use of these combined regimens in clinical practice.
Future Directions
Optimizing Delivery Systems
The development of advanced delivery systems represents a critical pathway for overcoming the pharmacological limitations of TCM monomers in cancer therapy [152]. Current research focuses on creating innovative formulations that can simultaneously address poor bioavailability, enhance tumor targeting, and minimize systemic toxicity. Nanotechnology-based approaches have emerged as particularly promising, with various nanoparticle platforms being explored to protect bioactive compounds from premature degradation and improve their pharmacokinetic profiles. Surface modification strategies, such as PEGylation or the conjugation of targeting ligands like EGFR antibodies, offer the potential for tumor-specific accumulation while reducing off-target effects. The integration of these delivery technologies must be carefully balanced with preserving the biological activity of complex natural product mixtures, particularly for TCM formulations where multiple active components may require co-delivery.
Personalized Medicine
Tailoring TCM formulations to individual patient profiles could maximize therapeutic benefits while minimizing adverse effects. Ongoing research focuses on identifying verifiable biomarkers that bridge TCM diagnostic patterns with modern molecular pathology, enabling truly evidence-based personalization. When fully realized, this approach promises to enhance EGFR-TKI efficacy while maintaining the holistic benefits of TCM and minimizing herb-drug interaction risks.
Efficacy and Cost Benefits
The combination of TCMs with EGFRIs is a promising strategy to increase therapeutic efficacy, reduce drug resistance, and mitigate side effects. While these treatment techniques can decrease costs through reduced treatment durations, fewer side effects, and improved patient outcomes, their economic viability depends on factors such as production costs, bioavailability, and long-term clinical benefits.
Conclusion
EGFR is one of the most promising and appealing clinical and scientific targets in cancer therapy, and EGFR-targeted therapy is considered to be a potential anticancer treatment strategy. The groundbreaking advancements in cancer treatment have been greatly aided by EGFR TKIs and mAbs. For example, some options limit the scope of use to cancer types with the overexpression of EGFR, and most patients become resistant to therapy within a year of starting treatment, leading to severe side effects, including rashes. These changes significantly decrease the effectiveness of medications. To increase antitumor effectiveness, more potent, safe, and inexpensive supplements, synergists, or additional anti-EGFR medications should be developed.
TCMs are a potential therapeutic approach to address these issues because of their diverse functions, including but not limited to blocking EGFR-related signaling pathways, sensitizing EGFR-targeted drugs, reversing resistance to EGFR-targeted drugs, and alleviating the side effects of targeted therapy. Owing to the unique pharmacological and biological activities of traditional Chinese medicine monomers, drug development based on the structure and properties of their components occupies an important position in the field of contemporary new drug development. Numerous traditional Chinese medicine monomers have been used in recent years for targeted EGFR therapy. Most of these drugs are only used as a pretreatment or supplement to EGFR-TKIs and mAbs, and studies on this topic are in their early stages. Several issues still need to be resolved. There are major obstacles to its widespread use in clinical practice due to its limited oral bioavailability, poor biological activity, severe toxic side effects, and limited supplies. Owing to the complexity of the components of natural compounds, in-depth studies on their pharmacological targets and mechanisms of action are challenging, which makes their optimal application in cancer therapy difficult to implement. Owing to the complexity of their pharmacology and individual differences, it is challenging to standardize the study of Chinese herbal remedies. Many traditional Chinese medicine monomers, such as curcumin and berberine, which have been extensively studied for their anticancer properties, can be used for EGFR-targeted therapy. Curcumin is very safe in humans, even at high doses, but its low bioavailability greatly limits its clinical use [69]. Berberine is also a promising traditional Chinese medicine monomer, but its anticancer efficacy is greatly hindered by its low bioavailability and water solubility, as well as its tendency to induce allergic reactions when it is injected intramuscularly and intravenously [61]. Therefore, better utilization of these TCMs is needed to treat cancer.
Natural products, especially many TCM products or their compound formulae that have a unique clinical application history and foundation in China should be continuously innovated using science and technology. These products should be combined with modern medicine to realize the innovation of Chinese medicines, the advancement of the pharmaceutical industry, and the health and well-being of people.
With continuous in-depth research on EGFR, TCMs that target EGFR will have greater potential for utilization in cancer treatment.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81473469); Belt and Road International Cooperation Project (No. 20400750600); Construction of an innovative flagship hospital of integrated traditional Chinese and Western medicine (No. ZY (2021-2023)-0205-05 and ZXXT-202203); The state administration of traditional Chinese medicine, a new type of coronavirus infection of traditional Chinese medicine emergency special (2023 ZYLCY J02-6). Graphic abstract support was provided by Figdraw.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63
2. Cao W, Qin K, Li F, Chen W. Comparative study of cancer profiles between 2020 and 2022 using global cancer statistics (GLOBOCAN). J Natl Cancer Cent. 2024;4:128-34
3. Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX. et al. [Cancer incidence and mortality in China, 2022]. Zhonghua Zhong Liu Za Zhi. 2024;46:221-31
4. Seres M, Spacayova K, Sulova Z, Spaldova J, Breier A, Pavlikova L. Dynamic Multilevel Regulation of EGFR, KRAS, and MYC Oncogenes: Driving Cancer Cell Proliferation Through (Epi)Genetic and Post-Transcriptional/Translational Pathways. Cancers (Basel). 2025;17:248
5. Zhou F, Guo H, Xia Y, Le X, Tan DSW, Ramalingam SS. et al. The changing treatment landscape of EGFR-mutant non-small-cell lung cancer. Nat Rev Clin Oncol. 2025;22:95-116
6. Rajaram P, Chandra P, Ticku S, Pallavi BK, Rudresh KB, Mansabdar P. Epidermal growth factor receptor: Role in human cancer. Indian J Dent Res. 2017;28:687-94
7. Pasha MCTY, Rahamathulla M. et al. Epidermal growth factor receptors unveiled: a comprehensive survey on mutations, clinical insights of global inhibitors, and emergence of heterocyclic derivatives as EGFR inhibitors. J of Drug Targeting. 2025;33:933-951
8. Deng R, Zong GF, Wang X, Yue BJ, Cheng P, Tao RZ. et al. Promises of natural products as clinical applications for cancer. Biochim Biophys Acta Rev Cancer. 2025;1880:189241
9. Sati P, Sharma E, Dhyani P, Attri DC, Rana R, Kiyekbayeva L. et al. Paclitaxel and its semi-synthetic derivatives: comprehensive insights into chemical structure, mechanisms of action, and anticancer properties. Eur J Med Res. 2024;29:90
10. Sun J, Luo D, Li H, Zhang D, Liu Y, Jin S. et al. The Effect of Fuzheng Yiai Decoction on the Transdifferentiation of Lung Adenocarcinoma in EGFR-TKI-Resistant Mice. Can Respir J. 2024;2024:8827810
11. Li S, Chen X, Shi H, Yi M, Xiong B, Li T. Tailoring traditional Chinese medicine in cancer therapy. Mol Cancer. 2025;24:27
12. Wang Z. ErbB Receptors and Cancer. Methods Mol Biol. 2017;1652:3-35
13. Roskoski R Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34-74
14. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117-34
15. Walton GM, Chen WS, Rosenfeld MG, Gill GN. Analysis of deletions of the carboxyl terminus of the epidermal growth factor receptor reveals self-phosphorylation at tyrosine 992 and enhanced in vivo tyrosine phosphorylation of cell substrates. J Biol Chem. 1990;265:1750-4
16. Ishii S, Xu YH, Stratton RH, Roe BA, Merlino GT, Pastan I. Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc Natl Acad Sci U S A. 1985;82:4920-4
17. Reiter JL, Maihle NJ. A 1.8 kb alternative transcript from the human epidermal growth factor receptor gene encodes a truncated form of the receptor. Nucleic Acids Res. 1996;24:4050-6
18. Reiter J, Maihle NJ. Characterization and expression of novel 60-kDa and 110-kDa EGFR isoforms in human placenta. Ann N Y Acad Sci. 2003;995:39-47
19. Chakkarappan SR, Umadharshini KV, Dhamodharan S, Rose MM, Gopu G, Murugan AK. et al. Super enhancer loci of EGFR regulate EGFR variant 8 through enhancer RNA and strongly associate with survival in HNSCCs. Mol Genet Genomics. 2024;299:3
20. Kamata T, Feramisco JR. Epidermal growth factor stimulates guanine nucleotide binding activity and phosphorylation of ras oncogene proteins. Nature. 1984;310:147-50
21. Hossain MA. Targeting the RAS upstream and downstream signaling pathway for cancer treatment. Eur J Pharmacol. 2024;979:176727
22. Dempke WC, Heinemann V. Ras mutational status is a biomarker for resistance to EGFR inhibitors in colorectal carcinoma. Anticancer Res. 2010;30:4673-7
23. He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW. et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6:425
24. Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin Cancer Biol. 2022;80:1-17
25. Sabbah DA, Hajjo R, Sweidan K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr Top Med Chem. 2020;20:815-34
26. Fontana F, Giannitti G, Marchesi S, Limonta P. The PI3K/Akt Pathway and Glucose Metabolism: A Dangerous Liaison in Cancer. Int J Biol Sci. 2024;20:3113-25
27. Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102:311-9
28. Li YJ, Zhang C, Martincuks A, Herrmann A, Yu H. STAT proteins in cancer: orchestration of metabolism. Nat Rev Cancer. 2023;23:115-34
29. Hu Y, Dong Z, Liu K. Unraveling the complexity of STAT3 in cancer: molecular understanding and drug discovery. J Exp Clin Cancer Res. 2024;43:23
30. Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218-30
31. Xie Z, Peng J, Pennypacker SD, Chen Y. Critical role for the catalytic activity of phospholipase C-gamma1 in epidermal growth factor-induced cell migration. Biochem Biophys Res Commun. 2010;399:425-8
32. Xie Z, Chen Y, Liao EY, Jiang Y, Liu FY, Pennypacker SD. Phospholipase C-gamma1 is required for the epidermal growth factor receptor-induced squamous cell carcinoma cell mitogenesis. Biochem Biophys Res Commun. 2010;397:296-300
33. Shaban N, Kamashev D, Emelianova A, Buzdin A. Targeted Inhibitors of EGFR: Structure, Biology, Biomarkers, and Clinical Applications. Cells. 2023;13:47
34. Quatrale AE, Petriella D, Porcelli L, Tommasi S, Silvestris N, Colucci G. et al. Anti-EGFR monoclonal antibody in cancer treatment: in vitro and in vivo evidence. Front Biosci (Landmark Ed). 2011;16:1973-85
35. Favorito V, Ricciotti I, De Giglio A, Fabbri L, Seminerio R, Di Federico A. et al. Non-small cell lung cancer: an update on emerging EGFR-targeted therapies. Expert Opin Emerg Drugs. 2024;29:139-54
36. Ayati A, Moghimi S, Salarinejad S, Safavi M, Pouramiri B, Foroumadi A. A review on progression of epidermal growth factor receptor (EGFR) inhibitors as an efficient approach in cancer targeted therapy. Bioorg Chem. 2020;99:103811
37. Douillard JY, Ostoros G, Cobo M, Ciuleanu T, McCormack R, Webster A. et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer. 2014;110:55-62
38. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239-46
39. Yasuda H, Park E, Yun CH, Sng NJ, Lucena-Araujo AR, Yeo WL. et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5:216ra177
40. Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10-i9
41. Ahmad I, Patel HM. From challenges to solutions: A review of fourth-generation EGFR tyrosine kinase inhibitors to overcome the C797S triple mutation in non-small cell lung cancer. Eur J Med Chem. 2025;284:117178
42. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160-74
43. Sentana-Lledo D, Academia E, Viray H, Rangachari D, Kobayashi SS, VanderLaan PA. et al. EGFR exon 20 insertion mutations and ERBB2 mutations in lung cancer: a narrative review on approved targeted therapies from oral kinase inhibitors to antibody-drug conjugates. Transl Lung Cancer Res. 2023;12:1590-610
44. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92
45. Saoudi González N, Ros J, Baraibar I, Salvà F, Rodríguez-Castells M, Alcaraz A. et al. Cetuximab as a Key Partner in Personalized Targeted Therapy for Metastatic Colorectal Cancer. Cancers (Basel). 2024;16:412
46. Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D. et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532-6
47. Lu X, Li Y, Li Y, Zhang X, Shi J, Feng H. et al. Prognostic and predictive biomarkers for anti-EGFR monoclonal antibody therapy in RAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2023;23:1117
48. Chua YJ, Cunningham D. Panitumumab. Drugs Today (Barc). 2006;42:711-9
49. Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M. et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-34
50. Liang R, Yang L, Zhu X. Nimotuzumab, an Anti-EGFR Monoclonal Antibody, in the Treatment of Nasopharyngeal Carcinoma. Cancer Control. 2021;28:1073274821989301
51. Zhou C, Tang KJ, Cho BC, Liu B, Paz-Ares L, Cheng S. et al. Amivantamab plus Chemotherapy in NSCLC with EGFR Exon 20 Insertions. N Engl J Med. 2023;389:2039-51
52. Díaz-Serrano A, Sánchez-Torre A, Paz-Ares L. Necitumumab for the treatment of advanced non-small-cell lung cancer. Future Oncol. 2019;15:705-16
53. Singha NC, Nekoroski T, Zhao C, Symons R, Jiang P, Frost GI. et al. Tumor-associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol Cancer Ther. 2015;14:523-32
54. Dong Q, Dai G, Quan N, Tong Q. Role of natural products in cardiovascular disease. Mol Cell Biochem. 2025;480:733-45
55. Gao K, Lv L, Li Z, Wang C, Zhang J, Qiu D. et al. Natural Products in the Prevention of Degenerative Bone and Joint Diseases: Mechanisms Based on the Regulation of Ferroptosis. Phytother Res. 2025;39:162-88
56. Su XL, Wang JW, Che H, Wang CF, Jiang H, Lei X. et al. Clinical application and mechanism of traditional Chinese medicine in treatment of lung cancer. Chin Med J (Engl). 2020;133:2987-97
57. Chen L, Chen WD, Xu YX, Ren YY, Zheng C, Lin YY. et al. Strategies for enhancing non-small cell lung cancer treatment: Integrating Chinese herbal medicines with epidermal growth factor receptor-tyrosine kinase inhibitors therapy. Eur J Pharmacol. 2024;980:176871
58. Yang Z, Liu J, Huang Q, Zhang Z, Zhang J, Pan Y. et al. Radiosynthesis and pharmacokinetics of [(18)F]fluoroethyl bufalin in hepatocellular carcinoma-bearing mice. Onco Targets Ther. 2017;10:329-38
59. Soumoy L, Ghanem GE, Saussez S, Journe F. Bufalin for an innovative therapeutic approach against cancer. Pharmacol Res. 2022;184:106442
60. Feng X, Wang K, Cao S, Ding L, Qiu F. Pharmacokinetics and Excretion of Berberine and Its Nine Metabolites in Rats. Front Pharmacol. 2020;11:594852
61. Cui Y, Zhou Q, Jin M, Jiang S, Shang P, Dong X. et al. Research progress on pharmacological effects and bioavailability of berberine. Naunyn Schmiedebergs Arch Pharmacol. 2024;397:8485-514
62. Shi M, Chen Z, Gong H, Peng Z, Sun Q, Luo K. et al. Luteolin, a flavone ingredient: Anticancer mechanisms, combined medication strategy, pharmacokinetics, clinical trials, and pharmaceutical researches. Phytother Res. 2024;38:880-911
63. Liu H, Chang G, Wang W, Ji Z, Cui J, Peng Y. Pharmacokinetics, Prostate Distribution and Metabolic Characteristics of Four Representative Flavones after Oral Administration of the Aerial Part of Glycyrrhiza uralensis in Rats. Molecules. 2022;27:3245
64. Zhu H, Zhu SC, Shakya S, Mao Q, Ding CH, Long MH. et al. Study on the pharmacokinetics profiles of polyphyllin I and its bioavailability enhancement through co-administration with P-glycoprotein inhibitors by LC-MS/MS method. J Pharm Biomed Anal. 2015;107:119-24
65. Ke F, Zhang R, Chen R, Guo X, Song C, Gao X. et al. The role of Rhizoma Paridis saponins on anti-cancer: The potential mechanism and molecular targets. Heliyon. 2024;10:e37323
66. Sun Q, Gong T, Liu M, Ren S, Yang H, Zeng S. et al. Shikonin, a naphthalene ingredient: Therapeutic actions, pharmacokinetics, toxicology, clinical trials and pharmaceutical researches. Phytomedicine. 2022;94:153805
67. Huang S, Yu L, Shen P. Simultaneous Quantitative Analysis of Shikonin and Deoxyshikonin in Rat Plasma by Rapid LC-ESI-MS-MS. Chromatographia. 2010;72:63-9
68. Hu Y, Sheng Y, Liu P, Sun J, Tang L. The pharmacokinetics and tissue distribution of curcumin following inhalation administration in rats-A comparative analysis with oral and intravenous routes. Biomed Chromatogr. 2024;38:e6003
69. Liu S, Liu J, He L, Liu L, Cheng B, Zhou F. et al. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules. 2022;27:4400
70. Fu Y, Yang L, Liu L, Kong L, Sun H, Sun Y. et al. Rhein: An Updated Review Concerning Its Biological Activity, Pharmacokinetics, Structure Optimization, and Future Pharmaceutical Applications. Pharmaceuticals (Basel). 2024;17:1665
71. Zhang S, Mao B, Cui S, Zhang Q, Zhao J, Tang X. et al. Absorption, metabolism, bioactivity, and biotransformation of epigallocatechin gallate. Crit Rev Food Sci Nutr. 2024;64:6546-66
72. Xie L, Zhao YX, Zheng Y, Li XF. The pharmacology and mechanisms of platycodin D, an active triterpenoid saponin from Platycodon grandiflorus. Front Pharmacol. 2023;14:1148853
73. Kwon M, Ji HK, Goo SH, Nam SJ, Kang YJ, Lee E. et al. Involvement of intestinal efflux and metabolic instability in the pharmacokinetics of platycodin D in rats. Drug Metab Pharmacokinet. 2017;32:248-54
74. Ma LY, Zhang YB, Zhou QL, Yang YF, Yang XW. Simultaneous Determination of Eight Ginsenosides in Rat Plasma by Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry: Application to Their Pharmacokinetics. Molecules. 2015;20:21597-608
75. Liu Z, Liu T, Li W, Li J, Wang C, Zhang K. Insights into the antitumor mechanism of ginsenosides Rg3. Mol Biol Rep. 2021;48:2639-52
76. Cheng Z, Cui H, Wang Y, Yang J, Lin C, Shi X. et al. The advance of the third-generation EGFR-TKI in the treatment of non-small cell lung cancer (Review). Oncol Rep. 2024;51:16
77. Tian X, Gu T, Lee MH, Dong Z. Challenge and countermeasures for EGFR targeted therapy in non-small cell lung cancer. Biochim Biophys Acta Rev Cancer. 2022;1877:188645
78. Fukuda A, Okuma Y. From Rarity to Reality: Osimertinib's Promising Horizon in Treating Uncommon EGFR Mutations in Non-Small Cell Lung Cancer. Clin Cancer Res. 2024;30:3128-36
79. Ma J, Huang L, Han C. Expert consensus on the use of third-generation EGFR-TKIs in EGFR-mutated advanced non-small cell lung cancer with various T790M mutations post-resistance to first-/second-generation EGFR-TKIs. Ther Adv Med Oncol. 2024;16:17588359241289648
80. Kaur G, Devi S, Sharma A, Sood P. Pharmacological insights and role of bufalin (bufadienolides) in inflammation modulation: a narrative review. Inflammopharmacology. 2024;32:3057-77
81. Dong Y, Yin S, Li J, Jiang C, Ye M, Hu H. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis. 2011;16:394-403
82. Liu J, Wei L, Miao Q, Zhan S, Chen P, Liu W. et al. MDM2 drives resistance to Osimertinib by contextually disrupting FBW7-mediated destruction of MCL-1 protein in EGFR mutant NSCLC. J Exp Clin Cancer Res. 2024;43:302
83. Cao F, Gong YB, Kang XH, Lu ZH, Wang Y, Zhao KL. et al. Degradation of MCL-1 by bufalin reverses acquired resistance to osimertinib in EGFR-mutant lung cancer. Toxicol Appl Pharmacol. 2019;379:114662
84. Sajeev A, Sailo B, Unnikrishnan J, Talukdar A, Alqahtani MS, Abbas M. et al. Unlocking the potential of Berberine: Advancing cancer therapy through chemosensitization and combination treatments. Cancer Lett. 2024;597:217019
85. Chen Z, Vallega KA, Chen H, Zhou J, Ramalingam SS, Sun SY. The natural product berberine synergizes with osimertinib preferentially against MET-amplified osimertinib-resistant lung cancer via direct MET inhibition. Pharmacol Res. 2022;175:105998
86. Mahwish Imran M, Naeem H Hussain M, Alsagaby SA Al Abdulmonem W. et al. Antioxidative and Anticancer Potential of Luteolin: A Comprehensive Approach Against Wide Range of Human Malignancies. Food Sci Nutr. 2025;13:e4682
87. Juszczak AM, Wöelfle U, Končić MZ, Tomczyk M. Skin cancer, including related pathways and therapy and the role of luteolin derivatives as potential therapeutics. Med Res Rev. 2022;42:1423-62
88. Huang G, Liu X, Jiang T, Cao Y, Sang M, Song X. et al. Luteolin overcomes acquired resistance to osimertinib in non-small cell lung cancer cells by targeting the HGF-MET-Akt pathway. Am J Cancer Res. 2023;13:4145-62
89. Feng F, Cheng P, Wang C, Wang Y, Wang W. Polyphyllin I and VII potentiate the chemosensitivity of A549/DDP cells to cisplatin by enhancing apoptosis, reversing EMT and suppressing the CIP2A/AKT/mTOR signaling axis. Oncol Lett. 2019;18:5428-36
90. Wu H, Zhao X, Hochrein SM, Eckstein M, Gubert GF, Knöpper K. et al. Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1α-mediated glycolytic reprogramming. Nat Commun. 2023;14:6858
91. Meng S, Wang G, Lu Y, Fan Z. Functional cooperation between HIF-1α and c-Jun in mediating primary and acquired resistance to gefitinib in NSCLC cells with activating mutation of EGFR. Lung Cancer. 2018;121:82-90
92. Zhang D, Tian X, Wang Y, Liu F, Zhang J, Wang H. et al. Polyphyllin I ameliorates gefitinib resistance and inhibits the VEGF/VEGFR2/p38 pathway by targeting HIF-1a in lung adenocarcinoma. Phytomedicine. 2024;129:155690
93. Lai L, Shen Q, Wang Y, Chen L, Lai J, Wu Z. et al. Polyphyllin I reverses the resistance of osimertinib in non-small cell lung cancer cell through regulation of PI3K/Akt signaling. Toxicol Appl Pharmacol. 2021;419:115518
94. Li RJ, Xu JJ, Zhang ZH, Chen MW, Liu SX, Yang C. et al. Rhein ameliorates transverse aortic constriction-induced cardiac hypertrophy via regulating STAT3 and p38 MAPK signaling pathways. Front Pharmacol. 2022;13:940574
95. Guo H, Hu Z, Yang X, Yuan Z, Gao Y, Chen J. et al. STAT3 inhibition enhances gemcitabine sensitivity in pancreatic cancer by suppressing EMT, immune escape and inducing oxidative stress damage. Int Immunopharmacol. 2023;123:110709
96. Zhao T, Jin F, Xiao D, Wang H, Huang C, Wang X. et al. IL-37/ STAT3/ HIF-1α negative feedback signaling drives gemcitabine resistance in pancreatic cancer. Theranostics. 2020;10:4088-100
97. Yang L, Lin S, Kang Y, Xiang Y, Xu L, Li J. et al. Rhein sensitizes human pancreatic cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. J Exp Clin Cancer Res. 2019;38:31
98. Zhuang Y, Bai Y, Hu Y, Guo Y, Xu L, Hu W. et al. Rhein sensitizes human colorectal cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. Onco Targets Ther. 2019;12:5281-91
99. Xi Z, Dai R, Ze Y, Jiang X, Liu M, Xu H. Traditional Chinese medicine in lung cancer treatment. Mol Cancer. 2025;24:57
100. Zhao W, Liu Z, Zhang Z, Chen Z, Liu J, Sun P. et al. Si Jun Zi decoction inhibits the growth of lung cancer by reducing the expression of PD-L1 through TLR4/MyD88/NF-κB pathway. J Ethnopharmacol. 2024;318:116948
101. Shang L, Wang Y, Li J, Zhou F, Xiao K, Liu Y. et al. Mechanism of Sijunzi Decoction in the treatment of colorectal cancer based on network pharmacology and experimental validation. J Ethnopharmacol. 2023;302:115876
102. Wang Z, Li T, Li R, Cao B, Wang S, Fei X. et al. Sijunzi Tang improves gefitinib resistance by regulating glutamine metabolism. Biomed Pharmacother. 2023;167:115438
103. Zhang L, Song J, Kong L, Yuan T, Li W, Zhang W. et al. The strategies and techniques of drug discovery from natural products. Pharmacol Ther. 2020;216:107686
104. Miao K, Liu W, Xu J, Qian Z, Zhang Q. Harnessing the power of traditional Chinese medicine monomers and compound prescriptions to boost cancer immunotherapy. Front Immunol. 2023;14:1277243
105. Duenas-Gonzalez A, Gonzalez-Fierro A, Bornstein-Quevedo L, Gutierrez-Delgado F, Kast RE, Chavez-Blanco A. et al. Multitargeted polypharmacotherapy for cancer treatment. theoretical concepts and proposals. Expert Rev Anticancer Ther. 2024;24:665-77
106. Zhu Y, Ouyang Z, Du H, Wang M, Wang J, Sun H. et al. New opportunities and challenges of natural products research: When target identification meets single-cell multiomics. Acta Pharm Sin B. 2022;12:4011-39
107. Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. 2018;13:20
108. Sun W, Yang Y, Wang C, Liu M, Wang J, Qiao S. et al. Epigallocatechin-3-gallate at the nanoscale: a new strategy for cancer treatment. Pharm Biol. 2024;62:676-90
109. Haque A, Rahman MA, Chen ZG, Saba NF, Khuri FR, Shin DM. et al. Combination of erlotinib and EGCG induces apoptosis of head and neck cancers through posttranscriptional regulation of Bim and Bcl-2. Apoptosis. 2015;20:986-95
110. Moon DO. Curcumin in Cancer and Inflammation: An In-Depth Exploration of Molecular Interactions, Therapeutic Potentials, and the Role in Disease Management. Int J Mol Sci. 2024 25
111. Liu C, Rokavec M, Huang Z, Hermeking H. Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023;30:1771-85
112. Mortezaee K, Salehi E, Mirtavoos-Mahyari H, Motevaseli E, Najafi M, Farhood B. et al. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J Cell Physiol. 2019;234:12537-50
113. Cao JF, Zhang X, Xia Q, Hang K, Men J, Tian J. et al. Insights into curcumin's anticancer activity in pancreatic ductal adenocarcinoma: Experimental and computational evidence targeting HRAS, CCND1, EGFR and AKT1. Bioorg Chem. 2025;157:108264
114. Hussain Y, Islam L, Khan H, Filosa R, Aschner M, Javed S. Curcumin-cisplatin chemotherapy: A novel strategy in promoting chemotherapy efficacy and reducing side effects. Phytother Res. 2021;35:6514-29
115. Chen CF, Lu CC, Chiang JH, Chiu HY, Yang JS, Lee CY. et al. Synergistic inhibitory effects of cetuximab and curcumin on human cisplatin-resistant oral cancer CAR cells through intrinsic apoptotic process. Oncol Lett. 2018;16:6323-30
116. Guo C, He J, Song X, Tan L, Wang M, Jiang P. et al. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol Res. 2019;149:104463
117. Wu B, Liang Z, Lan H, Teng X, Wang C. The role of PKM2 in cancer progression and its structural and biological basis. J Physiol Biochem. 2024;80:261-75
118. Tang JC, Ren YG, Zhao J, Long F, Chen JY, Jiang Z. Shikonin enhances sensitization of gefitinib against wild-type EGFR non-small cell lung cancer via inhibition PKM2/stat3/cyclinD1 signal pathway. Life Sci. 2018;204:71-7
119. Lu Z, Song W, Zhang Y, Wu C, Zhu M, Wang H. et al. Combined Anti-Cancer Effects of Platycodin D and Sorafenib on Androgen-Independent and PTEN-Deficient Prostate Cancer. Front Oncol. 2021;11:648985
120. Ye Y, Xie Y, Pei L, Jiang Z, Wu C, Liu S. Platycodin D induces neutrophil apoptosis by downregulating PD-L1 expression to inhibit breast cancer pulmonary metastasis. Int Immunopharmacol. 2023;115:109733
121. Liu Y, Tian S, Yi B, Feng Z, Chu T, Liu J. et al. Platycodin D sensitizes KRAS-mutant colorectal cancer cells to cetuximab by inhibiting the PI3K/Akt signaling pathway. Front Oncol. 2022;12:1046143
122. Lv Y, Wang W, Liu Y, Yi B, Chu T, Feng Z. et al. Platycodin D represses β-catenin to suppress metastasis of cetuximab-treated KRAS wild-type colorectal cancer cells. Clin Exp Metastasis. 2023;40:339-56
123. Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15:283-98
124. Li Z, Chu H, Huang S, Li R, Qiu B, Tan F. et al. Risk of treatment-related toxicity from EGFR tyrosine kinase inhibitors: a systematic review and network meta-analysis of randomized clinical trials in EGFR-mutant non-small cell lung cancer. J Thorac Dis. 2024;16:6579-94
125. Rhee J, Oishi K, Garey J, Kim E. Management of rash and other toxicities in patients treated with epidermal growth factor receptor-targeted agents. Clin Colorectal Cancer. 2005;5(Suppl 2):S101-6
126. Sharma AK, Sati DM, Murti Y, Ved A, Yadav S, Singh A. et al. A comprehensive review on Chinese honeysuckle (Qusqualis indica): A Traditional Chinese plant. Toxicol Rep. 2024;13:101768
127. Warner GT, Plosker GL. Clindamycin/benzoyl peroxide gel: a review of its use in the management of acne. Am J Clin Dermatol. 2002;3:349-60
128. Liu Z, Tian T, Wang B, Lu D, Ruan J, Shan J. Reducing Acneiform Rash Induced by EGFR Inhibitors with Honeysuckle Therapy: A Prospective, Randomized, Controlled Study. Front Pharmacol. 2022;13:835166
129. Miao J, Peng L. 10 cases study of EGFR inhibitors related skin rash with traditional Chinesedrug regimen-“San Liang San”. China Modern Medicine. 2009;16:66-8
130. Wang Y, Zhang Y, Ding C, Jia C, Zhang H, Peng T. et al. Exploration of the Potential Mechanism of Qi Yin San Liang San Decoction in the Treatment of EGFRI-Related Adverse Skin Reactions Using Network Pharmacology and In Vitro Experiments. Front Oncol. 2022;12:790713
131. Zhao HT. From traditional Chinese medicine formulations to effective anticancer agents: Insights from Calculus bovis. World J Gastroenterol. 2024;30:4011-3
132. Liu W, Wang L, Yuan Q, Hao W, Wang Y, Wu D. et al. Agaricus bisporus polysaccharides ameliorate ulcerative colitis in mice by modulating gut microbiota and its metabolism. Food Funct. 2024;15:1191-207
133. Gou H, Su H, Liu D, Wong CC, Shang H, Fang Y. et al. Traditional Medicine Pien Tze Huang Suppresses Colorectal Tumorigenesis Through Restoring Gut Microbiota and Metabolites. Gastroenterology. 2023;165:1404-19
134. Yan Y, Liu X, Zhuang Y, Zhai Y, Yang X, Yang Y. et al. Pien Tze Huang accelerated wound healing by inhibition of abnormal fibroblast apoptosis in Streptozotocin induced diabetic mice. J Ethnopharmacol. 2020;261:113203
135. Zhang M, Li Q, Sun Y. Skin rash caused by EGFR-TKI could be treated successfully by Pien Tze Huang Unguentum Compositum: a case report. J Biomed Res. 2022;36:440-5
136. Lai J, Jiang F, Zhuo X, Xu X, Liu L, Yin K. et al. Effects of Shenling Baizhu powder on pyrotinib-induced diarrhea: analysis of gut microbiota, metabonomics, and network pharmacology. Chin Med. 2022;17:140
137. Cárdenas-Fernández D, Soberanis Pina P, Turcott JG, Chávez-Tapia N, Conde-Flores E, Cardona AF. et al. Management of diarrhea induced by EGFR-TKIs in advanced lung adenocarcinoma. Ther Adv Med Oncol. 2023;15:17588359231192396
138. Yu W, Wang G, Lu C, Liu C, Jiang L, Jiang Z. et al. Pharmacological mechanism of Shenlingbaizhu formula against experimental colitis. Phytomedicine. 2022;98:153961
139. Ming W, Yun Z. Clinical value of modified Shenling Baizhu powder in treating targeted therapy-induced diarrhea in non-small cell lung cancer. J Tradit Chin Med. 2024;44:1000-5
140. Li Y, Wang Y, Niu K, Chen X, Xia L, Lu D. et al. Clinical benefit from EGFR-TKI plus ginsenoside Rg3 in patients with advanced non-small cell lung cancer harboring EGFR active mutation. Oncotarget. 2016;7:70535-45
141. Yang XB, Wu WY, Long SQ, Deng H, Pan ZQ. Effect of gefitinib plus Chinese herbal medicine (CHM) in patients with advanced non-small-cell lung cancer: a retrospective case-control study. Complement Ther Med. 2014;22:1010-8
142. Yang XB, Chai XS, Wu WY, Long SQ, Deng H, Pan ZQ. et al. Gefitinib plus Fuzheng Kang'ai Formula () in Patients with Advanced Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor Mutation: A Randomized Controlled Trial. Chin J Integr Med. 2018;24:734-40
143. Li L, Wang S, Zheng F, Wu W, Hann SS. Chinese herbal medicine Fuzheng Kang-Ai decoction sensitized the effect of gefitinib on inhibition of human lung cancer cells through inactivating PI3-K/Akt -mediated suppressing MUC1 expression. J Ethnopharmacol. 2016;194:918-29
144. Wang Y, Wu G, Li R, Luo Y, Huang X, He L. et al. Chinese Medicine Combined With EGFR-TKIs Prolongs Progression-Free Survival and Overall Survival of Non-small Cell Lung Cancer (NSCLC) Patients Harboring EGFR Mutations, Compared with the Use of TKIs Alone. Front Public Health. 2021;9:677862
145. Rodríguez Castaño P, Parween S, Pandey AV. Bioactivity of Curcumin on the Cytochrome P450 Enzymes of the Steroidogenic Pathway. Int J Mol Sci. 2019;20:4606
146. Li Z, Wang Y, Xu Q, Ma J, Li X, Yan J. et al. Berberine and health outcomes: An umbrella review. Phytother Res. 2023;37:2051-66
147. Li M, Wang XJ, Zhao Q, Wang JX, Xing HY, Zhang YZ. et al. Bufalin-induced cardiotoxicity: new findings into mechanisms. Chin J Nat Med. 2020;18:550-60
148. Wu J, Wei Z, Cheng P, Qian C, Xu F, Yang Y. et al. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics. 2020;10:10665-79
149. Cai Y, Huang C, Zhou M, Xu S, Xie Y, Gao S. et al. Role of curcumin in the treatment of acute kidney injury: research challenges and opportunities. Phytomedicine. 2022;104:154306
150. Zhang YT, Yu YQ, Yan XX, Wang WJ, Tian XT, Wang L. et al. Different structures of berberine and five other protoberberine alkaloids that affect P-glycoprotein-mediated efflux capacity. Acta Pharmacol Sin. 2019;40:133-42
151. Zheng Y, Wang Y, Xia M, Gao Y, Zhang L, Song Y. et al. The combination of nanotechnology and traditional Chinese medicine (TCM) inspires the modernization of TCM: review on nanotechnology in TCM-based drug delivery systems. Drug Deliv Transl Res. 2022;12:1306-25
152. Zhao B, Lin H, Jiang X, Li W, Gao Y, Li M. et al. Exosome-like nanoparticles derived from fruits, vegetables, and herbs: innovative strategies of therapeutic and drug delivery. Theranostics. 2024;14:4598-621
Author contact
![]() Corresponding author: Lihong Fan, E-mail: fanlihcom.
Corresponding author: Lihong Fan, E-mail: fanlihcom.

 Global reach, higher impact
Global reach, higher impact