Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(8):2492-2502. doi:10.7150/jca.94492 This issue Cite
Research Paper
In vitro antioxidant, anticholinesterase, and antiproliferative activities of methanol extracts of Crateva religiosa bark
1. Department of Pharmacy, Comilla University, Cumilla-3506, Bangladesh.
2. Institute for Biomedical Science, Georgia State University, Atlanta, Georgia.
3. Department of Chemistry, Comilla University, Cumilla-3506, Bangladesh.
4. Department of Pharmaceutics, College of Pharmacy, PO BOX 2457, King Saud University, Riyadh, 11451, Saudi Arabia.
5. Vitiligo Research Chair, Department of Dermatology, College of Medicine, King Saud University, Riyadh 11451, Saudi Arabia.
6. Department of Chemistry, University of Chittagong, Chittagong, 4331, Bangladesh.
7. Department of Chemical Engineering and Energy Sustainability, Faculty of Engineering, University Malaysia Sarawak, Malaysia.
*These authors contributed equally to this work.
Received 2024-1-20; Accepted 2025-3-23; Published 2025-4-28
Abstract

Background: C. religiosa has traditionally been applied to treat heart disease, chronic weight loss, improved digestion, memory loss, convulsion and epilepsy, psychological problems, and neurologic pains.
Purpose: Prior studies have already elucidated the potential therapeutic effects of C. religiosa. However, in this work, the bark extract of C. religiosa was studied systematically to investigate its antioxidant, anticholinesterase, and antiproliferative activities, focusing on potential applications in treating Alzheimer's disease (AD) and cancer.
Study Design and Methods: The dried coarse powder of C. religiosa bark was cold-extracted in methanol and evaporated to dryness. It was then successively fractionated into petroleum ether (PEF), dichloromethane (DMF), and ethyl acetate (EEF) fractions. The Folin-Ciocalteu reagent and AlCl3 approaches were utilized to evaluate the total phenol and flavonoid contents, respectively, and the DPPH (2,2-diphenyl-1-picrylhydrazyl) and phosphomolybdenum assays were employed to determine the antioxidant activity of each fraction. The DMF was tested against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) by employing the Ellman method, while all fractions were tested for antiproliferative activity against HepG2 and A549 cell lines by MTT assay.
Results: The DMF displayed the highest phenolic content (124.8 ± 17.5 mg gallic acid equivalent/g of dry extract) and flavonoid content (211.1 ± 4.8 mg quercetin equivalent/g of dry extract). In the phosphomolybdenum assay, its antioxidant IC50 value was 25 ± 1 µg/mL. Additionally, the DMF fraction exhibited significant inhibition activity against AChE and BChE, with IC50 values of 455 ± 1 and 450 ± 1 μg/mL, respectively. In terms of anti-proliferative activity, the DMF exhibited an IC50 value of 29.2 µM against the HepG2 cell line, while the EAF showed IC50 values of 24.7 µM in the A549 cell line.
Conclusion: The potent activity of C. religiosa as an antioxidant, along with its significant inhibition of AChE and BChE, positions it as a promising candidate for Alzheimer's disease treatment. Furthermore, its robust inhibition of human liver and lung cancer cells suggests its potential as a therapeutic agent against cancer.
Keywords: Crateva religiose, antioxidant, anticholinesterase, and antiproliferative
1. Introduction
Despite steady advances in the development of effective anticancer medications, cancer continues to be a fatal and destructive illness. With an estimated 9.6 million deaths and 18.1 million new cases in 2018, cancer is the second leading cause of mortality worldwide, behind only cardiovascular illnesses [1]. In 2010, due to cancer, a surprising total annual economic burden was estimated to be approximately USD 1.16 trillion [2]. The current standard of care for cancer treatment includes conventional methods, such as surgery, chemotherapy, and radiation therapy, and CAM approaches, including the use of natural products. More than 200 anticancer medications have been licensed worldwide in the last 60 years, and almost half of them are derived from natural sources [3]. Several promising, innovative natural compounds have undergone preclinical and clinical testing for anticancer activity. However, clinical trials for the vast majority of these candidate compounds have been terminated or abandoned [3]. Nearly 60 % and 80 % of the global population, especially in poor countries, rely on herbal medicine [4], demonstrating the widespread use of herbal medicine by indigenous communities around the world for the treatment of a wide range of pathological conditions. However, the presence of antineoplastic activity in more than 3000 different herbal plants has been established [5], and thirty natural compounds originating from plants have been tested as anticancer medications in clinical trials [6]. Several plant-based natural compounds, including camptothecin, etoposide phosphate, paclitaxel, vinblastine, podophyllotoxin, topotecan, and homo-harringtonine, have been successfully used as anticancer chemotherapeutic drugs [7]. Moreover, plant-derived anticancer molecules have fewer toxic effects on normal cells than their synthetic counterparts via alternative cell death-initiating mechanisms [8]. It has been reported that mixtures of phytochemicals exist in a balanced diet and show synergistic activity, preceding increased anticancer effects and health advantages. This cannot be achieved by using the consumption of a single functioning compound [9, 10]. As a result, there has been a surge in recent years in the study of herbal plants as potential resources of anticancer medications, with the hope of using them as natural therapeutic alternatives to conventional cancer treatments [6].
Alzheimer's disease (AD) is a complex multifactorial neurodegenerative disorder that is a leading cause of dementia and strongly affects brain function. It leads to severe memory loss and weakened language and judgment ability among older people. The disease can also affect younger individuals. According to an epidemiological survey, approximately 7-10 % of the population over 65 and 50-60 % of those over 85 are affected by AD, totalling almost 35 million people worldwide [11, 12]. AD can be identified by the development of senile plaques, which are composed of neurofibrillary tangles (NFTs), amyloid beta protein, and a deterioration of both cortical and cholinergic neurons [13,14]. The cognitive problem in AD patients occurs by the defeat of the neurotransmitter acetylcholine, which controls the function of cholinergic neurotransmission [15]. In the cerebral cortex, the AChE enzyme is liable for the failure of acetylcholine within synapses. Therefore, AChE inhibitors can be commonly used to treat AD [16, 17].
Numerous metabolic processes and a wide variation of external factors are liable for forming free radicals within the body[18]. The abundant accumulation of reactive oxygen species (ROS) in the body is responsible for several chronic diseases, including AD, which is caused by free radical-induced oxidative stress [19]. By generating antioxidants to destroy excess oxidants, the antioxidative defense scheme works to prevent the injurious influences of oxidative stress [20, 21]. At present, several AChE inhibitors are used to treat AD, which can cause severe adverse effects [22, 23]. Moreover, synthetic antioxidants are also applied in many cases, which have adverse effects, including liver damage and cancer [24]. On the other hand, natural plants contain a wide and largely unexplored source of compounds for drug discovery, including the advancement of novel antioxidants and cholinesterase inhibitors.
Crateva religiosa (C. religiosa), a member of the Capparaceae family, is a medium-sized deciduous tree also known as Varuna or the Garlic Pear Plant. Several such species are widely distributed in Australia, Bangladesh, Cambodia, China, Japan, Indonesia, India, Myanmar, Malaysia, New Guinea, Sri Lanka, Thailand, the Philippines, the Solomon Islands, and Vietnam [25]. There are several reports on the bioactive [26] and pharmacological effect of C. religiosa as traditional medicine, used by local practitioners to treat memory loss, psychological problems, neurologic pains, convulsion and epilepsy, heart disease, chronic weight loss, improved digestion, and swelling as well as burning sensations in the soles of feet [25, 27-28, 29]. There is a strong connection between some of these disorders and AD [30]. Parveen and co-workers recently reported on the natural compounds extracted from C. religiosa leaves and studied their structure clarification, DNA binding, as well as molecular docking [ 31]. Sahoo et al. demonstrated the antimycotic activity of C. religiosa hook and forst against designated fungal pathogens. In contrast, Bani and coworkers examined the inhibition of T lymphocyte activity using lupeol extracted from C. religiosa [32, 33]. Meenaloshini et al. studied the in vitro anticancer effectiveness of hydroalcoholic on human ovarian cancer cells extracted from C. religiosa G. Forst. bark [34], which efficiently suggested the vigorous study of this plant. Here, we report a systematic investigation of the antioxidant, anticholinesterase, and antiproliferative activities of C. religiosa bark extract to evaluate its activity for the treatment of AD as well as lung and liver cancer cells.
2. Materials and Method
2.1. Collection of plant materials
C. religiosa bark was gathered from Chauddagram, Cumilla, Bangladesh, in October 2022 and classified by Mr. Khandakar Kamrul Islam, a senior scientific officer, and the National Herbarium, Dhaka, Bangladesh, with a voucher specimen (DACB-86525). The bark was then cleaned with clean water to eliminate foul materials, after which, for several days, the plants were dried in the shade. The dried bark was then crushed into a coarse powder employing a crushing machine, and the resulting bark powder was stored at room temperature (RT).
2.2. Preparation of extract
A total of 800 g of dried powder bark was taken in an amber color extraction bottle (size: 2.5 L), and the materials were properly soaked in CH3OH. The bottle was sealed properly and preserved with occasional shaking for a week. The extracts were initially filtered through cotton and then again by filter paper. With a rotary evaporator at 50 °C under reduced pressure, it was then kept to get a concentrated solution. Finally, it was fractionated by petroleum ether (PEF), dichloromethane (DMF), and ethyl acetate (EAF) to gain the PEF (15.75 gm), DMF (6.23 g), and EAF (10.41 gm), as demonstrated in Flowchart S1 (Supplementary file).
2.3. Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), phosphate buffer, gallic acid (GA), catechin (CA), ascorbic acid (AA), AlCl3, potassium acetate, quercetin (QU), DMSO, and methanol were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Folin-Ciocalteu phenol reagent and Na2CO3 were gained from Merck (Darmstadt, Germany). MTT (3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyltetrazolium bromide), DMEM, fetal bovine serum (FBS), and penicillin/streptomycin were found from Gibco (Invitrogen, Eugene, OR, USA). Furthermore, 96-well plates, T-75, T-25 flasks, pipette tips, and serological pipettes were purchased from Corning, USA.
2.4. Determination of DPPH free radical scavenging action
The antioxidant capacity of the diverse fractions of C. religiosa was studied via the colorimetric technique using the DPPH reagent, where AA was employed as a standard [35]. The stock solution was serially diluted to achieve 200, 100, 50, 25, 12.5, and 6.25 μg/ml. Each test tube contained 2 ml of each concentration, and the media was properly marked. Three millilitres of 0.004 % DPPH solution were mixed in each test tube to a final volume of 5 ml, and the mixture was incubated at RT for 30 min in the dark. At 517 nm, the absorbance was subsequently evaluated against a blank. Without any sample, the control was set as previously mentioned. The free radical scavenging action was determined based on the percentage (%) of DPPH radicals scavenged utilizing the equation:
% inhibition = (1-) × 100
2.5. Phospho-molybdenum assay
For the estimation of total antioxidant activity, the Prieto et al. method was employed [36]. In this test, 0.1 ml of the sample at diverse concentrations (6.25-200 μg/ml) was reacted with 1 ml of reagent containing 0.6 M H2SO4, 4 mM ammonium molybdate, and 28 mM sodium phosphate. In a water bath, the test tubes were incubated at 95 °C for 90 min. The absorbance of the samples was subsequently estimated at 765 nm after cooling to RT, where AA was used as the control. The antioxidant activity was measured by employing the equation:
Antioxidant activity, % = (1-) × 100
2.6. Determination of total phenolics
The total phenolic content of the diverse fractions of C. religiosa was measured using the technique illustrated by Veliogluet in 1998, relating Folin-Ciocalteu reagent (FCR) as an oxidizing agent and catechin as a standard [37]. A total of 0.5 mL of each serially diluted plant extract and standard of diverse concentration of solutions was reserved in the test tubes. In a test tube, 3 ml of diluted FCR solution with 4 ml of 7 % sodium carbonate was added and mixed well. To complete the reaction, the test tubes were incubated at RT for 30 min. Employing a spectrophotometer, the absorbance of the solution was subsequently estimated at 765 nm against a blank. The total phenolic content was measured from the calibration curve, which was developed by formulating a GA solution. The total phenolic content was stated in mg of gallic acid equivalents (GAE) per g of the dried sample.
2.7. Determination of total flavonoids
The total flavonoid content of the diverse fractions of C. religiosa was estimated by the AlCl3 colorimetric technique, where quercetin was used as a standard [38]. In test tubes, two milliliters of each diluted plant extract and standard solutions of diverse concentrations were mixed. 2 ml of AlCl3 solution, 1 M potassium acetate (0.20 ml), and distilled H2O (5 ml) were further added. Then, to complete the reaction, the mixture was incubated for 60 min at RT. Using a spectrophotometer, the absorbance of the mixture was subsequently measured against a blank at 415 nm. The total flavonoid content was calculated from the calibration curve, which was developed by preparing a quercetin solution. The total flavonoid content was stated in mg of quercetin equivalents (QE) per g of the dried sample.
2.8. Determination of cholinesterase inhibitory activity
Since DMF had the best antioxidant capacity in the antioxidant tests, it was selected for the in vitro AChE inhibitory activity assay at 25, 50, 100, 200, and 500 μg/ml. With acetylthiocholine iodide as a substrate, the AChE inhibitory capacity of DMF was determined following the colorimetric technique [39]. By a homogenizer with five volumes of ice-cold homogenization buffer, rat brains were homogenized (10 mM Tris-HCl (pH 7.2), which included 50 mM MgCl2, 1 M NaCl, and 1 % Triton X-100) and centrifuged at 4 °C for 30 min at 10000 × g, which was employed as the enzyme resource. As an enzyme source, the supernatant was employed after centrifugation. The protein concentration was confirmed by applying a bicinchoninic acid kit (Sigma Co., St. Louis, MO, USA) in the presence of bovine serum albumin (BSA), which served as a protein standard. DMF (500 μL), used as a standard, was combined with an enzyme (500 μL) and incubated at body temperature for 15 min. After mixing Ellman's reagent in 50 mM sodium phosphate buffer (pH = 8), the absorbance of the reaction was measured at 405 nm thoroughly. To confirm that the reaction ensued linearly, the readings were repeated 5 times at 2-minute intervals. Donepezil was applied as a positive control in this test. The % inhibition of AChE capacity was determined utilizing the following formula:
% inhibition of AChE capacity =× 100
During this experiment, acetylthiocholine iodide was substituted with butyrylthiocholine iodide, and BChE inhibition was determined, as illustrated earlier, by altering the volume of the enzyme solution to 50 μl. Galantamine was applied as a positive control in this test. The % inhibition of BChE capacity was obtained by applying the same formula for AChE activity.
2.9. Cell lines and culture conditions
Two human cancer cell lines were employed in this research, namely, adenocarcinomic human alveolar basal epithelial cells (A549) and hepatocellular carcinoma (HepG2) cells. For the cytotoxicity comparison study, noncancerous alveolar basal epithelial cells and normal hepatocyte cell lines were used. A549 and HepG2 cells were cultured in DMEM enhanced with 10 % FBS and 1 % penicillin/streptomycin at 37 °C, 5 % CO2, and 95 % air in a 75 cm2 tissue culture flask.
2.10. MTT assay
The alteration of MTT (yellow) to formazan crystals (purple) by mitochondrial dehydrogenase of viable cell enzymes was applied to evaluate the degree of cellular cytotoxicity employed by Com1, DMF, PEE, and EAF, as described by Kazi et al. [40] and improved by Kumar et al. [41]. Concisely, in 96-well plates (2 × 103 cells/well), cancer cells were placed by keeping in a complete cell growth medium and incubated at 37 °C for a day under a humidified atmosphere of 5 % CO2. Then, the cell medium was put back to the cell growth medium in the presence of 5 % FBS (5 % medium) and different concentrations of Com1, DMF, PEE, and EAF, such as 1.95, 3.9, 7.8, 15.6, 31.25, 62.5, 125 and 250 µg/ml, with the control (no further treatment). After 72 hours of incubation, the medium in all test and control wells was replaced with 100 µL/well MTT solution (0.5 mg/mL in PBS), and the cells were incubated at 37 °C for an additional 3 h. Afterwards, the MTT solution was replaced with 100 µL of isopropanol/well to disperse the formazan (purple) crystals that had been produced at the bottom of the wells, and the mixture was shaken at RT for at least 2 h. Later, with a Bio-Tek microplate reader (ELX 800; Bio-Tek Instruments, Winooski, VT, USA), the color intensity at 549 nm in the wells was computed. The outcomes were examined in triplicate, and the % of viable cells was calculated. The data obtained are represented as % of viable cells in the test wells in comparison to the control group. To get the cell viability, the subsequent equation was applied [41]:
% cell viability = [] × 100
2.11. Statistical analysis
The data were studied using Microsoft Excel 2016 (Roselle, IL, USA), IBM SPSS Statistics 26, and the Statistical Package for Windows version 17.0 for the Social Sciences (SPSS; IL, USA). All tests were done in triplicate, and most of the obtained data are denoted as the mean ± standard deviation.
One-way ANOVA was employed to compare the mean values of quantitative variables across the categorical variable. A post hoc Tukey HSD test was applied to validate whether there were any significant changes between the diverse concentrations of the treated groups and the control. A p-value of < 0.05 was measured as statistically important. Data were studied employing GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA), as shown in Tables S1 and S2.
3. Results
3.1. DPPH free radical scavenging assay
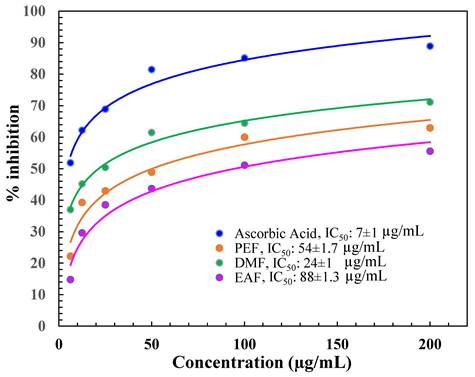
The antioxidant capacity of the diverse fractions of C. religiosa was expressed as the IC50 value. The IC50 is the concentration of the fraction essential to result in 50 % greater antioxidant activity than that of the control (AA). The outcomes represented that the IC50 value of AA was (7 ± 1) µg/mL as displayed in Figure 1. Among the tested fractions, the IC50 values were in the following order: DMF (24 ± 1), PEF (54 ± 1.7), and EAF (88 ± 1.3) µg/mL, which were determined from the % of inhibition value (Figure 1).
3.2. Phosphomolybdenum assay
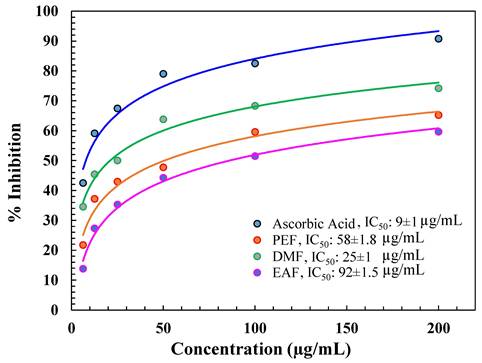
In this assay, the decrease of Mo(VI) to Mo(V) by a reductant was determined by the development of a green phosphate-Mo(V) complex. Among the fractions, DMF showed the best total antioxidant activity, i.e., the IC50 value was 25 µg/ml, as shown in Figure 2. Other fractions did not show good IC50 values or molybdenum ion percentage reductions (Figure 2) compared with AA (9 µg/ml).
3.3. Determination of total phenolics
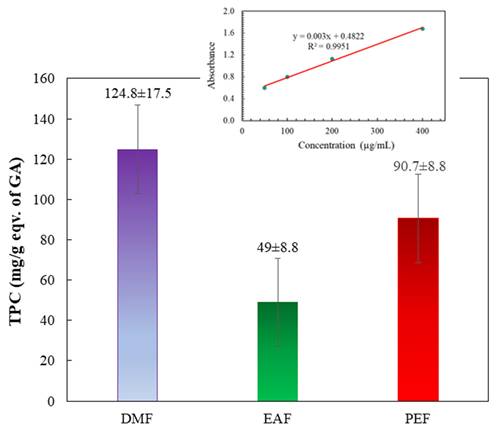
Figure 3 and its insert depict the total phenolic contents of the fractions. These contents were determined by applying the standard curve of GA (y = 0.003x + 0.4822; R² = 0.9951). The findings revealed that the DMF treatment showed the highest phenolic content, followed by the PEF treatment. Conversely, the EAF exhibited the lowest phenolic content. The phenol contents of the fractions followed this descending order: DMF > PEF > EAF.
3.4. Determination of total flavonoids
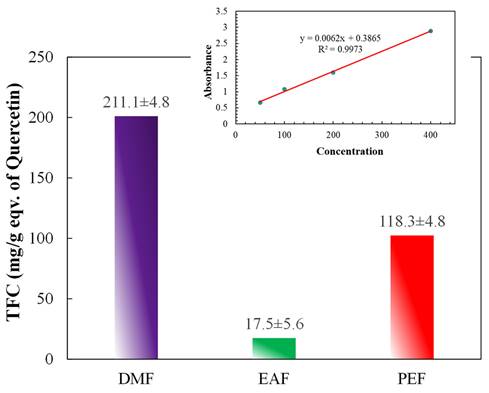
The total flavonoid contents are demonstrated in Figure 4 and the inset of Figure 4. The contents were computed by employing the standard curve of quercetin (y = 0.0062x + 0.3895; R² = 0.9973) and were stated as QE per gm of plant extract. The outcomes revealed that the maximum flavonoid content was in the DMF fraction, then in the PEF, whereas the lowest content was found in the EAF. Thus, the flavonoid contents of the fractions decreased in the order of DMF > PEF > EAF.
DPPH free radical scavenging activity of DMF, PEF, and EAF.
% inhibition of different fractions in the phosphomolybdenum assay.
Total phenol contents of different fractions of C. religiosa. The inset shows the standard curve of gallic acid.
3.5. In vitro AChE and BChE enzyme activity
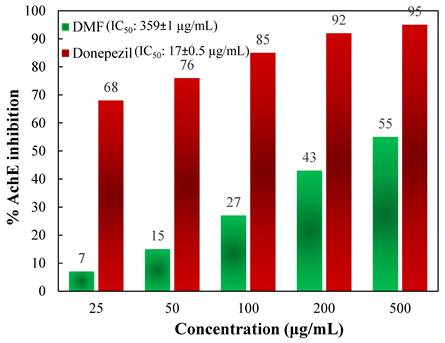
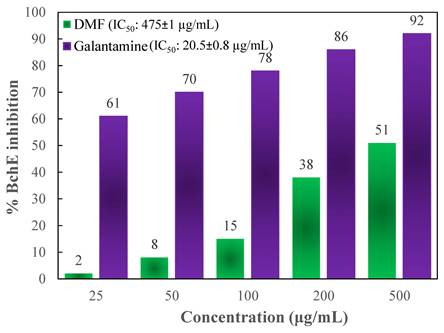
To determine the activity of the DMF fraction as an anti-AD drug, its AChE as well as BChE inhibitory actions were measured. In comparison to those of the standards, Figures 5 and 6 depict the AChE and BChE inhibitory activities, respectively, and the cholinesterase inhibitory action showed dose-dependent changes. As demonstrated in Figure 5, the AChE inhibitory capacities of the DMF fraction were 7 ± 0.8, 15 ± 2.3, 27 ± 3.7, 43 ± 1.9, and 55 ± 2.2 % at concentrations of 25, 50, 100, 200 and 500 μg/mL, respectively, with an IC50 value of 359 ± 1 μg/mL. Besides, Figure 6 demonstrated the BChE inhibitory activities of DMF were 2 ± 1.3, 8 ± 0.6, 15 ± 1.5, 38 ± 1.9, and 51 ± 3.7 % at concentrations of 25, 50, 100, 200 and 500 μg/mL, respectively, with an IC50 value of 475 ± 1 μg/mL. In these experiments, donepezil and galantamine were applied as reference standards, and their IC50 values against AChE and BChE were 17 ± 0.5 and 20.5 ± 0.8 μg/mL, respectively.
Total flavonoid contents of different fractions of C. religiosa. Inset shows the standard curve of quercetin.
% inhibition of AChE activity at different concentrations of DMF and the reference standard donepezil.
3.6. Effects of different fractions on the MTT assay
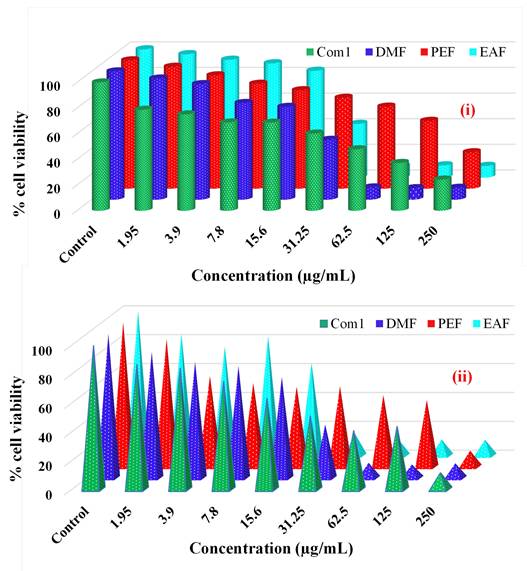
The effectiveness of the crude methanolic extract (Com1) and three fractions resultant from the bark of C. religiosa in combating cancer was estimated by employing the MTT assay. The findings are presented as % of viable cells compared to the control after 72 hours of exposure, as demonstrated in Figure 7 (i and ii).
For the liver cancer (HepG2) cell line, Com1 exhibited cell viability % of 78.67, 75.09, 68.7, 68.61, 60.08, 47.84, 37.16, and 24.2 at doses of 1.95, 3.9, 7.8, 15.6, 31.25, 62.5, 125, and 250 µg/mL, respectively, compared with controls (0.5 % DMSO in a 5 % serum-containing DMEM medium). In contrast, the viability % in the presence of DMF were 94.4, 90.07, 75.35, 72.3, 46.57, 9.44, 8.88, and 9.16 across the same dosage range. Similarly, the % of viable cells for the PEF treatment were 94.98, 88.29, 81.73, 76.68, 70.78, 63.88, 52.79, and 28.27, while for the EAFs, they were 96.22, 91.88, 89.12, 83.27, 41.85, 8.77, 9.45, and 9.02. Finally, for this cell line, the DMF fraction showed the best efficacy.
In the case of the lung cancer (A549) cell line, Com1 demonstrated the viability of 86.93, 84.34, 75.23, 63.45, 51.13, 41.04, 43.92, and 11.62 % at doses mentioned earlier, compared with the controls. The presence of DMF resulted in the viability of 87.36, 80.67, 77.79, 70.04, 37.17, 10.68, 9.5, and 10.34 % across the same dosage range. The PEF treatment showed the viability of 88.48, 62.89, 58.15, 55.59, 55.87, 49.75, 46.35, and 11.28 %, while the EAF treatment displayed 84.11, 75.41, 82.66, 64.21, 15.44, 11.58, 11.29, and 11.17 %. Thus, EAF was the most active fraction against this cell line.
% inhibition of BChE at different concentrations of DMF and the reference standard galantamine.
Effects of different concentrations of crude methanolic extract (Com1), DMF, PEF, EAF as % cell viability on (i) liver cancer cell line (ii) lung cancer cell line as measured by MTT 72 h following exposure.
4. Discussion
Oxidative stress is basically determined by the uncontrolled synthesis of ROS and reactive nitrogen species (RNS). Oxidative stress originated through free radicals, for instance, superoxide anion radical (O2)•-, hydroxyl radical (•OH), H2O2, singlet oxygen (1O2), and peroxyl radicals (ROO•), which have great affinity for accepting an electron by combining with biological macromolecules, for example, lipids, proteins, and DNA. This affinity for oxidative stress leads to many human degenerative diseases [42]. The antioxidative defence system works by removing ROS and preventing cellular destruction by neutralizing free radicals, thus defending against diseases like AD [43]. However, severe biological destruction can happen when the rate of free radical synthesis is greater than the activity of the protection system, which increases the ROS level in the body [44]. Increased levels of ROS perform an action in the pathogenesis of AD [45]. In this study, as demonstrated in Figure 1, DMF demonstrated the highest % of DPPH radical scavenging, which reflects its potent antioxidant activity. The phosphomolybdenum assay also revealed similar findings, as demonstrated in Figure 2. Therefore, the scavenging potential of DMF was quantified via the use of DPPH and Phosphomolybdenum reagent to determine antioxidant activity [46]. The degree of color variation in this assay is directly proportional to the concentration of antioxidants in the sample [47].
Further, phenolic compounds are very important alternatives to synthetic chemotherapeutic drugs and have been found to demonstrate effective antineoplastic effects against numerous steps of carcinogenesis and related inflammation. Phenolic and flavonoid compounds are the most vibrant classes of antioxidants that can scavenge free radicals to prevent cellular damage [48, 49]. Figures 3 and 4 showed that the TPC and TFC for DMF are 124.8 ± 17.5 (mg/g eqv. of GA) and 211.1 ± 4.8 (mg/g eqv. of Quercetin), respectively, and high as compared to other fractions of C. religiosa. So, the phenolic and flavonoid contents increased with growing concentrations of the methanol extract, signifying that the bark of C. religiosa can reduce the threat of several degenerative diseases [50], including AD, by working as an antioxidant to prevent oxidative stress-induced cell damage.
Moreover, AD is a multifactorial chronic as well as a neurodegenerative syndrome that is diagnosed by the progressive deterioration of cognition, memory, and behavior; this disease typically has a very slow onset without rapid damage and ultimately leads to death. There are no available drugs that treat AD, but two hypothetical methods for the medication of AD have been advanced. The initial method works by quarantining prime progenitors to stop AD growth. The other mechanism includes symptomatic medication of the tertiary mental indications of AD [51]. Currently, only a few cholinesterase inhibitors, for instance, donepezil, galantamine, and rivastigmine, as well as one fractional NMDA receptor antagonist, memantine, are FDA-permitted medicines for treating AD. Cholinesterase inhibitors are drugs that prevent the breakdown of acetylcholine inside synapses, resulting in a high level of acetylcholine. Therefore, researchers have tried discovering novel medicines from natural resources, like plants, that are highly effective at treating AD. In this present study, DMF was found to prevent AChE as well as BChE in a dose-dependent mode, as demonstrated in Figures 5 and 6, where the IC50 of DMF for AChE and BChE tests were 359 ± 1 μg/mL and 475 ± 1 μg/mL, respectively. The findings reveal that the high phenolic content in DMF not only enhances its antioxidant capacity but also suggests potential neuroprotective effects through cholinesterase inhibition. Studies have proven that the phenolic compounds in DMF can bind to the active sites of AChE and BChE, thereby inhibiting their activity. This dual action - antioxidant activity and cholinesterase inhibition - positions DMF as a promising candidate for further evaluation in AD research [52, 53].
Similarly, plant-derived products have been reported to be biologically safe for use with normal cells in the human body [3, 7]. In this context, this was the first report in which EAF and DMF derived from the fresh bark of C. religiosa demonstrated potent antineoplastic activity. As demonstrated in Figure 7 (i) and (ii), this study was designed to govern the antiproliferative activity of these compounds against two selected types of cancer cells, A549 and HepG2, which represent lung adenocarcinoma and liver hepatocarcinoma, respectively, that are currently responsible for high mortality in humans [54, 55]. In this study, healthy lung and hepatic cells were used as positive controls for these two cell lines. Our phytochemical fractions have shown selective efficacy on two types of cancer cells. For the HepG2 cell line, DMF was the most effective fraction, as the cell viability was the least among the fractions with IC50 29.2 µM, and EAF was the best fraction worked against A549 cell lines, where the IC50 concentration was 24.7 µM.
These outcomes propose the significant action of the polyphenolic components of the methanolic extract in free radical neutralization and of DMF in the antiproliferative activity and inhibition of AChE action. Additional investigations are anticipated to identify the functioning compounds and leads that may be useful as candidate drugs.
5. Conclusion
The potent antioxidant activity and significant inhibition of AChE and BChE by the DMF fraction and the inhibition of cell growth by the EAF and DMF fractions of C. religosa focus on its prospect of affording an efficient treatment for AD and cancer, respectively. This work is the first effort to scrutinize the antioxidant, AChE/BChE inhibitory and antiproliferative activities of C. religiosa. It was found that the DMF fraction exhibited significant inhibition action against AChE and BChE. In the case of anti-proliferative activity, the DMF showed an IC50 value of 29.2 µM against the HepG2 cell line, while the EAF exhibited IC50 values of 24.7 µM in the A549 cell line. However, additional investigation in an animal model of AD and cancer is warranted to elucidate the in vivo efficacy and isolation of active metabolites of this plant.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Author contributions
M. S. H - Formal analysis, investigation, data curation, original draft preparation, visualization, review, and editing.
M. A. M. P. - Conceptualization, supervision, methodology, validation, formal analysis, resources, data curation, original draft preparation, review, and editing.
S. A. R.- formal analysis, investigation, data curation, review, and editing.
M. K - validation, formal analysis, data curation, review and editing.
A. K - formal analysis, data curation, investigation, review, and editing.
K. A- formal analysis, data curation, investigation, review, and editing.
M. M. M- data curation, resources, review, and editing.
M. R. R.- data curation, resources, visualization, review, and editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211-1259 doi: 10.1016/S0140-6736(17)32154-2
2. Stewart BW, Wild CP. Eds.; International Agency for Research on Cancer. WHO Press: Geneva, Switzerland, 2014; ISBN 978-92-832-0443-5
3. Butler MS, Robertson AAB, Cooper MA. Natural product and natural product-derived drugs in clinical trials. Nat Prod Rep. 2014;31:1612-61 https://doi.org/10.1039/C4NP00064A
4. Mussarat S, Abdel-Salam NM, Tariq A. et al. Use of Ethnomedicinal Plants by the People Living around Indus River. Evid Based Complement. Altern Med. 2014 https://doi.org/10.1155/2014/212634
5. Tariq A, Sadia S, Pan K. et al. A systematic review on ethnomedicines of anti-cancer plants. Phytother Res. 2017;31:202-264 https://doi.org/10.1002/ptr.5751
6. Gali-Muhtasib H, Hmadi R, Kareh M. et al. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis. 2015;20:1531-1562 https://doi.org/10.1007/s10495-015-1169-2
7. Newman DJ, Cragg GM, Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod. 2016; 79: 629-661. https://doi.org/10.1021/acs.jnatprod.5b01055.
8. Mishra BB, Tiwari VK, Natural products. An evolving role in future drug discovery. Eur J Med Chem. 2011;46:4769-4807 https://doi.org/10.1016/j.ejmech.2011.07.057
9. Liu RH, Health benefits of fruit, vegetables are from additive, synergistic combinations of phytochemicals. Am J Clin Nutr. 2003; 78: 517S-520S.
10. Liu RH, Potential synergy of phytochemicals in cancer prevention. Mechanism of action. J Nutr. 2004;134:3479S-3485S
11. Reitz C, Brayne C, Mayeux R, Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011; 7 (3): 137-52.
12. Silva T, Reis J, Teixeira J. et al. Alzheimer's disease, enzyme targets and drug discovery struggles: From natural products to drug prototypes. Ageing Res Rev. 2014;15:116-45 https://doi.org/10.1016/j.arr.2014.03.008
13. Asaduzzaman M, Uddin M, Kader M. et al. In vitro acetylcholinesterase inhibitory activity and the antioxidant properties of Aegle marmelos leaf extract: implications for the treatment of Alzheimer's disease. Psychogeriatrics. 2014;14(1):1-10 https://doi.org/10.1111/psyg.12031
14. Varadarajan S, Yatin S, Aksenova M. et al. Review: Alzheimer's amyloid β-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000;130(2-3):184-208 https://doi.org/10.1006/jsbi.2000.4274
15. Vinutha B, Prashanth D, Salma K. et al. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J Ethnopharmacol. 2007;109(2):359-63 https://doi.org/10.1016/j.jep.2006.06.014
16. Bierer LM, Haroutunian V, Gabriel S. et al. Neurochemical correlates of dementia severity in Alzheimer's disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64(2):749-60 https://doi.org/10.1046/j.1471-4159.1995.64020749.x
17. Mukherjee PK, Kumar V, Mal M. et al. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14(4):289-300 https://doi.org/10.1016/j.phymed.2007.02.002
18. López V, Martan S, Gómez-Serranillos MP. et al. Neuroprotective and neurological properties of Melissa officinalis. Neurochem Res. 2009;34(11):1955-61 https://doi.org/10.1007/s11064-009-9981-0
19. Matsuda T, Kanki T, Tanimura T. et al. Effects of overexpression of mitochondrial transcription factor a on lifespan and oxidative stress response in Drosophila melanogaster. Biochem Biophys Res Commun. 2013;430(2):717-21 https://doi.org/10.1016/j.bbrc.2012.11.084
20. Mamelona J, Pelletier Ã, Girard-Lalancette K. et al. Quantification of phenolic contents and antioxidant capacity of Atlantic Sea cucumber, Cucumaria frondosa. Food Chem. 2007;104(3):1040-7 https://doi.org/10.1016/j.foodchem.2007.01.016
21. Tang Y, Gao C, Xing M. et al. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol. 2012;50(5):1194-200 https://doi.org/10.1016/j.fct.2012.02.008
22. Melzer D, New drug treatment for Alzheimer's disease. lessons for healthcare policy. BMJ. 1998;316(7133):762 https://doi.org/10.1136/bmj.316.7133.762
23. Small GW, Rabins PV, Barry PP. et al. Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. JAMA. 1997;278(16):1363-71 doi:10.1001/jama.1997.03550160083043
24. Wichi H, Enhanced tumor development by butylated hydroxyanisole (BHA) from the prospective of effect on fore stomach, oesophageal squamous epithelium. Food Chem Toxicol. 1988; 26: 717-23. https://doi.org/10.1016/0278-6915(88)90072-5.
25. Srinivas V, Surendra G, Anjana M. et al. A scientific review on Crateva religiosa. Int J Adv Pharm. Biology and Chemistry. 2018 7(1)
26. Cantrell CL, Berhow MA, Phillips BS, Duval SM, Weisleder D, Vaughn SF. Bioactive crude plant seed extracts from the NCAUR oilseed repository. Phytomedicine. 2003;10(4):325-33 doi: 10.1078/094471103322004820
27. Wagay NA, Khan NA, Rothe SP. Profiling of secondary metabolites and antimicrobial activity of Crateva religiosa G. Forst. Bark - A rare medicinal plant of Maharashtra India. Int J Biosciences. 2017;10(5):DOI 10.12692/ijb/10.5.343-354
28. Prabha VC, Prabha KC, Vijayakumar S. Phytochemical analysis and Invitro antioxidant activity of silver nanoparticle synthesized Crateva religiosa bark extract. J Uni of Shanghai for Sci Tech. 2021;23:190-198 ISSN: 1007-6735
29. Shinsy P, Thomas J. A Systematic Review of Crateva Species for It's Traditional and Modern Medicinal Uses. Eur Chem Bull. 2023 12, 7, 5822- 5841
30. Mendez MF, Martin RJ, Smyth KA. et al. Psychiatric symptoms associated with Alzheimer's disease. J Neuropsychriatary Clin Neurosci. 1990;2(1):28-33 https://doi.org/10.1176/jnp.2.1.28
31. Parveen M, Aslam A, Siddiqui S. et al. Structure elucidation, DNA binding and molecular docking studies of natural compounds isolated from Crateva religiosa leaves. J Molecular Structure. 2022;1251:131976 https://doi.org/10.1016/j.molstruc.2021.131976
32. Sahoo S, Mishra SK, Panda PK, Tripathy S, Mishra SR, Ellaiah P, Dash SK, Antimycotic potential of Crataeva religiosa Hook, Forst against some selected fungal pathogens. Acta Pol Pharm. 2008 Mar-Apr;65(2):245-7.
33. Bani S, Kaul A, Khan B, Ahmad SF, Suri KA, Gupta BD, Satti NK, Qazi GN, Suppression of T lymphocyte activity by lupeol isolated from Crataeva religiosa. Phytother Res. 2006. Apr;20(4): 279-87. doi: 10.1002/ptr.185.
34. Meenaloshini P, Thirumurugan A, Mohan PK. et al. In Vitro Anticancer Efficacy of Hydroalcoholic Extract From Crateva religiosa G. Forst. Bark on Human Ovarian Cancer Cells. J Herbal Medicine. 2024;45:100870 https://doi.org/10.1016/j.hermed.2024.100870
35. Rahman F, Patwary MAM, Siddique MAB. et al. Green synthesis of ZnO nanoparticles using Cocos nucifera leaf extract: Characterization, antimicrobial, antioxidant, and photocatalytic activity. Royal Society Open Science. 2022;9:220858 https://dx.doi.org/10.1098/rsos.220858
36. Prieto P, Pineda M, Aguilar M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Analytical Biochemistry. 1999; 269, 2: 337-341, https://doi.org/10.1006/abio. 1999 4019
37. Veliogluet YS, Mazza G, Gao L. et al. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem. 1998;46:4113-17 https://doi.org/10.1021/jf9801973
38. Wang SY, Jiao H, Correlation of antioxidant capacities to oxygen radical scavenging enzyme activities in blackberry. J Agric Food Chem. 2000; 48 (11): 5672-76. https://doi.org/10.1021/jf000765q.
39. Ellman GL, Courtney KD, Andres V. et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88-95 https://doi.org/10.1016/0006-2952(61)90145-9
40. Kazi M, Alanazi Y, Kumar A. et al. Oral Bioactive Self-Nanoemulsifying Drug Delivery Systems of Remdesivir and Baricitinib: A Paradigmatic Case of Drug Repositioning for Cancer Management. Molecules. 2023 28 (5). https://doi.org/10.3390/molecules28052237
41. Kumar A, Alfhili MA, Bari A. et al. Apoptosis-mediated anti-proliferative activity of Calligonum comosum against human breast cancer cells, and molecular docking of its major polyphenolics to Caspase-3. Frontiers in Cell and Developmental Biology. 2022; 10. https://doi.org/10.3389/fcell. 2022 972111
42. Hossain MS, Reza AA, Rahaman MM. et al. Evaluation of morning glory (Jacquemontia tamnifolia (L.) Griseb leaves for antioxidant, antinociceptive, anticoagulant and cytotoxic activities. J Basic Clin Physiol Pharmacol. 2018;28(3):1-9 https://doi.org/10.1515/jbcpp-2017-0042
43. Braca A, Sortino C, Politi M. et al. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002;79(3):379-81 https://doi.org/10.1016/S0378-8741(01)00413-5
44. Hasan SR, Hossain MM, Akter R. et al. DPPH free radical scavenging activity of some Bangladeshi medicinal plants. J Med Plants Res. 2009;3(11):875-9 http://www.academicjournals.org/jmpr
45. Manoharan S, Guillemin GJ. et al. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer's Disease, Parkinson's Disease, and Huntington's Disease: A Mini Review. Oxid Med Cell Longev. 2016. 2016 (8590578) 1-15. https://doi.org/10.1155/2016/8590578
46. Nunes XP, Silva FS, Almeida JRGDS. et al. Biological oxidations and antioxidant activity of natural products: Phytochemicals as nutraceuticals-Global Approaches to Their Role in Nutrition and Health. London: InTech. 2012
47. Von GA, Joubert E, Hansmann C. Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong, and black tea. Food Chem. 1997;60(1):73-7 https://doi.org/10.1016/S0308-8146(96)00312-3
48. Shahidi F, Janitha P, Wanasundara P. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32(1):67-103 https://doi.org/10.1080/10408399209527581
49. Islam MR, Reza AA, Chawdhury KA. et al. Evaluation of in vitro antioxidant activity and cytotoxicity of methanolic extract of Sida cordata leaves. Int J Biol Pharm Res. 2014;5(2):196-200
50. Bravo L, Polyphenols. chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998; 56 (11), 317-33. https://doi.org/10.1111/j.1753-4887. 1998 tb01670.x
51. Feng Y, Wang X, Antioxidant therapies for Alzheimer's disease. Oxidative Med Cell Longev. 2012; 1-17. https://doi.org/10.1155/2012/472932.
52. Williams P, Sorribas A. et al. Natural products as a source of Alzheimer's drug leads. Natural Product Reports. 2011 28(1), 48-77. doi: 10.1039/C0NP00027B
53. Anekonda TS, Reddy PH. Can herbs provide a new generation of drugs for treating Alzheimer's disease? Brain Research Reviews, 2005; 50(2), 361-376. doi: 10.1016/j.brainresrev. 2005 08.003
54. El-Shemy HA, Aboul-Enein AM, Aboul-Enein MI. et al. The effect of willow leaf extracts on human leukemic cells in vitro. J Biochem Mol Biol. 2003;36:387-389
55. El-Shemy HA, Aboul-Enein AM, Aboul-Enein KM. et al. Willow Leaves' Extracts Contain Anti-Tumor Agents Effective against Three Cell Types. PLOS ONE. 2007;2:178 https://doi.org/10.1371/journal.pone.0000178
Author contact
![]() Corresponding authors: (M.A.M.P.) mampatwaryac.bd; (K.A.) kmgdermcom.
Corresponding authors: (M.A.M.P.) mampatwaryac.bd; (K.A.) kmgdermcom.

 Global reach, higher impact
Global reach, higher impact