Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(11):3539-3546. doi:10.7150/jca.91701 This issue Cite
Research Paper
The Efficacy and Safety of Anlotinib Plus Etoposide with Cisplatin/Carboplatin in the First-Line Treatment of Lung Cancer: A Phase II Clinical Study
1. Department of Thoracic Surgery, Linfen Central Hospital, Linfen, Shanxi, 041000, China.
2. Department of Cardiovascular Surgery, Xijing Hospital, Air Force Military Medical, University, Xincheng, Xi'an, 710032, China.
3. Department of medical, Xi'an Fourth Hospital, Xincheng, Xi'an, 710004, China.
4. Department of Cardiovascular Surgery, Xi'an Fourth Hospital, Xincheng, Xi'an, 710004, China.
Received 2023-10-30; Accepted 2024-4-13; Published 2024-5-5
Abstract
Background: The primary aim of this phase II clinical study was to assess the safety and efficacy of combining anlotinib, etoposide, and platinum-based drugs as a first-line treatment for ES-SCLC.
Methods: Patients underwent the standard chemotherapeutic regimen, consisting of four courses of etoposide plus cisplatin/carboplatin. Additionally, each patient received a 2-week intervention with anlotinib (12 mg/day, once daily). Anlotinib was continued until disease progression, occurrence of unbearable adverse events (AEs), or withdrawal from the research. Progression-free survival (PFS) served as the primary prognostic measure. Secondary measures included the disease control rate (DCR), objective response rate (ORR), overall survival time (OS), and the incidence of AEs.
Results: The DCR and ORR were 97.6% and 91.0%, respectively. Estimated PFS and OS were 5.0 months (95% CI: 1.0-10.8 months) and 13.0 months (95% CI: 8.4-18.6 months), respectively. No unexpected adverse effects were reported during the trial. The most common adverse reactions included anemia (42.22%), hypertension (53.33%), alopecia (40.00%), elevated transaminase (24.40%), and elevated alkaline phosphatase (24.44%). Sixteen cases (35.56%) were classified as AEs of grades 3-5. No deaths attributed to treatment-related causes occurred in any patient during the trial.
Conclusion: Combination chemotherapy is currently the first-line therapy for extensive small-cell lung cancer (ES-SCLC). Combining anlotinib with conventional platinum-based chemotherapy demonstrated promising therapeutic outcomes and prognosis in the management of ES-SCLC.
Keywords: Anlotinib, Etoposide, Clinical trials, Lung cancer
Introduction
A particular form of lung cancer, clinically referred to as small cell lung cancer (SCLC), belongs to the category of highly malignant neuroendocrine tumors, constituting approximately 15% of all lung cancers. The tumor cells exhibit rapid multiplication and a propensity for invasive growth and distant metastasis [1, 2]. SCLC primarily manifests in individuals who smoke. It is a malignancy that tends to metastasize widely in its early stages, resulting in relatively short patient survival times. Although responsive to chemotherapy and radiotherapy, it is prone to secondary resistant relapses despite high remission rates during initial treatment. Currently, systemic chemotherapy forms the foundation of treatment [3, 4]. Due to its robust up-regulation and early metastasis, approximately 70% of patients receive an initial diagnosis of extensive-stage small cell lung cancer (ES-SCLC), with a median overall survival (OS) of only ten months [5].
When patients are diagnosed with SCLC, approximately 30% are present with localized tumors, while the majority are already in the extensive stage, characterized by the tumor spreading beyond the supraclavicular region [6]. In comparison to several other types of lung cancer, chemotherapy and radiotherapy are less effective for treating SCLC [7-10]. However, due to the widespread distribution of SCLC at the time of diagnosis, achieving a cure is often challenging [11, 12]. Various techniques utilizing artificial intelligence or CT scans may efficiently and promptly detect this form of lung cancer [13-16].
Recently, the standard primary therapy for patients with SCLC has been the combination chemotherapy of etoposide and platinum [3, 17]. Despite the initially high response rate, a significant number of patients experience rapid progression. More recently, the addition of immune checkpoint inhibitors (such as atezolizumab or bevacizumab) to platinum-etoposide therapy in ES-SCLC patients has shown a transformative effect on OS, leading to a significant paradigm shift in the primary treatment model for SCLC [3, 17].
Angiogenesis plays a crucial role in tumor development. A promising therapeutic strategy for SCLC involves targeting endothelial cells therapeutically. Bevacizumab, which blocks VEGF, has shown an extended progression-free survival (PFS) when combined with platinum-etoposide chemotherapy for the treatment of ES-SCLC. However, this improvement is observed primarily in PFS rather than OS [18, 19]. In comparison to other angiogenesis class inhibitors, Patina, Zivlivet, Pazopanib, and sunitinib have demonstrated potential for maintenance therapy or as second-line medical interventions in SCLC. However, there are no reports indicating their efficacy in improving the survival of previously untreated patients [20, 21].
Anlotinib, a small molecule tyrosine kinase inhibitor currently administered orally to patients, has demonstrated potent antiangiogenic and antitumor effects by targeted inactivation of vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), and platelet-derived growth factor receptor (PDGFR) [4, 22]. Anlotinib, independently registered for both first- and third-line relapsed SCLC in China, has demonstrated significant improvements in OS and PFS. Another study has also shown similar clinical benefits of anlotinib in recurrent cases of SCLC, highlighting its remarkable efficacy and safety in the treatment of this cancer subtype [23-25].
The integration of anlotinib, a potent small-smolecule tyrosine kinase inhibitor targeting VEGFR, FGFR, and PDGFR, into first-line treatment regimens alongside etoposide and platinum-based chemotherapy presents a novel approach with promising therapeutic outcomes in ES-SCLC [26, 27]. Therefore, this trial aims to evaluate the efficacy of combining etoposide and platinum with anlotinib in ES-SCLC and assess any associated adverse effects.
Methods
Main indicators of clinical trial evaluation and ethical review
The research project was conducted at Linfen Central Hospital from February 2019 to February 2021. The evaluated performance indicators included the objective control rate (ORR), OS, adverse events (AEs), and disease control rate (DCR). PFS was the primary metric for assessing outcomes. Ethical approval for this study was granted by the Linfen Central Hospital's ethics committee. Prior to participation, written informed consent was obtained from each participant. All procedures were carried out in strict adherence to applicable guidelines and regulations.
Exclusion and inclusion criteria of clinical research subjects
Inclusion criteria: (1) Adult patients (over 18 years old but under 75 years old); (2 Histological or cytological clinical diagnosis of ES-SCLC; (3) No prior systematic treatment for metastatic diseases; however, adjuvant chemotherapy is permissible, provided it was completed at least six months before registration; (4) Performance status (PS) according to the Eastern Cooperative Oncology Group (ECOG) is 0 or 1; (5) Presence of at least one measurable lesion established in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1); (6) Patients not indicated for corticosteroid treatment for central nervous system metastases; (7) No radiotherapy received within seven days before registration; (8) Absence of radiological progression from the end of radiotherapy to the time of registration.
Exclusion criteria: (1) Prior anlotinib therapy received; (2) Previous antineoplastic therapy, including signal transduction inhibitors [28], such as systemic chemotherapy, hormonal, targeted therapy, and endocrine therapy; (3) Diagnosis of other malignant tumors within five years prior to enrollment; (4) Presence of any unresolved toxicity related to treatment at level 2 or higher according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4.0), except for hair loss; (5) Difficulties in oral administration or absorption, including conditions like chronic diarrhea, intestinal obstruction, various dysphagia, and gastrointestinal resection; (6) Asymptomatic central nervous system metastasis; (7) Radiological findings indicating tumor invasion into perivascular tissue or, according to researchers' judgment in subsequent studies, a high likelihood of fatal bleeding due to invasion of vital blood vessels; (8) Uncontrollable pleural, pericardial, or peritoneal effusion requiring repeated drainage.
Treatments
All patients underwent a treatment regimen involving two consecutive weeks of anlotinib administration (12 mg/day) followed by a one-week break (one treatment session comprised two weeks of treatment and one week of rest). Etoposide (100 mg/m2) was administered intravenously from the 1st to the 3rd day as part of each treatment course. At the beginning of each course, patients received intravenous carboplatin or cisplatin (75 mg/m2), with the calculated area under the concentration-time curve for carboplatin set at 5 mg/ml/min. The treatment protocol included the assessment of tumor response, categorized as stable disease (SD), complete remission (CR), or partial remission (PR). Anlotinib was continued until disease progression, the occurrence of intolerable adverse reactions, or the patient's withdrawal from the study. A total of four chemotherapy courses were administered, involving a combination of etoposide with cisplatin/carboplatin.
Assessments
We evaluated the therapeutic outcomes using several criteria, including OS, PFS, DCR, and ORR. PFS, often referred to as Progressive Disease (PD)-free survival, measures the time from the experiment's initiation until death from any cause or the onset of PD. In the final follow-up, we examined the number of patients who survived throughout the analysis period. OS, the duration from the registration date until mortality attributed to a specific cause, was assessed, commonly known as the survival duration. Patients who were still alive by the deadline were examined. ORR is determined as the percentage of patients with CR and PR. The establishment of DCR relies on the percentage of patients with CR, PR, and Stable Disease SD. The RECIST (Version 1.1), which evaluates tumor response every two treatment courses, was employed in this study. Patients without brain metastasis and symptoms underwent chest CT, abdominal ultrasonography, and CT/MR every two courses, with brain MR/CT performed at 6-month intervals. For patients with brain metastases, abdominal ultrasound, MR/CT, chest CT, and CT/MR were conducted every two courses. Additionally, patients underwent Positron Emission Tomography (PET) CT and routine brain MR/CT examinations.
Statistical analysis
The projected increase in the median PFS value is anticipated to be from 4.0 months to 6.5 months. The enrollment period spanned 24 months, with a follow-up duration of 6.5 months. However, owing to the COVID-19 epidemic, progress might have been expedited. Enrollment was concluded due to the IMPAWER133 and Caspian studies, which endorsed the use of chemotherapy plus immunotherapy to enhance survival—typically considered the standard of care.
The summarized data includes the frequency and percentage of categorical variables, as well as the median and range for continuous variables. The calculation of Hazard Ratios (HR) for OS and estimation of PFS were performed using the Kaplan-Meier algorithm. All result measurements are accompanied by 95% confidence intervals (CIs), which are approximated using the Cox proportional hazard model. Differences in patients' baseline clinicopathological characteristics were assessed using either Fisher's exact test or Pearson χ2.
The Log-Rank test was employed in exploratory univariate analysis. To determine statistical significance, the pre-established threshold for testing was set at P < 0.05, indicating a noteworthy distinction. The p-values and CIs for all analyses are two-tailed. Summary statistics for AEs were presented using percentages and frequency counts. IBM SPSS Statistics software (version 24) was utilized for the analysis of all statistical results.
Results
Baseline characteristics of clinical subjects
The study was conducted at Linfen Central Hospital from February 2019 to February 2021. A total of 45 previously untreated patients with ES-SCLC were included, and the baseline characteristics of patients in the intention-to-treat set (ITT set; n=45) are presented in Table 1. The mean age was 61 years (range: 44-75), with 66.6% of patients being under the age of 65 (30/45). Of the participants, 77.7% were male (35/45), and 71.2% were either past or present smokers (32/45). All 45 patients in the ECOG had a baseline ECOG Performance Status (PS) of 1. The most commonly observed metastatic sites at baseline were the liver (28.9%, 13/45), brain (26.7%, 12/45), bone (28.9%, 13/45), pleura (24.4%, 11/45), and bilateral lungs (24.4%, 11/45). Among them, 20 cases received cisplatin (44.4%, 20/45), and 25 cases received carboplatin (55.5%, 25/45).
Five participants were excluded from the efficacy analysis as they did not complete two courses of treatment, which was necessary for data collection. Despite being under continuous care at a local hospital, these five patients did not undergo efficacy evaluation due to reasons unrelated to the study. The baseline characteristics of patients in the per-protocol set (PP set; n=40) are presented in Table 1. The data indicate that the mean age of the 40 patients was 61 years (range: 44-74), with 62.5% of them being under 65 years old (25/40). Among the participants, 77.5% were male (31/40), and 70.0% were former or current smokers (28/40). The Eastern Cooperative Oncology Group Performance Status (ECOG PS) for all 40 patients was 1 at baseline. The most frequently observed metastatic sites at baseline were the liver (32.5%, 13/40), bone (32.5%, 13/40), brain (30.0%, 12/40), and bilateral lungs (27.5%, 11/40). Of the patients, 42.5% received cisplatin (17/40), and 57.5% received carboplatin (23/40).
Tumor responses
Table 2 presents the tumor response results. In the PP group, thirty-six patients achieved a PR, constituting approximately 90% of the total SD was observed in 3 cases (7.5%), PD in 2 cases (5.0%). The ORR was 91.0% (95% CI: 78.5%-100.6%), and the DCR was 97.6% (95% CI: 91.8%-103.3%). In the ITT group (n=45), thirty-two patients achieved PR, accounting for approximately 71.1% of the total. Two patients (4.4%) exhibited SD, and one patient (2.2%) reported PD as a response. Nine cases (20.0%) were deemed ineligible for curative effect analysis. The ORR in this group was 75.0% (95% CI: 57.6%-86.4%), and the DCR was 77.8% (95% CI: 64.1%-92.0%).
Analysis of PFS time for patients in the PP group (n = 40)
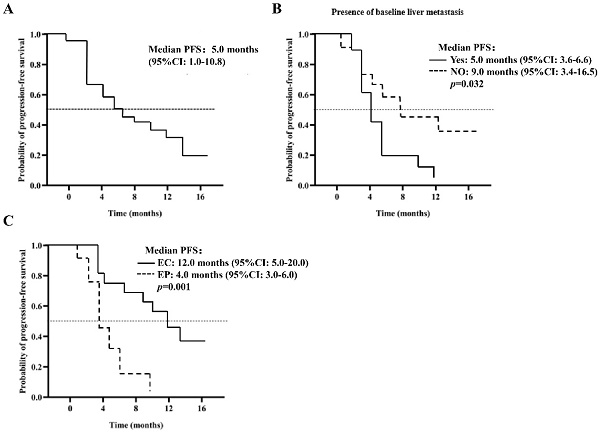
As of the March 21, 2022 deadline, progression was observed in 27 patients (67.5%). The median PFS is estimated at 5.0 months (95% CI: 1.0-10.8), as illustrated in Figure 1A. Various factors were analyzed to predict PFS, detailed in Table 3. The Log-Rank test revealed a significant association between baseline liver metastasis (p=0.032), chemotherapy regimen (p=0.001), and PFS. The PFS for patients with baseline liver metastasis was notably shorter than that for those with extraneous liver metastasis at baseline (5.0 months, 95% CI: 3.6-6.6 vs. 9.0 months, 95% CI: 3.4-16.5, p=0.032), as depicted in Figure 1B. In multivariate analysis, the chemotherapy regimen emerged as an independent risk factor for PFS (12.0 months, 95% CI: 5.0-20.0 vs. 4.0 months, 95% CI: 3.0-6.0, p=0.021), as shown in Figure 1C. Analysis of 27 patients with PD revealed that 14 (51.8%) received second-line treatment, 10 (37.0%) received the best supportive therapy, and 3 (11.2%) were lost to follow-up.
Analysis of OS time of patients (PP set)
The median OS is estimated to be 13.0 months (95% CI: 8.4-18.6), as depicted in Figure 2A. The analysis, based on the Log-Rank test, indicated a significant correlation between OS and the chemotherapy treatment (p=0.016) (Table 4). Patients who received EP chemotherapy had a substantially shorter OS than those who received EC chemotherapy (EP vs. EC, 9.0 months, 95% CI: 7.5-10.5 vs. 14.0 months, 95% CI: 9.6-19.6, p=0.016) (Figure 2B).
Report statistics of adverse events and safety in ITT set (n = 45)
The most prevalent AEs were hypertension (24/45, 53.33%), anemia (19/45, 42.22%), alopecia (18/45, 40.00%), elevation of transaminases (11/45, 24.40%), and elevation of alkaline phosphatase (11/45, 24.44%) (Table 5). There were 16 cases of grade 5 adverse reactions (35.56%). No unexpected adverse reactions were observed, and no patient was found to have died as a result of treatment in this study.
Baseline characteristics of patients with ITT (n= 45) set and PP set (n= 40)
| Characteristics | ITT set | PP set | P value |
|---|---|---|---|
| Age, years | |||
| Mean | 61 (44-75) | 61 (44-74) | 0.76 |
| Age group | |||
| <65 years | 30 (66.6%) | 25 (62.5%) | 0.43 |
| ≥65 years | 15 (33.4%) | 15 (37.5%) | 0.85 |
| Smoking history | |||
| Never-smoker | 13 (28.8%) | 12 (30.0%) | 0.33 |
| Former or current smoker | 32 (71.2%) | 28 (70.0%) | 0.35 |
| Sex | |||
| Male | 35 (77.7%) | 31 (77.5%) | 0.91 |
| Female | 10 (22.3%) | 9 (22.5%) | 0.57 |
| ECOG PS at baseline | |||
| 1 | 45 (100.0%) | 40 (100.0%) | 0.68 |
| Metastatic sites at baseline | |||
| Bilateral lung | 11 (24.4%) | 11 (27.5%) | 0.66 |
| Liver | 13 (28.9%) | 13 (32.5%) | 0.74 |
| Bone | 13 (28.9%) | 13 (32.5%) | 0.15 |
| Brain | 12 (26.7%) | 12 (30.0%) | 0.26 |
| Supraclavicular lymph node | 9 (20.0%) | 8 (20.0%) | 0.39 |
| Adrenal gland | 6 (13.3%) | 6 (15.0%) | 0.66 |
| Pleural | 11 (24.4%) | 8 (20.0%) | 0.19 |
| Others | 13 (28.9%) | 8 (20.0%) | 0.78 |
| Chemotherapy | |||
| EP | 20 (44.4%) | 17 (42.5%) | 0.49 |
| EC | 25 (55.5%) | 23 (57.5%) | 0.83 |
Therapeutic efficacy and tumor response of ITT set (n= 45) and PP set (n= 40)
| Responses | ITT set (n= 45) [n or %] | PP set (n= 40) [n or %] | P values |
|---|---|---|---|
| PR | 32 (71.1) | 36 (90.0) | 0.84 |
| PD | 1 (2.2) | 2 (5.0) | 0.22 |
| Ineligible | 9 (20.0) | 0 (0.0) | 0.15 |
| SD | 2 (4.4) | 3 (7.5) | 0.32 |
| DCR | 77.8% [95% CI: 64.1% - 92.0%] | 97.6% [95% CI: 91.8% - 103.3%] | 0.58 |
| ORR | 75.0% [95% CI: 57.6% - 86.4%] | 91.0% [95% CI: 78.5% - 100.6%] | 0.92 |
Kaplan Meier curves for PFS (A) 40 patients in the PP group (n = 40); (B) does the patient develop baseline liver metastases; (C) the patient was given EP / EC plus anlotinib.
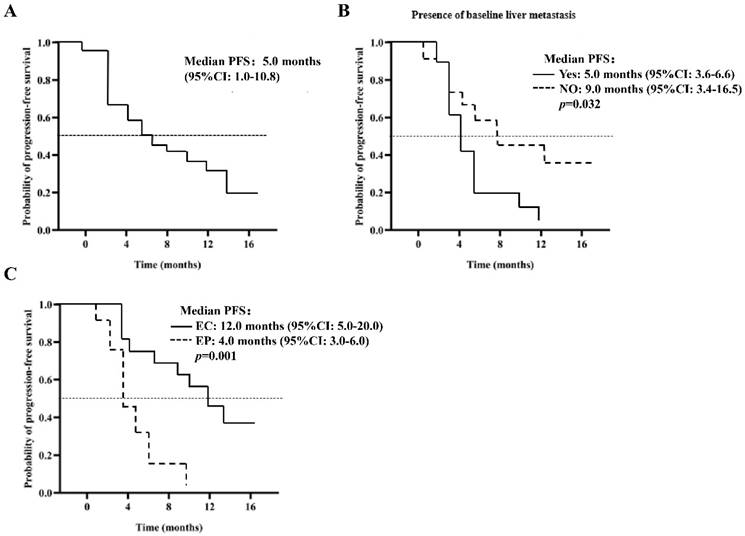
Kaplan-Meier curve of OS (A) 40 patients in the PP group; (B) patients were given EP / EC in combination with anlotinib.
Discussion
This study has significantly contributed to understanding the role of anlotinib combined with etoposide and platinum-based chemotherapy in treating ES-SCLC. Our findings, indicating a median PFS of 5.0 months and OS of 13.0 months, underscore the potential of this combination therapy. These survival rates, coupled with a high DCR and ORR, are promising, especially considering the aggressive nature and traditionally poor prognosis associated with ES-SCLC [29, 30]. The primary adverse event observed, hypertension, is a known effect of antiangiogenic therapies and aligns with findings from other studies in lung cancer treatments [31, 32]. The manageability of this side effect is crucial for patient care, suggesting that anlotinib can be incorporated into ES-SCLC treatment protocols without significantly increasing treatment-related toxicity [33].
Evaluation of PFS of PP set by Cox multivariate analysis
| Multivariate analysis | ||||
|---|---|---|---|---|
| Variables | Log-rank test | HR | 95% CI | p-value |
| Age (<65 vs. ≥ 65 years) | 0.061 | 0.432 | 0.412-1.264 | 0.254 |
| Lung metastasis (yes vs. no) | 0.373 | 0.961 | 0.865-2.654 | 0.425 |
| Adrenal gland metastasis (yes vs. no) | 0.672 | 0.857 | 0.236-0.935 | 0.234 |
| Bone metastasis (yes vs. no) | 0.187 | 1.067 | 0.968-3.935 | 0.135 |
| Pleural metastasis (yes vs. no) | 0.259 | 1.265 | 0.868-1.896 | 0.785 |
| Smoking history (yes vs. no) | 0.314 | 0.587 | 0.457-0.824 | 0.657 |
| Liver metastasis (yes vs. no) | 0.032 | 0.587 | 0.312-1.884 | 0.531 |
| Sex (male vs. female) | 0.897 | 0.256 | 0.157-0.482 | 0.681 |
| Brain metastasis (yes vs. no) | 0.471 | 0.759 | 0.582-0.897 | 0.279 |
| Chemotherapy (EP vs. EC) | 0.001 | 4.367 | 1.457-11.89 | 0.021 |
| Supraclavicular lymph node metastasis (yes vs. no) | 0.453 | 2.215 | 1.256-4.658 | 0.658 |
Evaluation of OS of PP set by Cox multivariate analysis
| Variables | Log-rank test |
|---|---|
| Brain metastasis (yes vs. no) | 0.772 |
| Sex (male vs female) | 0.714 |
| Age (<65 vs. ≥65 years) | 0.223 |
| Bone metastasis (yes vs. no) | 0.451 |
| Lung metastasis (yes vs. no) | 0.670 |
| Smoking history (yes vs. no) | 0.891 |
| Supraclavicular lymph node metastasis (yes vs. no) | 0.278 |
| Chemotherapy (EP vs. EC) | 0.016 |
| Adrenal gland metastasis (yes vs. no) | 0.217 |
| Pleural metastasis (yes vs. no) | 0.289 |
| Liver metastasis (yes vs. no) | 0.254 |
Report statistics of AEs in ITT set (n= 45)
| AEs | All grades [no. (%)] | ≥Grade 3 [no. (%)] |
|---|---|---|
| Any | 43(95.56%) | 16(35.56%) |
| Anemia | 19(42.22%) | |
| Hypertension | 24(53.33%) | 2(4.44%) |
| Alopecia | 18(40.00%) | |
| Elevated alkaline phosphatase | 11(24.44%) | |
| Neutropenia | 7(15.56%) | 6(13.33%) |
| Leukopenia/hyponatremia | 9(20.00%) | |
| Elevated transaminases | 11(24.40%) | |
| Thrombocytopenia | 10(22.22%) | |
| Fatigue | 6(13.33%) | 5(11.11%) |
| Elevated white blood cells | 5(11.11%) | |
| Oral pain | 5(11.11%) | |
| Diarrhea | 4(8.89%) | |
| Constipation | 4(8.89%) | |
| Nausea | 4(8.89%) | |
| Rash | 4(8.89%) | 1(2.22) |
| Thrombosis | 2(4.44%) | |
| Hyperuricemia | 2(4.44%) | |
| Hemorrhage | 2(4.44%) | |
| Vomiting | 1(2.22%) | |
| Hyperkalemia | 1(2.22%) | |
| Hypocalcemia | 1(2.22%) | 1(2.22) |
| Decreased appetite | 1(2.22%) | |
| Hypophosphatemia | 1(2.22%) | 1(2.22) |
The rapid progression and development of chemotherapy resistance in ES-SCLC remain substantial challenges in oncology [34]. Our study takes a novel approach by introducing anlotinib in first-line treatment, providing a potential avenue to enhance outcomes in ES-SCLC patients. This strategy aligns with the evolving landscape of lung cancer treatment, where integrating novel agents with traditional therapies is increasingly recognized as a viable approach to overcoming treatment resistance and improving survival rates [35, 36]. While NSCLC has witnessed substantial advancements with targeted therapies and immunotherapies, ES-SCLC has not experienced the same level of therapeutic evolution due to its unique biological characteristics [37, 38]. Our study, focusing on anlotinib for ES-SCLC, contributes to bridging this gap, suggesting that targeted approaches can yield positive outcomes in this challenging cancer subtype [39].
Our findings suggest that anlotinib's antiangiogenic properties may offer a distinct advantage in treating ES-SCLC. Angiogenesis plays a crucial role in tumor growth and metastasis, and its inhibition could be particularly effective in combating the rapid progression characteristic of ES-SCLC [40]. This hypothesis aligns with the observed efficacy in our study, where anlotinib, combined with chemotherapy, led to significant tumor control and extended survival times.
Further, the observed DCR and ORR in our study are notable. The high response rates suggest that anlotinib, combined with etoposide and platinum-based chemotherapy, can induce substantial tumor regression, an essential factor in managing ES-SCLC [41]. This is particularly relevant given the often-limited response of ES-SCLC to conventional chemotherapy after initial treatment [42].
The potential for anlotinib in ES-SCLC raises questions about the optimal integration of targeted therapies in the standard treatment regimen for this cancer subtype. Our results suggest that earlier introduction of targeted agents like anlotinib in the treatment protocol could be beneficial. This approach could potentially transform the current therapeutic strategy for ES-SCLC, where advancements have been slower than other lung cancer subtypes [43, 44].
Furthermore, the implications of our findings extend beyond the immediate scope of ES-SCLC treatment. They highlight the importance of continuing to explore and understand the molecular pathways involved in ES-SCLC. Investigating the mechanisms through which anlotinib exerts its anti-cancer effects could lead to discovering novel biomarkers for predicting treatment response and identifying patients who would most benefit from this therapy [45].
In conclusion, our study presents anlotinib combined with etoposide and platinum-based chemotherapy as a promising new treatment option for newly diagnosed ES-SCLC—our study's favorable survival outcomes and manageable safety profile warrant further investigation. Larger-scale, randomized controlled trials are essential to confirm these findings. Such studies will be crucial in establishing a new standard of care for ES-SCLC, offering hope for improved treatment outcomes in this challenging and aggressive form of lung cancer [46].
Conclusion
In general, our results demonstrate the efficacy and assurance of anlotinib combined with chemotherapy for managing newly diagnosed ES-SCLC. In the future, numerous randomized phase III clinical trials will be necessary to further validate the therapeutic effectiveness of anlotinib in the treatment of ES-SCLC.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Peng P, Luan Y, Sun P, Wang L, Zeng X, Wang Y. et al. Prognostic factors in stage IV colorectal cancer patients with resection of liver and/or pulmonary metastases: a population-based cohort study. Frontiers in Oncology. 2022;12:850937
2. Wang X, Chen G, Zhang Y, Ghareeb WM, Yu Q, Zhu H. et al. The impact of circumferential tumour location on the clinical outcome of rectal cancer patients managed with neoadjuvant chemoradiotherapy followed by total mesorectal excision. European Journal of Surgical Oncology. 2020;46:1118-23
3. No HJ, Raja N, Von Eyben R, Das M, Roy M, Myall N. et al. Characterization of Metastatic Non-Small Cell Lung Cancer and Oligometastatic Incidence in an Era of Changing Treatment Paradigms. Int J Radiat Oncol Biol Phys. 2022;114:603-610
4. Recondo G, Mezquita L. Tiragolumab and atezolizumab in patients with PD-L1 positive non-small-cell lung cancer. Lancet Oncol. 2022;23:695-7
5. Cao X, Ganti A, Stinchcombe T, Wong M, Ho J, Shen C. et al. Predicting risk of chemotherapy-induced severe neutropenia: A pooled analysis in individual patients data with advanced lung cancer. Lung Cancer. 2020;141:14-20
6. Matutino A, Mak M, Takahashi T, Bitton R, Nakazato D, Fraile N. et al. Prophylactic Cranial Irradiation for Extensive-Stage Small-Cell Lung Cancer: A Retrospective Analysis. Front Oncol. 2018;4:1-7
7. Cui Y, Wang X, Lin F, Li W, Zhao Y, Zhu F. et al. MiR-29a-3p improves acute lung injury by reducing alveolar epithelial cell PANoptosis. Aging and disease. 2022;13:899
8. Chen X, Liao Y, Long D, Yu T, Shen F, Lin X. The Cdc2/Cdk1 inhibitor, purvalanol A, enhances the cytotoxic effects of taxol through Op18/stathmin in non-small cell lung cancer cells in vitro. International Journal of Molecular Medicine. 2017;40:235-42
9. Chen S, Zhao Y, Shen F, Long D, Yu T, Lin X. Introduction of exogenous wild-type p53 mediates the regulation of oncoprotein 18/stathmin signaling via nuclear factor-κB in non-small cell lung cancer NCI-H1299 cells. Oncology Reports. 2019;41:2051-9
10. Zhao Y, Chen S, Shen F, Long D, Yu T, Wu M. et al. In vitro neutralization of autocrine IL-10 affects Op18/stathmin signaling in non-small cell lung cancer cells. Oncology Reports. 2019;41:501-11
11. Potti A, Ganti A, Tuchman S, Sholes K, Langness E, Koka V. et al. HER-2/neu and CD117 (c-kit) overexpression in patients with pesticide exposure and extensive stage small cell lung carcinoma (ESSCLC). J Carcinog. 2005;4:8
12. Potti A, Moazzam N, Ramar K, Hanekom D, Kargas S, Koch M. CD117 (c-KIT) overexpression in patients with extensive-stage small-cell lung carcinoma. Ann Oncol. 2003;14:894-7
13. Lu S, Yang B, Xiao Y, Liu S, Liu M, Yin L. et al. Iterative reconstruction of low-dose CT based on differential sparse. Biomedical Signal Processing and Control. 2023;79:104204
14. Lu S, Liu S, Hou P, Yang B, Liu M, Yin L. et al. Soft Tissue Feature Tracking Based on DeepMatching Network. CMES-Computer Modeling in Engineering & Sciences. 2023 136
15. Zhu Y, Huang R, Wu Z, Song S, Cheng L, Zhu R. Deep learning-based predictive identification of neural stem cell differentiation. Nature communications. 2021;12:2614
16. Lu S, Yang J, Yang B, Yin Z, Liu M, Yin L. et al. Analysis and Design of Surgical Instrument Localization Algorithm. CMES-Computer Modeling in Engineering & Sciences. 2023 137
17. Zhu Y, Wu H, Yang X, Xiong Z, Zhao T, Gan XJB. LINC00514 facilitates cell proliferation, migration, invasion, and epithelial-mesenchymal transition in non-small cell lung cancer by acting on the Wnt/β-catenin signaling pathway. Bioengineered. 2022;13:13654-66
18. Nokihara H, Ogino H, Mitsuhashi A, Kondo K, Ogawa E, Ozaki R. et al. Efficacy of osimertinib in epidermal growth factor receptor-mutated non-small-cell lung cancer patients with pleural effusion. BMC Cancer. 2022;22:597
19. Cui J, Li L, Yuan S. The Value of Radiotherapy for Advanced Non-Small Cell Lung Cancer With Oncogene Driver-Mutation. Front Oncol. 2022;12:863715
20. Yang X, Zeng Y, Tan Q, Huang Z, Jia J, Jiang G. Efficacy of PD-1/PD-L1 Inhibitors versus Chemotherapy in Lung Cancer with Brain Metastases: A Systematic Review and Meta-Analysis. J Immunol Res. 2022;2022:4518898
21. Chang J, Huang C, Fang Y, Chang C, Yang C, Kuo C. et al. Epidermal growth factor receptor tyrosine kinase inhibitors for de novo T790M mutation: A retrospective study of 44 patients. Thorac Cancer. 2022;13:1888-1897
22. Gan Y, Liu P, Luo T. Successful Treatment of an Elderly Patient With Combined Small Cell Lung Cancer Receiving Anlotinib: A Case Report. Front Oncol. 2021;11:775201
23. Gong J, Wan Q, Shang J, Qian X, Su D, Sun Z. et al. Cost-Effectiveness Analysis of Anlotinib as Third- or Further-Line Treatment for Relapsed Small Cell Lung Cancer (SCLC) in China. Adv Ther. 2021;38:5116-26
24. Wu Y, Zhang T, Liu Y, Wang J, Bi N. Anlotinib combined with durvalumab in a patient with recurrent multifocal brain metastases of small cell lung cancer after definitive concurrent chemoradiotherapy and palliative radiotherapy of the lung and brain: a case report. Ann Palliat Med. 2021;10:2379-86
25. Chen D, Xu J, Zhao Y, Chu T, Zhong H, Han B. et al. Prognostic value of tumor cavitation in extensive-stage small-cell lung cancer patients treated with anlotinib. J Cancer Res Clin Oncol. 2020;146:401-6
26. Liu C, Liao J, Wu X, Zhao X, Sun S, Wang H. et al. A phase II study of anlotinib combined with etoposide and platinum-based regimens in the first-line treatment of extensive-stage small cell lung cancer. Thorac Cancer. 2022;13:1463-70
27. Deng P, Hu C, Chen C, Cao L, Gu Q, An J. et al. Anlotinib plus platinum-etoposide as a first-line treatment for extensive-stage small cell lung cancer: A single-arm trial. Cancer Med. 2022;11:3563-3571
28. Zhang Y, Mengnan Z, Benke L, Zhang B, Bing C, Yuanyuan W. et al. Ephedra Herb extract ameliorates adriamycin-induced nephrotic syndrome in rats via the CAMKK2/AMPK/mTOR signaling pathway. Chinese Journal of Natural Medicines. 2023;21:371-82
29. Valadares BN, Stephano MA. Small cell lung cancer: an overview of the targets. Brazilian Journal of Pharmaceutical Sciences. 2022;58:e19114
30. Demedts I, Vermaelen K, Van Meerbeeck J. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. European Respiratory Journal. 2010;35:202-15
31. Chan SHY, Khatib Y, Webley S, Layton D, Salek S. Identification of cardiotoxicity related to non-small cell lung cancer (NSCLC) treatments: A systematic review. Frontiers in Pharmacology. 2023;14:1137983
32. Gould Rothberg BE, Quest TE, Yeung SCJ, Pelosof LC, Gerber DE, Seltzer JA. et al. Oncologic emergencies and urgencies: A comprehensive review. CA: A Cancer Journal for Clinicians. 2022;72:570-93
33. Yan X, Zhao Z, Tang H. Current status and future of anti-angiogenic drugs in lung cancer. Clinical and Experimental Medicine. 2023:1-15
34. Upadhyay P, Ghosh A, Sarangthem V, Singh TD. Nanocarrier mediated co-delivery of phytochemicals and chemo-drugs: an emerging strategy to combat lung cancer in a systemic way. Phytochemistry Reviews. 2023:1-43
35. Khurshid H, Ismaila N, Bian J, Dabney R, Das M, Ellis P. et al. Systemic therapy for small-cell lung cancer: ASCO-Ontario health (cancer care Ontario) guideline. Journal of Clinical Oncology. 2023;41:5448-5472
36. De Sanctis JB, Charris J, Blanco Z, Ramírez H, Martínez GP, Mijares MR. Molecular Mechanisms of Chloroquine and Hydroxychloroquine Used in Cancer Therapy. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents). 2023;23:1122-44
37. Lum C, Alamgeer M. Technological and therapeutic advances in advanced small cell lung cancer. Cancers. 2019;11:1570
38. Megyesfalvi Z, Gay CM, Popper H, Pirker R, Ostoros G, Heeke S. et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA: A Cancer Journal for Clinicians. 2023
39. Kumar H, Gupta NV, Jain R, Madhunapantula SV, Babu CS, Kesharwani SS. et al. A review of biological targets and therapeutic approaches in the management of triple-negative breast cancer. Journal of Advanced Research. 2023
40. Zhang C, Wang H. Accurate treatment of small cell lung cancer: Current progress, new challenges and expectations. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2022: 188798.
41. Li Y, Sun Z, Sun W, Wang H, Zu J. Effectiveness and safety of anlotinib monotherapy for patients with extensive-stage small-cell lung cancer who progressed to chemotherapy: a real-world exploratory study. Clinical Medicine Insights: Oncology. 2022;16:11795549211067184
42. Guaitoli G, Neri G, Cabitza E, Natalizio S, Mastrodomenico L, Talerico S. et al. Dissecting Immunotherapy Strategies for Small Cell Lung Cancer: Antibodies, Ionizing Radiation and CAR-T. International Journal of Molecular Sciences. 2022;23:12728
43. Cani M, Napoli VM, Garbo E, Ferrari G, Del Rio B, Novello S. et al. Targeted Therapies in Small Cell Lung Cancer: From Old Failures to Novel Therapeutic Strategies. International Journal of Molecular Sciences. 2023;24:8883
44. El Sayed R, El Darsa H. Therapeutic modalities in small cell lung cancer: a paradigm shift after decades of quiescence. Expert Opinion on Pharmacotherapy. 2022;23:583-97
45. Yuan M, Zhao Y, Arkenau H-T, Lao T, Chu L, Xu Q. Signal pathways and precision therapy of small-cell lung cancer. Signal Transduction and Targeted Therapy. 2022;7:187
46. Yu L, Lai Q, Gou L, Feng J, Yang J. Opportunities and obstacles of targeted therapy and immunotherapy in small cell lung cancer. Journal of Drug Targeting. 2021;29:1-11
Author contact
![]() Corresponding author: Weiwei Zhi, Email: weiwei.zhicom.
Corresponding author: Weiwei Zhi, Email: weiwei.zhicom.

 Global reach, higher impact
Global reach, higher impact