3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(11):3539-3546. doi:10.7150/jca.91701 This issue Cite
Research Paper
The Efficacy and Safety of Anlotinib Plus Etoposide with Cisplatin/Carboplatin in the First-Line Treatment of Lung Cancer: A Phase II Clinical Study
1. Department of Thoracic Surgery, Linfen Central Hospital, Linfen, Shanxi, 041000, China.
2. Department of Cardiovascular Surgery, Xijing Hospital, Air Force Military Medical, University, Xincheng, Xi'an, 710032, China.
3. Department of medical, Xi'an Fourth Hospital, Xincheng, Xi'an, 710004, China.
4. Department of Cardiovascular Surgery, Xi'an Fourth Hospital, Xincheng, Xi'an, 710004, China.
Abstract
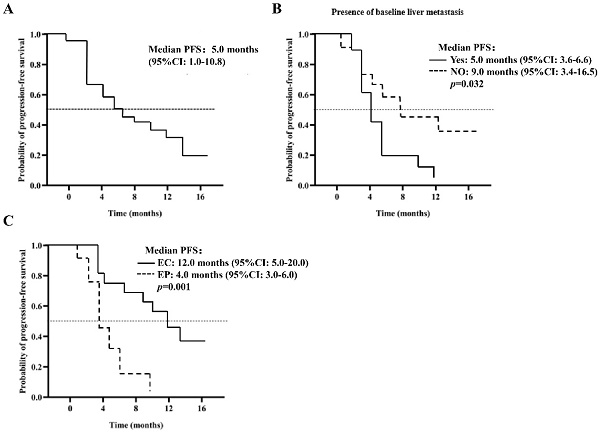
Background: The primary aim of this phase II clinical study was to assess the safety and efficacy of combining anlotinib, etoposide, and platinum-based drugs as a first-line treatment for ES-SCLC.
Methods: Patients underwent the standard chemotherapeutic regimen, consisting of four courses of etoposide plus cisplatin/carboplatin. Additionally, each patient received a 2-week intervention with anlotinib (12 mg/day, once daily). Anlotinib was continued until disease progression, occurrence of unbearable adverse events (AEs), or withdrawal from the research. Progression-free survival (PFS) served as the primary prognostic measure. Secondary measures included the disease control rate (DCR), objective response rate (ORR), overall survival time (OS), and the incidence of AEs.
Results: The DCR and ORR were 97.6% and 91.0%, respectively. Estimated PFS and OS were 5.0 months (95% CI: 1.0-10.8 months) and 13.0 months (95% CI: 8.4-18.6 months), respectively. No unexpected adverse effects were reported during the trial. The most common adverse reactions included anemia (42.22%), hypertension (53.33%), alopecia (40.00%), elevated transaminase (24.40%), and elevated alkaline phosphatase (24.44%). Sixteen cases (35.56%) were classified as AEs of grades 3-5. No deaths attributed to treatment-related causes occurred in any patient during the trial.
Conclusion: Combination chemotherapy is currently the first-line therapy for extensive small-cell lung cancer (ES-SCLC). Combining anlotinib with conventional platinum-based chemotherapy demonstrated promising therapeutic outcomes and prognosis in the management of ES-SCLC.
Keywords: Anlotinib, Etoposide, Clinical trials, Lung cancer

 Global reach, higher impact
Global reach, higher impact