3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(11):3452-3465. doi:10.7150/jca.92588 This issue Cite
Research Paper
S100A8-Mediated Inflammatory Signaling Drives Colorectal Cancer Progression via the CXCL5/CXCR2 Axis
1. Department of Gastroenterology, The Third Xiangya Hospital of Central South University, Changsha, Hunan, China.
2. Hunan Key Laboratory of Nonresolving Inflammation and Cancer, Central South University, Changsha, Hunan, China.
3. Department of Gastroenterology, Anhui No.2 Provincial People's Hospital.
4. Department of Pathology, Liuzhou People's Hospital Affiliated to Guangxi Medical University, No. 8 Wenchang Road, Liuzhou 545006, China.
5. Hunan Key Laboratory of Cancer Metabolism, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.
6. Key Laboratory of Carcinogenesis and Cancer Invasion of the Chinese Ministry of Education, NHC Key Laboratory of Carcinogenesis, Cancer Research Institute, School of Basic Medical Science, Central South University, Changsha, China.
* These authors contributed equally to this work and should be considered co-first authors.
Abstract
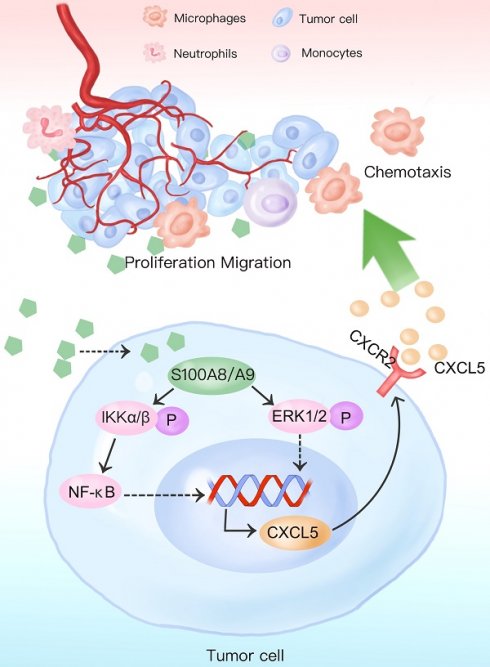
Background: S100A8/S100A9 belong to the S100 calcium-binding protein family and play an essential role in the progression of chronic inflammation in diseases. It also regulates various biological processes such as tumor cell survival, apoptosis, and invasive metastasis. The extracellular form of S100A8/S100A9 functions by modulating cellular oxidative metabolism and facilitating inflammation-to-cancer progression. This modulation occurs through specific binding to receptors like RAGE and TLR4 and activation of signaling pathways including STAT3 and NF-κB. In tumor cells, S100A8 and S100A9 induce phenotypic changes by influencing calcium ion concentrations and other pathways. However, the precise function of high S100A8/S100A9 expression in colorectal cancer cells remains unclear.
Methods: To explore the role of S100A8/S100A9 in colorectal cancer, we used immunohistochemistry and data from GEO and TCGA databases to analyze its expression in colorectal cancer cells, normal intestinal mucosa, and adjacent tissues. Functional models of high S100A8/S100A9 expression in colorectal cancer cells were established through transfection with overexpression plasmids. Protein microarrays, enzyme-linked immunosorbent assays (ELISAs), and real-time PCR were employed to assess the expression and secretion of 40 cytokines. MTT and Transwell invasion assays were conducted to evaluate changes in cell proliferation, invasion, and chemotaxis. Finally, tail vein and subcutaneous tumorigenesis assays assessed cell proliferation and migration in vivo.
Results: We observed significantly higher expression of S100A8/S100A9 in colorectal cancer epithelial cells compared to normal intestinal mucosa and adjacent tissues. Overexpression of S100A8/S100A9 in mouse colon cancer cells CT26.WT led to differential increases in the secretion levels of various cytokines (CXCL5, CXCL11, GM-CSF, G-CSF, IL1a, IL1b, sTNF RI, and CCL3). Additionally, this overexpression activated signaling pathways such as STAT3, NF-κB, and ERK-MAPK. The synthesis and secretion of inflammatory factors could be inhibited by using NF-κB and ERK-MAPK pathway inhibitors. Moreover, S100A8 promotes the proliferation and invasion of colon cancer cells. Notably, the CXCR2 inhibitor (SB265610) effectively reversed the phenotypic changes induced by the CXCL5/CXCR2 biological axis.
Conclusions: Our findings indicate that increased expression of S100A8 and S100A9 in colon cancer epithelial cells enhances the secretion of inflammatory factors by activating NF-κB, ERK-MAPK, and other signaling pathways. S100A8 facilitates colon cancer cell proliferation, invasion, and metastasis through the CXCL5/CXCR2 biological axis.
Keywords: S100A8/S100A9, colorectal cancer, inflammatory cytokine, signaling pathways, NF-κB, ERK-MAPK, CXCL5/CXCR2

 Global reach, higher impact
Global reach, higher impact