Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(6):1770-1778. doi:10.7150/jca.90620 This issue Cite
Research Paper
Exosome-mediated delivery of artificial circular RNAs for gene therapy of bladder cancer
1. The Affiliated Nanhua Hospital, Department of Urology, Hengyang Medical School, University of South China, Hengyang, 421002, Hunan, China.
2. Department of Nursing, School of Medicine, Huainan Union University, Huainan, 232038, Anhui, China.
# Equal contributors.
Abstract
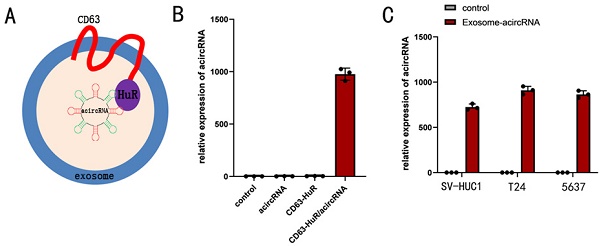
Bladder cancer (BCa) is one of the most common malignancies affecting men. Oncogenic transcription factors function as an important regulator in the progression of human cancer. In our study, we aimed to construct artificial circular non-coding RNAs (acircRNAs) consisting of three functional units that mimic the CRISPR-Cas system and elucidate its therapeutic role in bladder cancer. Additionally, the compare of the efficiency in regulating gene expression between acircRNA and CRISPR-dCas systems was performed. We connected the cDNA sequences of TFs aptamer and constructed a circRNA. To demonstrate the platform's practicality, β-catenin and NF-κB were chosen as functional targets, while T24 and 5637 cell lines served as test models. Real-time Quantitative PCR (qPCR), double luciferase assay and related phenotype assay were used to detect the expression of related genes and the therapeutic effect. To elucidate the functionality of acircRNAs, luciferase vectors capable of detecting β-catenin and NF-κB expression were employed to assess the inhibitory impact of acircRNA on β-catenin and NF-κB. Consequently, the optimal combination involving acircRNA-3 was determined. Next, qPCR assay was employed to assess the relative expression levels of target downstream genes following acircRNA treatment. The expression of c-myc and cyclin D1 were used to determine the function of β-catenin, while Bcl-XL and TRAF1 were used to determine that of NF-κB. The acircRNAs inhibited the β-catenin and NF-κB related signaling in BCa cells specifically. CD63-HuR fusion protein was used to loading acircRNA into exosomes. The results showed that acircRNA could inhibit the activity of the target transcription factors, and the inhibitory effect was better than that of CRIPSR-dCas9-KRAB. Furthermore, functional experiments demonstrated that the transfection of acircRNA in bladder cells resulted in decreased proliferation, enhanced apoptosis, and suppressed migration. In conclusion, our synthetic gene device exhibited anti-tumor regulatory capabilities and showed greater efficiency in tumor suppression compared to the CRISPR-dCas9-KRAB system. Therefore, our device provides a new strategy for cancer treatment and could be a useful strategy for cancer cells.
Keywords: CRISPR-Cas9, AcircRNA, β-catenin, NF-κB, Bladder Cancer

 Global reach, higher impact
Global reach, higher impact