3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(6):1583-1592. doi:10.7150/jca.91716 This issue Cite
Research Paper
Contribution of a Circulating 2'-O-methylated MicroRNA Panel to the Diagnosis of Pancreatic Ductal Adenocarcinoma
1. Department of Gastroenterology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
2. Department of Gastroenterology, Ruijin Hospital North, Shanghai Jiao Tong University School of Medicine, Shanghai, 201801, China.
3. Department of Gastroenterology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 201620, China.
4. State Key Laboratory of Pharmaceutical Biotechnology, Jiangsu Engineering Research Center for MicroRNA Biology and Biotechnology, NJU Advanced Institute for Life Sciences (NAILS), School of Life Sciences, Nanjing University, Nanjing, Jiangsu, 210023, China.
*These authors contributed equally to this work.
Abstract
Background: We conducted an assessment of 2'-O-methylated (2'OMe) microRNAs (miRNAs) present in the circulation of individuals suffering from pancreatic ductal adenocarcinoma (PDAC). Subsequently, we devised a set of circulating 2'OMe miRNAs that can be utilized for the screening of PDAC patients within a group at increased risk.
Methods: A four-step, multicenter research was carried out. The initial screening phase involved analyzing 10 samples from patients with pancreatic ductal adenocarcinoma (PDAC) and 10 specimens from donors who were in good health. RNA sequencing was performed on these specimens after pre-treatment via NaIO4. The instruction and confirmation phases involved the use of 2'OMe miRNA profiling and multivariate analysis to examine applicant 2'OMe miRNAs in a sample of 248 individuals. In a prospective registration population of 135 individuals, a clinical screening panel was created and confirmed. The performance of individual 2'OMe miRNAs or the biomarker panel was evaluated using the receiver operating characteristic curve.
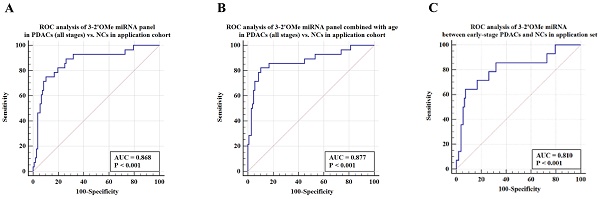
Results: Abnormal circulating 2'OMe miRNAs were detected in individuals suspected of pancreatic ductal adenocarcinoma (PDAC). A circulating panel of 3-2'OMe miRNAs, namely miR-28-3p, miR-143-3p, and miR-151a-3p, was subsequently identified. These miRNAs continually exhibited up-regulation in plasma samples of patients with pancreatic ductal adenocarcinoma (PDAC). The panel's area under the curve (AUC) was 1.0 in the experimental group and 0.928 in the verification cohort when comparing PDAC patients in all clinical stages to normal controls. During the application study, both the specificity and sensitivity were found to be 75.00% and 89.72% respectively, with an AUC value of 0.868. In the comparison between early-stage (I-II) PDAC patients and control subjects, the panel demonstrated an AUC of 1.0 in the training cohort and 0.924 in the validation population. In the application group the AUC was 0.810 (95% CI 0.729-0.876) in comparison to the high-risk symptomatic group.
Conclusion: Abnormal circulating 2'OMe miRNAs were detected in individuals with pancreatic ductal adenocarcinoma (PDAC). Consequently, we devised a 2'OMe miRNA biological marker panel that holds promise as an initial detection tool for PDAC.
Keywords: 2'-O-methylation, Circulating microRNA, Diagnosis, Pancreatic ductal adenocarcinoma, Plasma biomarkers

 Global reach, higher impact
Global reach, higher impact