3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(6):1568-1582. doi:10.7150/jca.91504 This issue Cite
Research Paper
Identification and Validation of a Hypoxia and Glycolysis Prognostic Signatures in Lung Adenocarcinoma
1. Department of Lung Cancer Surgery, Tianjin Medical University General Hospital, Tianjin, China.
2. Department of Dermatovenereology, Tianjin Medical University General Hospital/ Tianjin Institute of Sexually Transmitted Disease, Tianjin, China.
3. Tianjin Key Laboratory of Lung Cancer Metastasis and Tumor Microenvironment, Tianjin Lung Cancer Institute, Tianjin Medical University General Hospital, Tianjin, China.
†These authors have contributed equally to this work.
Abstract
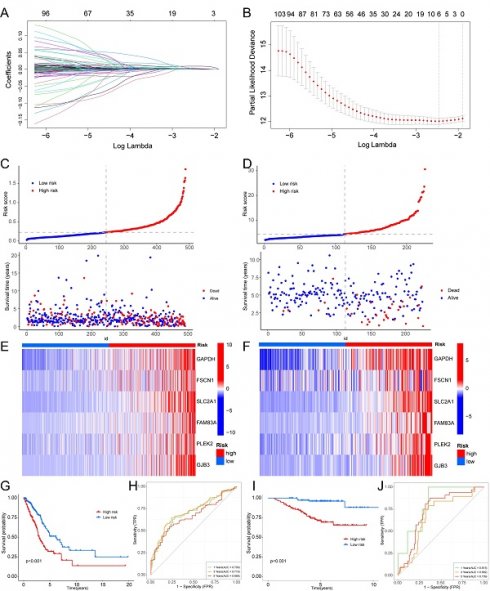
Background: Lung adenocarcinoma (LUAD) represents a prevalent subtype of non-small cell lung cancer with a complex molecular landscape. Dysregulated cellular energetics, notably the interplay between hypoxia and glycolysis, has emerged as a hallmark feature of LUAD tumorigenesis and progression. In this study, we aimed to identify hypoxia and glycolysis related gene signatures and construct a prognostic model to enhance the clinical management of LUAD.
Methods: A gene signature associated with hypoxia and glycolysis was established within the The Cancer Genome Atlas (TCGA) cohort and subsequently validated in the GSE31210 cohort. Additionally, a nomogram was formulated to aid in predictive modeling. Subsequently, an evaluation of the tumor microenvironment and immune checkpoints expression levels was conducted to discern disparities between low risk and high risk groups. Lastly, an exploration for drugs with potential effectiveness was carried out.
Results: Our analyses revealed a distinct hypoxia and glycolysis related gene signature consisting of 6 genes significantly associated with LUAD patient survival. Integration of these genes into the prognostic model demonstrated superior predictive accuracy for patient outcomes. Furthermore, we developed a user-friendly nomogram that effectively translates the model's prognostic information into a practical tool for clinical decision-making.
Conclusion: This study elucidates the critical role of hypoxia and glycolysis related genes in LUAD and offers a novel prognostic model with promising clinical utility. This model has the potential to refine risk stratification and guide personalized therapeutic interventions, ultimately improving the prognosis of LUAD patients.
Keywords: lung adenocarcinoma, hypoxia, glycolysis, immune, prognosis, immunotherapy.

 Global reach, higher impact
Global reach, higher impact