Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(2):281-289. doi:10.7150/jca.80955 This issue Cite
Review
Low Pretreatment Albumin-to-Globulin Ratios Predict Poor Survival Outcomes in Patients with Head and Neck Cancer: A Systematic Review and Meta-analysis
1. Department of Otorhinolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital, Chiayi, Taiwan.
2. College of Medicine, Chang Gung University, Taoyuan, Taiwan.
3. Division of Sports Medicine, Department of Orthopedic Surgery, Chang Gung Memorial Hospital, Chiayi, Taiwan.
4. Department of Radiation Oncology, Chang Gung Memorial Hospital, Chiayi, Taiwan.
5. Department of Diagnostic Radiology, Chang Gung Memorial Hospital, Chiayi, Taiwan.
6. Department of Otorhinolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital, Keelung, Taiwan.
7. Department of Otorhinolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital, Taoyuan, Taiwan.
Received 2022-11-17; Accepted 2022-12-25; Published 2023-1-9
Abstract
Background: Studies have indicated that a low albumin-to-globulin ratio (AGR) before treatment is linked to poor prognosis of many cancers, but the prognostic impact of AGR remains controversial in head and neck cancer (HNC). This meta-analysis examined the prognostic value of AGR in HNC.
Methods: We systematically searched the Embase, PubMed, and Cochrane library for relevant articles from inception to July 22, 2022. Studies conducted from 2000 to 2022 exploring the prognostic value of AGR in HNC were retrieved. We employed a random-effects model and calculated pooled hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) to examine the associations of AGR with survival outcome.
Results: Our analysis included nine studies involving 3211 patients with HNC. The pooled results revealed significant associations between low pretreatment AGRs and poor disease-free survival (HR = 1.97, 95% CI 1.58-2.45, p < 0.001), distant metastasis-free survival (HR = 1.64, 95% CI 1.25-2.16, p < 0.001), overall survival (HR = 2.18, 95% CI 1.65-2.88, p < 0.001), T3-T4 status (OR = 2.22, 95% CI 1.43-3.44, p < 0.001), stage III-IV disease (OR = 2.62, 95% CI 1.62-4.23, p < 0.001), and lymph node metastasis (OR = 1.95, 95% CI 1.29-2.82, p = 0.001) in patients with HNC.
Conclusion: AGR can serve as a prognostic biomarker in managing HNC, and a low pretreatment AGR is strongly associated with adverse survival outcomes and advanced cancer status. Additional large-scale prospective trials must be conducted to assess the validity of our findings.
Keywords: albumin-to-globulin ratio, head and neck cancer, meta-analysis, survival outcomes, biomarker
Introduction
Head and neck cancers (HNCs), including tumors that originate from the epithelium of the nasopharynx, larynx, hypopharynx, oropharynx, and oral cavity, are heterogeneous [1]. HNCs are the sixth most common malignancy worldwide and result in more than 200,000 deaths annually [2]. Although the prognosis of HNCs has improved, the 5-year survival rate is approximately 60% and varies significantly among patients with tumors at different locations [1]. The main reasons for treatment failure in HNCs are locoregional recurrence and distant metastasis [3], and the high risk of second primary cancers in head and neck regions create a considerable therapeutic challenge [4]. The tumor-node-metastasis (TNM) staging system is commonly used for HNC prognostics and treatment planning. However, the TNM system accounts only for anatomical tumour extension, and the survival outcomes of patients with the same disease stage often vary. Therefore, the identification of effective biomarkers that facilitate the early identification of patients with poor prognosis and the development of personalised treatment plans are crucial.
Because host nutrition and systemic inflammatory responses play major roles in tumorigenesis, disease progression, and cancer management [5, 6], numerous studies have explored the impacts of these factors on the prognosis of patients with cancer. Humans' main serum protein components, albumin (ALB) and globulin (GLB), have essential functions in systemic inflammatory responses and ligand transportation [7]. Researchers have identified ALB and GLB levels and the albumin-to-globulin ratio (AGR) as valuable prognostic markers in patients with various cancers, including colorectal cancer [8], brain tumor [9], urothelial cancer [10], and gastric and lung cancer [11, 12]. Given that malnutrition is frequently observed in patients with HNC before treatment [13], studies have investigated the relationship between pretreatment AGR and survival in patients with HNC [3, 14, 15]. However, because of the single-institution nature of these studies with small sample sizes as well as variations in study design, the results of these studies have remained inconclusive. A meta-analysis including 24 studies and 13,890 patients with solid tumours indicated that a high AGR was linked to better survival in patients with all disease stages and tumor locations, except for patients with oesophageal cancer [16]. However, this meta-analysis included only two studies on nasopharyngeal carcinoma (NPC), and to date, there is no quantitative and comprehensive assessment on the prognostic role of AGR in HNC. Therefore, we conducted this systematic review and meta-analysis to verify whether the AGR can predict prognosis and clinicopathological features of HNC, so as to render more cogent evidence to support its use in HNC management. Because AGRs can reflect both host nutrition status and systemic inflammatory responses, we hypothesised that AGR would be significantly associated with HNC prognosis.
Materials and Methods
Search strategy
We conducted the systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guidelines [17], and the study was registered in PROSPERO (CRD42022365813). Because the collected data were obtained from published articles, approval from an institutional review board was not necessary. Studies that included patients with HNC and investigated the prognostic value of AGR were eligible for inclusion. We conducted a search for eligible studies in Embase, Medline, PubMed, and the Cochrane Central Register of Controlled Trials from inception to July 22, 2022. We also searched the registry of trials administered by the US National Institutes of Health (http://clinicaltrials.gov) for ongoing clinical trials. Details regarding the search strategy and keywords used to search each database are listed in the Supplementary Materials (Table S1). The reference lists of the selected studies were independently and manually screened for potentially relevant publications by two review authors (Y.T.W. and Y.T.T.). We did not apply restrictions related to publication language, publication year, or participant ethnicity.
Study selection
To be eligible for inclusion in our review, articles were required to (1) report studies including patients with pathological diagnoses of HNC, (2) report studies investigating the association between AGR and survival outcomes, and (3) report relevant hazard ratios (HRs) and 95% confidence intervals (CIs) or sufficient information for their calculation. We excluded (1) case reports, letters, conference abstracts, reviews, and opinions; (2) articles that provided insufficient data for survival analysis; and (3) articles reporting nonhuman trials. The studies identified through the searches were independently evaluated by two authors (Y.T.W. and Y.T.T.). First, the titles and abstracts of all the selected articles were screened, and irrelevant studies were excluded. Thereafter, the full texts of the remaining articles were reviewed, and were screened texts for eligibility according to exclusion and inclusion criteria. Any inconsistencies were resolved through consultation with the third author (L.T.K.).
Data extraction
The two authors who conducted the search (Y.T.W. and Y.T.T.) independently extracted the following information from the included articles: (1) study information (year of publication, study region, study design, name of the first author, sample size, and follow-up period); (2) patient clinicopathological characteristics (cancer stage, tumour location, T status, lymph node metastasis, cancer cell differentiation, and treatment modality); (3) AGR cutoff values and the methods used to determine them; and (4) HRs with 95% CIs for survival analysis, including those for overall survival (OS), local relapse-free survival (LRFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and locoregional recurrence-free survival (LRRFS). The HRs and 95% CIs were extracted from the articles or were obtained from the survival curves and raw data through the use of methods proposed by Parmar and Tierney [18, 19]. If both univariate and multivariate data were provided, the adjusted HRs were used for a pooled analysis. The third author (L.T.K.) checked the extracted data.
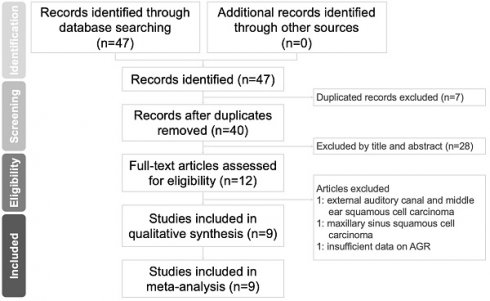
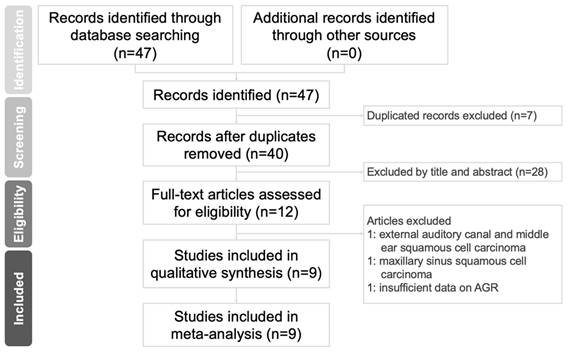
Flowchart of study selection.
Quality assessment
The two authors who extracted the data (Y.T.W. and Y.T.T.) used the Newcastle-Ottawa quality assessment scale (NOS) [20] to independently evaluate the methodological quality of the included studies. Scores on the NOS range from 0 to 9 points; we considered studies with scores of >6 to have high methodological quality. Any disagreements regarding the scores were resolved through consultation with the third author (L.T.K.).
Statistical analysis
Comprehensive Meta-Analysis software (version 3; Biostat, Englewood, NJ, USA), was applied to conduct all statistical analyses, and a p value of <0.05 represented statistical significance. To account for potential heterogeneity among the included studies, we employed a random-effects model [21]. We used Cochran's Q test and I2 values to measure heterogeneity among the included studies; a p value of <0.10 was considered statistically significant. I2 values of 75%-100%, 50%-74%, 25-49.9%, and 0%-24.9% indicated high heterogeneity, moderate heterogeneity, low heterogeneity, and no heterogeneity, respectively [22]. If significant heterogeneity was detected, we performed subgroup analyses to explore factors that may influence the prognostic value of AGR in HNC. We also performed sensitivity analysis involving sequential removal of each study to examine the robustness of the pooled results. We calculated odds ratios (ORs) and corresponding 95% CIs to measure the associations between AGR and clinicopathological characteristics, and a funnel plot test was performed to identify potential publication bias [23].
Results
Search results
A flowchart of the study selection procedure is presented in Figure 1. We retrieved 47 relevant published articles through our initial search. After excluding seven duplicates, we excluded 28 more articles after the abstracts and titles were screened. After the full texts of the remaining 12 articles were screened, three records were excluded; the reasons for exclusion are specified in Figure 1. Ultimately, nine studies involving 3211 patients with HNC [3, 14, 15, 24-29] met the eligibility criteria and were entered in our meta-analysis.
Study characteristics
The characteristics of enrolled studies are summarised in Table 1. All the included studies were retrospective and published between 2014 and 2021. The sample sizes ranged from 76 to 792 (median, 255). All the studies included patients with a stage I-IV disease; four, three, and two included patients with NPC [3, 25-27], oral cavity cancer [14, 24, 29], and laryngeal cancer [15, 28], respectively. The AGR cutoff values ranged from 1.19 to 1.6 (median, 1.34) and were determined using receiver operating characteristics curve analysis in six studies [3, 14, 15, 25, 26, 28], X-tile software in two studies [24, 29], and a method proposed by Igarashi et al. [30] in one study [27]. Only two of the selected studies investigated the association between AGR and LRFS/LRRFS; therefore, OS [3, 14, 15, 24, 25, 27-29], DFS [14, 15, 25, 28, 29], and DMFS [3, 26, 27] were selected as the primary outcomes of interest in our meta-analysis. All the included studies had NOS scores of >6 points (Table S2).
Association of AGR with OS
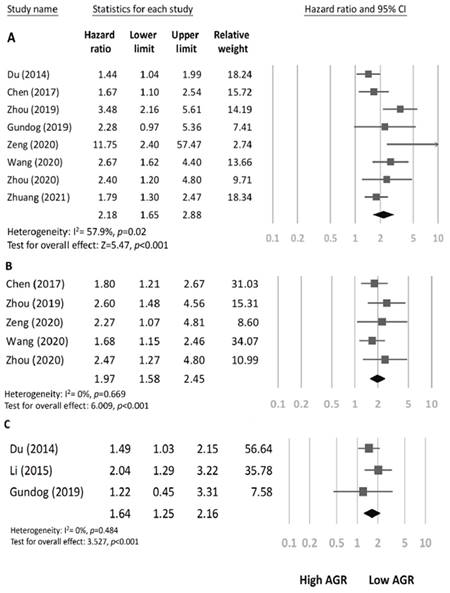
We detected moderate heterogeneity among the eight studies (involving 2691 patients) reporting a link between AGR and OS (I2 = 57.9%; PH = 0.02). According to the pooled results, a low AGR before treatment is linked with poor OS in HNC (pooled HR 2.18, CI 1.65-2.88, p < 0.001; Figure 2A).
In our search for sources of heterogeneity, we performed subgroup analyses stratified by tumor location (nasopharynx, oral cavity, or larynx), sample size (≤255 or >255), AGR cutoff value (≤1.34 or >1.34), and the method used to determine the cutoff value (ROC or others; Table 2). The results indicated that a low AGR had a significant association with poor OS in patients with laryngeal cancer (pooled HR=2.38, 95%CI 1.29-4.40, p = 0.006), oral cavity cancer (pooled HR=2.21, 95%CI 1.31-3.71, p = 0.003), or NPC (pooled HR=2.14, 95%CI 1.15-3.98, p = 0.016). Furthermore, the heterogeneity among subgroups stratified by tumor location varied, suggesting that tumor location may have caused the observed heterogeneity. Regarding sample size, AGR cutoff values, and methods used to determine the cutoff values, AGR had a significant association with OS in all the subgroups, suggesting that our results were both robust and reliable.
Association of AGR with DFS/DMFS
The pooled HR from the five studies (involving 1110 patients) that reported an association between AGR and DFS exhibited minimal heterogeneity (I2 = 0.0%; PH = 0.669; Figure 2B) and indicated that a low AGR could predict poor DFS (pooled HR=1.97, 95%CI 1.58-2.45, p < 0.001). The three studies (involving 1309 patients) that reported an association between AGR and DMFS also exhibited low heterogeneity (I2= 0.0%; PH = 0.484; Figure 2C) and demonstrated that a low AGR has a significant association with poor DMFS in HNC (pooled HR=1.64, 95%CI 1.25-2.16, p < 0.001).
AGR and clinicopathological factors
We analyzed the relationships between AGR and seven clinicopathological variables: gender, age, smoking status, T status, TNM stage, lymph node metastasis, and cancer cell differentiation (Table 3). The results revealed that a low AGR had a significant association with stage III-IV disease (OR=2.62, 95%CI 1.62-4.23, p < 0.001), T3-4 status (OR=2.22, 95%CI 1.43-3.44, p < 0.001), and lymph node metastasis (OR=1.95, 95%CI 1.29-2.82, p = 0.001). No significant associations were observed between AGR and age (OR=1.29, 95%CI 0.91-1.82, p = 0.154), gender (OR=0.76, 95%CI 0.51-1.14, p = 0.182), smoking status (OR=0.84, 95%CI 0.40-1.74, p = 0.63), or cancer cell differentiation (OR=0.85, 95%CI 0.61-1.22, p = 0.38).
Sensitivity analysis and Publication bias
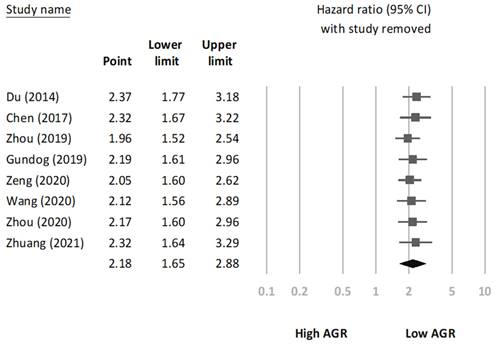
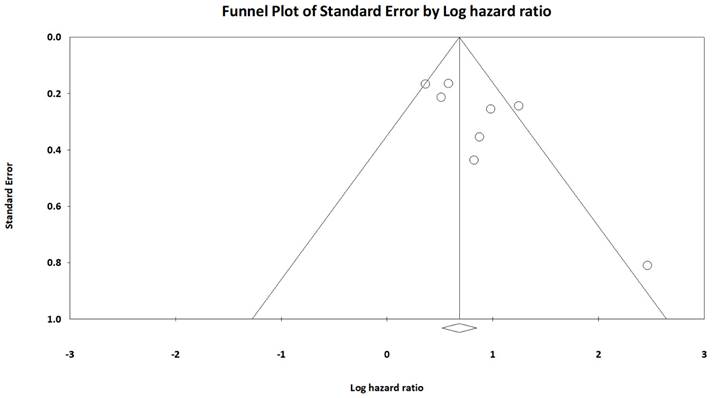
We conducted a sensitivity analysis to assess the robustness of the association between AGR and OS through the sequential omission of each study from the pooled analysis (Figure 3). The results revealed that the association between AGR and OS did not change significantly, indicating that our findings were reliable. In addition, we constructed a Begg's funnel plot (Figure 4) and detected no significant publication bias (p = 0.22).
Characteristics of included studies
| First author | Year | Study region | Sample Size | Tumor location | Cancer Stage | Treatment | Cut-off value resource | AGR Cut-off | Survival outcome | HR analysis | Follow-up (months) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Du | 2014 | China | 694 | Nasopharynx | I-IV | RT or CRT | Others* | 1.4 | OS, DMFS, DFS, LRFS | M | N/A | 7 |
| Li | 2015 | China | 520 | Nasopharynx | I-IV | RT or CRT | ROC | 1.34 | DMFS | M | 88.4 | 8 |
| Chen | 2017 | China | 241 | Larynx | I-IV | Surgery +/- adjuvant | ROC | 1.28 | OS, DFS | U | 73 | 8 |
| Gundog | 2019 | Turkey | 95 | Nasopharynx | I-IV | RT or CRT | ROC | 1.19 | OS, LRRFS, DMFS | U | 41 | 8 |
| Zhou | 2019 | China | 232 | Larynx | I-IV | Surgery +/- adjuvant | ROC | 1.31 | OS, DFS | M | 27.3 | 7 |
| Zeng | 2020 | China | 255 | Nasopharynx | I-IV | RT or CRT | ROC | 1.5 | OS, DFS | M | 33.5 | 6 |
| Wang | 2020 | Taiwan | 306 | Oral cavity | I-IV | Surgery +/- adjuvant | ROC | 1.55 | OS, DFS | M | 49.2 | 8 |
| Zhou | 2020 | China | 76 | Oral cavity | I-IV | Surgery +/- adjuvant | X-tile software | 1.6 | OS, DFS | M | 48 | 7 |
| Zhuang | 2021 | China | 792 | Oral cavity | I-IV | Surgery +/- adjuvant | X-tile software | 1.34 | OS | M | 27.48 | 8 |
*Method proposed by Igarashi et al. [30].
Abbreviations: CRT: chemo-radiotherapy; DFS: disease- free survival; DMFS: distant metastasis-free survival; LRFS: local relapse-free survival; LRRFS: loco-regional recurrence-free survival; M: multivariate analysis; N/A: not applicable; NOS: Newcastle- Ottawa scale; OS: overall survival; RT: radiotherapy; ROC: receiving operating characteristics; U: univariate analysis.
Associations between AGR and survival outcomes. A Forest plot of the association between overall survival and AGR in patients with HNC. B Forest plot of the association between disease-free survival and AGR in patients with HNC. C Forest plot of the association between distant metastasis-free survival and AGR in patients with HNC.
Summary of subgroup analysis results of AGR in OS
| Subgroup | No. of studies | No. of patients | Pooled HR (95%CI) | p value | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | Ph | |||||
| Overall | 8 | 2691 | 2.18 (1.65-2.88) | <0.001 | 57.9 | 0.02 |
| Tumor sites | ||||||
| Nasopharynx | 3 | 1044 | 2.14 (1.15-3.98) | 0.016 | 71.83 | 0.029 |
| Larynx | 2 | 473 | 2.38 (1.29-4.40) | 0.006 | 80.49 | 0.024 |
| Oral cavity | 3 | 1174 | 2.21 (1.31-3.71) | 0.003 | 0 | 0.377 |
| Sample size | ||||||
| >255 | 4 | 2047 | 2.05 (1.37-3.05) | <0.001 | 68.62 | 0.023 |
| ≤255 | 4 | 644 | 2.36 (1.55-3.59) | <0.001 | 41.64 | 0.162 |
| Cut-off of AGR | ||||||
| >1.34 | 4 | 1331 | 2.28 (1.43-3.63) | <0.001 | 70.18 | 0.018 |
| ≤1.34 | 4 | 1360 | 2.16 (1.42-3.28) | <0.001 | 52.85 | 0.095 |
| Methods for determining AGR cutoff | ||||||
| ROC | 5 | 1129 | 2.60(1.84-3.67) | <0.001 | 56.36 | 0.057 |
| Others | 3 | 1562 | 1.73 (1.2-2.49) | 0.003 | 1.24 | 0.363 |
Abbreviations: AGR: albumin-to-globulin ratio; ROC: receiving operating characteristics.
Discussion
According to our literature review, this was the first meta-analysis to examine the utility of the AGR for predicting survival outcomes and investigate the relationships between AGR and clinicopathologic characteristics in HNC. Our analysis included nine studies involving 3211 patients, and the pooled results revealed that a low pretreatment AGR is significantly associated with poor DFS, OS, and DMFS in patients with HNC. Because HNC is a heterogeneous disease, the tumor location may strongly affect the survival of patients with HNC. However, our results demonstrated that AGR had a significant association with OS in patients with tumours originating in the oral cavity, nasopharynx, or larynx, suggesting the common applicability of AGR in HNC prognosis. In addition, our subgroup analyses stratified by sample size, AGR cutoff value, and the method used to determine the AGR cutoff value achieved significant and consistent results. The heterogeneity among the studies involving patients with oral cavity cancer was minimal, but that among the studies involving patients with NPC and laryngeal cancer was high, suggesting that the observed heterogeneity may be attributable to tumor location. Our sensitivity analysis further supported the reliability of our findings. In addition, we discovered that a low pretreatment AGR is associated with advanced pathological characteristics of HNC, such as neck lymph node metastasis and advanced T status, implicating that measuring a patient's AGR before treatment may facilitate the early detection of adverse tumour features. Based on these findings, we suggested that the AGR may be clinically applicable as a cost-effective prognostic marker in the management of HNC, and the patients with HNC and low pretreatment AGRs may benefit from more careful and personalized treatment planning and careful follow-up. To facilitate the use of AGR in clinical practice, several researchers have proposed the use of AGR-based nomograms that integrate AGR and clinicopathological characteristics, which have been demonstrated to accurately predict the OS of patients with HNC [14].
Odds ratio analysis between clinicopathological and AGR
| Variables | No. of studies | No. of patients | OR (95%CI) | p value | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | Ph | |||||
| Age (>60 vs. <60) | 3 | 549 | 1.29 (0.91-1.82) | 0.154 | 0 | 0.752 |
| Gender (Male vs. Female) | 6 | 1644 | 0.76 (0.51-1.14) | 0.182 | 42.39 | 0.123 |
| Smoking (yes vs. no) | 4 | 644 | 0.84 (0.40-1.74) | 0.63 | 75.37 | 0.007 |
| T stage (T3-4 vs. T1-2) | 5 | 1568 | 2.22 (1.43-3.44) | <0.001 | 71.53 | 0.007 |
| Lymph node metastasis (yes vs. no) | 4 | 1243 | 1.95 (1.29-2.82) | 0.001 | 47.29 | 0.128 |
| TNM stage (III-IV vs. I-II) | 5 | 1403 | 2.62 (1.62-4.23) | <0.001 | 63.92 | 0.026 |
| Differentiation (P-D vs. W-D/M-D) | 4 | 855 | 0.85 (0.61-1.22) | 0.38 | 0 | 0.953 |
The mechanisms underlying the relationship between AGR and prognosis of HNC remain uncertain but may be related to inflammatory responses and nutritional status, both of which are reflected in a patient's AGR. ALB, which is synthesized by hepatocytes, is the most abundant protein in human serum and is frequently used to assess patients' nutritional statuses. In addition, ALB helps maintain vascular integrity and permeability and serves as a major free radical scavenger in the human body [31]. The antioxidant and antitumor effects of ALB help ensure the stability of cell DNA replication and inhibit the proliferation of cancer cells [32]. Therefore, patients with low ALB levels are considered to have a poor nutritional status and weakened antitumor immunity. Patients with HNC frequently experienced cachexia and hypoalbuminemia [33]; both of which are associated with poor prognosis [34]. In addition, serum ALB levels may be correlated with host inflammatory responses [35], which are involved in the tumorigenesis and progression of HNC [36]. Aggressive cancer invasion may increase cancer-related inflammation and cytokine production, suppressing the biosynthesis of ALB [37]. Various proinflammatory proteins, including immunoglobulins, complement components, and C-reactive protein, fall under the umbrella of GLB [38]. The upregulation of some inflammatory cytokines in serum GLBs, such as tumor necrosis factor, IL-6, and IL-8, could promote tumor progression, immune escape, and metastasis [39], which are closely linked to poor prognoses in patients with HNC [40]. Low AGR levels may reflect a poor nutritional status, increased inflammatory responses, and weak antitumor immunity, which may contribute to poor prognosis in HNC. However, the mechanisms underlying the associations between AGR and prognosis in HNC warrant further investigation.
Sensitivity analysis.
Funnel plot of hazard ratios for overall survival based on AGR.
Researchers have conducted meta-analyses to explore the associations between AGR and the prognoses of various human malignancies. One meta-analysis of 28 studies involving 15,356 patients with various types of cancer revealed significant associations between a low pretreatment AGR and poor PFS, DFS, and OS [41]; however, it only included two studies involving patients with HNC. A meta-analysis focusing on gastric cancer had similar results [42]. Our results were also consistent with the aforementioned findings, suggesting that AGR may be used as a prognostic marker in cancer management. In addition, the clinical value of AGRs extends beyond the prediction of survival outcomes. In one large retrospective study involving 26,974 healthy adults, the participants with low AGRs were at a higher risk of various malignancies, including HNC [43]. Regarding the associations between AGR and clinicopathological factors, a meta-analysis of 14 studies involving 4136 patients with 11 types of cancer demonstrated that low AGRs were significantly associated with an elevated risk of lymph node metastasis [44]. Another meta-analysis of eight cohort studies involving 2668 patients with urologic cancer revealed that lymphovascular invasion and T and N status differed significantly between patients with high and low AGRs [45]. In another study involving 306 patients with oral cavity cancer, a low AGR (<1.55) was associated with adverse clinicopathological characteristics, such as advanced cancer stage, extranodal extension, and lymph node metastasis as well as a depth of invasion of ≥10 mm [14]. The aforementioned results had similar trend with our findings, which may be attributable to several factors. First, a substantial tumor burden may be associated with cachexia [46] and decreased serum ALB levels [47], resulting in a lower AGR. Moreover, patients with large head and neck tumours have higher serum levels of proinflammatory and proangiogenic cytokines [48], which may lead to a low serum ALB concentration [49] and, in turn, a low AGR. However, the mechanisms underlying the aforementioned associations warrant further investigation.
Although ALB and GLB can be used to predict the clinical outcomes of patients with cancer [50, 51], the measurement of a single indicator may be affected by host factors, such as body fluid changes, hepatic dysfunction, and acute infection or inflammation. Therefore, ALB or GLB levels alone may not serve as an effect predictor of survival outcomes in patients with HNC. However, AGR may be less influenced by variations attributable to measurement methods or the aforementioned individual factors and is therefore a more reliable prognostic marker than ALB or GLB alone. In addition, the sensitivity of AGR in survival prediction is high. Suh et al. conducted a retrospective study involving 26,974 healthy adults and discovered that some individuals with normal total serum protein and albumin levels had low AGRs (<1.1) [43]. Analysing patients' AGRs may help health-care providers identify patients at risk of unfavourable survival outcomes who cannot be identified on the basis of their serum ALB levels.
The strength of our meta-analysis is represented by its quantitative and systematic evaluation of the clinical role of AGR in patients with HNC, which is, to our knowledge, the first meta-analysis in this field. The enrollment of large numbers of patients enabled us to verify the prognostic effect of AGR in different tumor locations and cut-off intervals, further increasing the general applicability of AGR in HNC management. Based on the observed associations between a low AGR and adverse pathological characteristics of HNC, our findings suggested that pretreatment AGR may serve as a useful tool for early detection of advanced HNC. As a literature-based meta-analysis, this study has several limitations of note. First, all the included studies employed retrospective designs and may therefore have been subject to bias. Second, most of the included studies involved Asian populations; therefore, our findings may be applicable to Asian populations but not generalisable to individuals of other races. Third, no consensus has been reached regarding the optimal AGR cutoff value for predicting survival in patients with HNC; the resulting heterogeneity among the included studies may limit the applicability of our findings. Furthermore, although most of the HRs were derived from multivariate analyses, different sets of variables may have been included in different studies, which may have influenced the reliability of our results. Because of these limitations, additional large-scale well-designed prospective studies are warranted to assess the validity of our findings and further explore the optimal AGR cutoff value for use in predicting the survival outcomes of patients with HNC.
In conclusion, our study results indicated that a low pretreatment AGR is significantly associated with poor DFS, DMFS, and OS as well as stage III-IV disease and neck lymph node metastasis in patients with HNC. Because measuring a patient's AGR is convenient and simple, AGR may serve as a cost-effective biomarker for prognostics and individualized treatment planning. Researchers should conduct additional randomized and multicentre prospective trials to assess the utility of AGR in clinical practice and the associations between AGR and other clinicopathological biomarkers.
Abbreviations
AGR: albumin-to-globulin ratio; ALB: albumin; CI: confidence interval; DFS: disease-free survival; DMFS: distant metastasis-free survival; GLB: globulin; HNC: head and neck cancer; HR: hazard ratio; LRFS: local relapse-free survival; LRRFS: locoregional recurrence-free survival; NOS: Newcastle-Ottawa quality assessment scale; NPC: nasopharyngeal carcinoma; OR: odds ratio; OS: overall survival; TNM: tumor-node-metastasis.
Supplementary Material
Supplementary tables.
Acknowledgements
Funding information
This research was funded by the Research Project of Chang Gung Medical Foundation in Chiayi with grant numbers of CMRPG6L0231.
Author contributions
- Conceptualization: Yao-Te Tsai, Yun-Ting Wang.
- Data collection and analysis: Yun-Ting Wang, Liang-Tseng Kuo, Chun-Ta Liao, Chung-Jan Kang.
- Literature assessments: Yun-Ting Wang, Liang-Tseng Ku, Ethan I. Huang, Ming-Shao Tsa.
- Visualization: Chia-Hsuan Lai, Yuan-Hsiung Tsai, Yi-Chan Lee, Cheng-Ming Hsu, Geng-He Chang.
- Article writing and editing: Yao-Te Tsai, Yun-Ting Wang.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: A period analysis. Oncologist. 2010;15:994-1001
2. Sung H, Ferlay J, Siegel RL. et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249
3. Gundog M, Basaran H. Pretreatment low prognostic nutritional index and low albumin-globulin ratio are predictive for overall survival in nasopharyngeal cancer. Eur Arch Otorhinolaryngol. 2019;276:3221-3230
4. Morris LG, Sikora AG, Patel SG. et al. Second primary cancers after an index head and neck cancer: Subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29:739-746
5. Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity. 2019;51:27-41
6. Kim DH. Nutritional issues in patients with cancer. Intest Res. 2019;17:455-462
7. Don BR, Kaysen G. Serum albumin: Relationship to inflammation and nutrition. Semin Dial. 2004;17:432-437
8. Quan L, Jiang X, Jia X. et al. Prognostic value of the albumin-to-globulin ratio in patients with colorectal cancer: A meta-analysis. Nutr Cancer. 2022;74:3329-3339
9. Liu M, Wang L. Prognostic significance of preoperative serum albumin, albumin-to-globulin ratio, and prognostic nutritional index for patients with glioma: A meta-analysis. Medicine (Baltimore). 2020;99:e20927
10. Xia Z, Fu X, Li J. et al. Prognostic value of pretreatment serum albumin-globulin ratio in urothelial carcinoma: A systematic review and meta-analysis. Front Oncol. 2022;12:992118
11. Bozkaya Y, Erdem GU, Demirci NS. et al. Prognostic importance of the albumin to globulin ratio in metastatic gastric cancer patients. Curr Med Res Opin. 2019;35:275-282
12. Wang Y, Li S, Hu X. et al. The prognostic value of serum albumin-globulin ratio in early-stage non-small cell lung cancer: A retrospective study. Cancer Manag Res. 2019;11:3545-3554
13. Kono T, Sakamoto K, Shinden S. et al. Pre-therapeutic nutritional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy. Clin Nutr. 2017;36:1681-1685
14. Wang YT, Fang KH, Hsu CM. et al. Retrospective study on the potential of albumin/globulin ratio as a prognostic biomarker for oral cavity cancer patients. Eur Arch Otorhinolaryngol. 2021;278:227-238
15. Zhou T, Yu ST, Chen WZ. et al. Pretreatment albumin globulin ratio has a superior prognostic value in laryngeal squamous cell carcinoma patients: A comparison study. J Cancer. 2019;10:594-601
16. He J, Pan H, Liang W. et al. Prognostic effect of albumin-to-globulin ratio in patients with solid tumors: A systematic review and meta-analysis. J Cancer. 2017;8:4002-4010
17. McInnes MDF, Moher D, Thombs BD. et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The prisma-dta statement. JAMA. 2018;319:388-396
18. Tierney JF, Stewart LA, Ghersi D. et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16
19. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834
20. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605
21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188
22. Cumpston M, Li T, Page MJ. et al. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142
23. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101
24. Zhuang Z, Li Y, Hong Y. et al. A novel prognostic score based on systemic inflammatory biomarkers for patients with oral squamous cell carcinoma. Oral Dis. 2022;28:631-638
25. Zeng X, Liu G, Pan Y. et al. Prognostic value of clinical biochemistry-based indexes in nasopharyngeal carcinoma. Front Oncol. 2020;10:146
26. Li AC, Xiao WW, Wang L. et al. Risk factors and prediction-score model for distant metastasis in nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Tumour Biol. 2015;36:8349-8357
27. Du XJ, Tang LL, Mao YP. et al. The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. PLoS One. 2014;9:e94473
28. Chen WZ, Yu ST, Xie R. et al. Preoperative albumin/globulin ratio has predictive value for patients with laryngeal squamous cell carcinoma. Oncotarget. 2017;8:48240-48247
29. Zhou C, Wei ZC, Ju GP. et al. Prognostic significance of preoperative peripheral blood parameters in 76 cases of oral squamous cell carcinoma. Chin J Cancer Prev Treat. 2020;27:1902-1907
30. Igarashi T, Tobe T, Kuramochi H. et al. Serum immunosuppressive acidic protein as a potent prognostic factor for patients with metastatic renal cell carcinoma. Jpn J Clin Oncol. 2001;31:13-17
31. Garcia-Martinez R, Caraceni P, Bernardi M. et al. Albumin: Pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836-1846
32. Seaton K. Albumin concentration controls cancer. J Natl Med Assoc. 2001;93:490-493
33. Solis-Martinez O, Alvarez-Altamirano K, Cardenas D. et al. Cancer cachexia affects patients with head and neck cancer in all stages of disease: A prospective cross-sectional study. Nutr Cancer. 2022;74:82-89
34. Hayashi N, Sato Y, Fujiwara Y. et al. Clinical impact of cachexia in head and neck cancer patients who received chemoradiotherapy. Cancer Manag Res. 2021;13:8377-8385
35. Barbosa-Silva MC. Subjective and objective nutritional assessment methods: What do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11:248-254
36. Bonomi M, Patsias A, Posner M. et al. The role of inflammation in head and neck cancer. Adv Exp Med Biol. 2014;816:107-127
37. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448-454
38. Afshar-Kharghan V. The role of the complement system in cancer. J Clin Invest. 2017;127:780-789
39. Mantovani A, Allavena P, Sica A. et al. Cancer-related inflammation. Nature. 2008;454:436-444
40. Uz U, Eskiizmir G. Association between interleukin-6 and head and neck squamous cell carcinoma: A systematic review. Clin Exp Otorhinolaryngol. 2021;14:50-60
41. Lv GY, An L, Sun XD. et al. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: A meta-analysis. Clin Chim Acta. 2018;476:81-91
42. Wei C, Yu Z, Wang G. et al. Low pretreatment albumin-to-globulin ratio predicts poor prognosis in gastric cancer: Insight from a meta-analysis. Front Oncol. 2020;10:623046
43. Suh B, Park S, Shin DW. et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol. 2014;25:2260-2266
44. Chi J, Xie Q, Jia J. et al. Prognostic value of albumin/globulin ratio in survival and lymph node metastasis in patients with cancer: A systematic review and meta-analysis. J Cancer. 2018;9:2341-2348
45. Zhang Y, Wang L, Lin S. et al. Preoperative albumin-to-globulin ratio as a significant prognostic indicator in urologic cancers: A meta-analysis. Cancer Manag Res. 2018;10:4695-4708
46. De Lerma Barbaro A. The complex liaison between cachexia and tumor burden (review). Oncol Rep. 2015;34:1635-1649
47. Schwarz S, Prokopchuk O, Esefeld K. et al. The clinical picture of cachexia: A mosaic of different parameters (experience of 503 patients). BMC Cancer. 2017;17:130
48. Chen Z, Malhotra PS, Thomas GR. et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5:1369-1379
49. Milan Manani S, Virzi GM, Clementi A. et al. Pro-inflammatory cytokines: A possible relationship with dialytic adequacy and serum albumin in peritoneal dialysis patients. Clin Kidney J. 2016;9:153-157
50. Yoshino Y, Taguchi A, Shimizuguchi T. et al. A low albumin to globulin ratio with a high serum globulin level is a prognostic marker for poor survival in cervical cancer patients treated with radiation based therapy. Int J Gynecol Cancer. 2019;29:17-22
51. Tsai MH, Chuang HC, Lin YT. et al. Clinical impact of albumin in advanced head and neck cancer patients with free flap reconstruction-a retrospective study. PeerJ. 2018;6:e4490
Author contact
![]() Corresponding author: Yao-Te Tsai, MD, Department of Otorhinolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital, Chiayi, Taiwan. Address: No.6, W. Sec., Jiapu Rd., Puzih City, Chiayi County 613, Taiwan. E-mail: yaote1215com; Tel: +886 975014653.
Corresponding author: Yao-Te Tsai, MD, Department of Otorhinolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital, Chiayi, Taiwan. Address: No.6, W. Sec., Jiapu Rd., Puzih City, Chiayi County 613, Taiwan. E-mail: yaote1215com; Tel: +886 975014653.

 Global reach, higher impact
Global reach, higher impact