3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(2):193-199. doi:10.7150/jca.79797 This issue Cite
Research Paper
Effectiveness Without Efficacy: Cautionary Tale from a Landmark Breast Cancer Randomized Controlled Trial
1. Department of Biostatistics, The University of Texas, MD Anderson Cancer Center, Houston TX, USA.
2. Department of Radiation Oncology, The University of Texas, MD Anderson Cancer Center, Houston TX, USA.
3. Department of Breast Surgical Oncology, The University of Texas, MD Anderson Cancer Center, Houston TX, USA.
Abstract
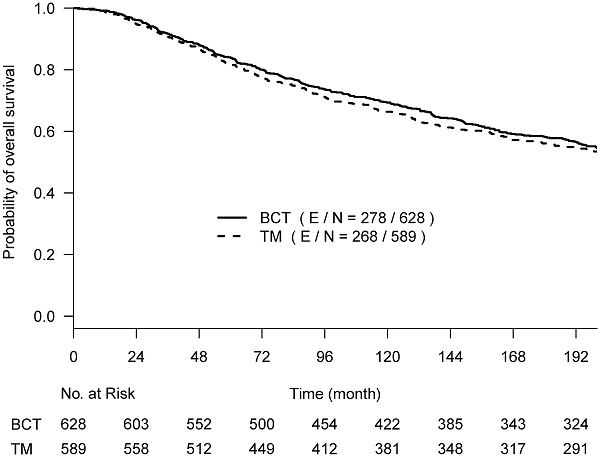
Background: “Old” randomized controlled trials established breast conserving therapy (BCT) and total mastectomy (TM) equivalence for treating early breast cancer, whereas recent literature report improved survival with BCT. To reconcile this, we performed a simulation study and re-analyzed B-06 trial data.
Methods: We estimated the distributions for overall survival (OS), cumulative incidence functions for breast-cancer-specific death (BCSD) and other causes-specific death (OCSD) by BCT and TM. The restricted mean survival time (RMST) difference and hazard ratio between the two arms were estimated. Given the estimated distributions, we simulated cause-specific death times from each arm, evaluating the power to test treatment difference in OS, BCSD, and OCSD with different sample sizes, follow-up times, and a modified setting by simulating BCT-arm OCSD times from the distribution of patients not receiving radiation.
Results: With 200 months follow-up, the average BCT-over-TM gain measured by RMST was 3.7 months for OS and 4.5 months for BCSD. Increasing the trial size to 5,000 per arm, there is a 79.2% chance to detect the OS benefit with RMST and 92.4% for BCSD. A nonproportional increase of OCSD in BCT compared to TM was observed after 144 months, and particularly after 200 months post treatments. When OCSD times of BCT were simulated using patients not receiving radiation, the estimated OS gain increased to 4.4 months, and the power increased to 92.2%.
Conclusions: The late excess other-cause-death, likely due to radiation, in the BCT arm and sample size constraints limited the power to report BCT superiority. Given radiation delivered in the era of B-06 trial, BCT and TM remain largely equivalent.
Keywords: Breast conserving therapy, Competing risks analysis, Randomized clinical trial, Total mastectomy

 Global reach, higher impact
Global reach, higher impact