3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(14):3539-3553. doi:10.7150/jca.77247 This issue Cite
Review
Hepatitis B Virus Reactivation in Cancer Patients Undergoing Immune Checkpoint Inhibitors Therapy: A Systematic Review
1. Clinical Trial Center, National Medical Products Administration Key Laboratory for Clinical Research and Evaluation of Innovative Drugs, West China Hospital, Sichuan University, Chengdu, Sichuan, 610041 P.R. China.
2. West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan 610041, P.R. China.
3. State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, and West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, and Collaborative Innovation Center for Biotherapy, Chengdu, 610041, P.R. China
4. Department of Thoracic Oncology, Cancer Center and State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, China.
# These authors contributed equally to this work.
Abstract
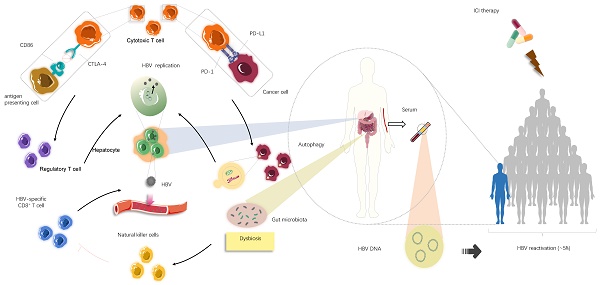
Background: Immune checkpoint inhibitor (ICI) therapy is now administered to patients with advanced cancers. However, the safety and efficacy of ICIs in cancer patients with hepatitis B virus (HBV) infection is unknown. Therefore, we performed this systematic review to examine the safety and efficacy of ICIs in patients with HBV infection, with particular focus on HBV reactivation.
Methods: Studies examining ICI treatment in patients with advanced cancer and HBV infection in PubMed from database inception to April 2022 were retrieved in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. In addition, reports of individuals diagnosed with HBV reactivation were supplemented through the Food and Drug Administration Adverse Event Reporting System.
Results: We identified 20 articles (8 case reports, 10 retrospective case series, and 2 prospective clinical trials) and 2 meeting abstracts including 633 patients with advanced cancer and HBV infection treated with ICIs. The overall rate of HBV reactivation was 4.1% (26/633), and no HBV-related fatal events were reported. Among patients with HBV reactivation with known baseline data (20/26), HBV-DNA returned to undetectable status in 15 of 17 patients (88.2%) after a median 5.5 weeks (range, 1-14 weeks). Therapeutic responses to ICIs were observed in 14 of 88 patients (15.91%) with hepatocellular carcinoma, 6 of 45 patients (13.33%) with non-small cell lung cancer, and 3 of 13 patients (23.08%) with melanoma.
Conclusion: ICIs may be safe and effective in patients with advanced cancer and HBV infection. However, there is still a need for clinical monitoring of liver enzymes and HBV-DNA during ICI therapy. Prospective trials are necessary to elucidate the appropriate antiviral therapy in these patients.
Keywords: immune checkpoint inhibitors (ICIs), immunotherapy, hepatitis B virus (HBV), reactivation, Food and Drug Administration Adverse Event Reporting System (FAERS)

 Global reach, higher impact
Global reach, higher impact