Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(14):3539-3553. doi:10.7150/jca.77247 This issue Cite
Review
Hepatitis B Virus Reactivation in Cancer Patients Undergoing Immune Checkpoint Inhibitors Therapy: A Systematic Review
1. Clinical Trial Center, National Medical Products Administration Key Laboratory for Clinical Research and Evaluation of Innovative Drugs, West China Hospital, Sichuan University, Chengdu, Sichuan, 610041 P.R. China.
2. West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan 610041, P.R. China.
3. State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, and West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, and Collaborative Innovation Center for Biotherapy, Chengdu, 610041, P.R. China
4. Department of Thoracic Oncology, Cancer Center and State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, China.
# These authors contributed equally to this work.
Received 2022-7-19; Accepted 2022-10-14; Published 2022-10-31
Abstract
Background: Immune checkpoint inhibitor (ICI) therapy is now administered to patients with advanced cancers. However, the safety and efficacy of ICIs in cancer patients with hepatitis B virus (HBV) infection is unknown. Therefore, we performed this systematic review to examine the safety and efficacy of ICIs in patients with HBV infection, with particular focus on HBV reactivation.
Methods: Studies examining ICI treatment in patients with advanced cancer and HBV infection in PubMed from database inception to April 2022 were retrieved in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. In addition, reports of individuals diagnosed with HBV reactivation were supplemented through the Food and Drug Administration Adverse Event Reporting System.
Results: We identified 20 articles (8 case reports, 10 retrospective case series, and 2 prospective clinical trials) and 2 meeting abstracts including 633 patients with advanced cancer and HBV infection treated with ICIs. The overall rate of HBV reactivation was 4.1% (26/633), and no HBV-related fatal events were reported. Among patients with HBV reactivation with known baseline data (20/26), HBV-DNA returned to undetectable status in 15 of 17 patients (88.2%) after a median 5.5 weeks (range, 1-14 weeks). Therapeutic responses to ICIs were observed in 14 of 88 patients (15.91%) with hepatocellular carcinoma, 6 of 45 patients (13.33%) with non-small cell lung cancer, and 3 of 13 patients (23.08%) with melanoma.
Conclusion: ICIs may be safe and effective in patients with advanced cancer and HBV infection. However, there is still a need for clinical monitoring of liver enzymes and HBV-DNA during ICI therapy. Prospective trials are necessary to elucidate the appropriate antiviral therapy in these patients.
Keywords: immune checkpoint inhibitors (ICIs), immunotherapy, hepatitis B virus (HBV), reactivation, Food and Drug Administration Adverse Event Reporting System (FAERS)
Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte antigen 4 (CTLA-4) have become a well-established therapeutic option for cancer [1].
The World Health Organization estimates that there were approximately 296 million chronic hepatitis B virus (HBV) infections worldwide in 2019 [2]. A large proportion of patients with cancer may also have HBV infections. A multicentre prospective study reported that 0.6% of patients with newly diagnosed cancer had chronic HBV infections, whereas 6.5% had previously had HBV infections [3]. However, clinical trials of immunotherapy for cancer exclude patients with HBV infection, and few prospective clinical studies include individuals with chronic HBV infection [4,5]. Therefore, the safety and efficacy of ICIs in cancer patients with HBV are unclear.
Basic studies have shown that chronic HBV infection leads to virus-specific T cell exhaustion [6,7], which could theoretically affect the efficacy of ICIs. Furthermore, reactivation of HBV during treatment can result in treatment delay or termination. However, whether ICIs increase the risk of HBV reactivation and whether there is ongoing need for antiviral prophylaxis are unknown. Therefore, we performed a systematic review of the current literature to provide evidence for clinical decision making.
Methods
Data source and search strategy
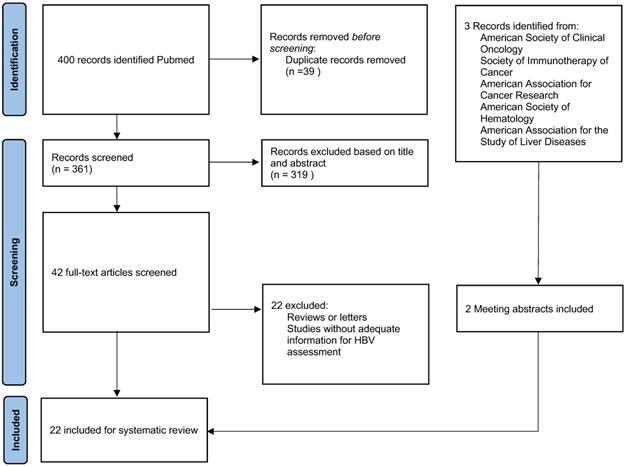
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We comprehensively searched the PubMed, Medline and Embase databases for studies examining ICIs and HBV infection from database inception to April 2022 using the following terms: ("Hepatitis B virus*" OR "HBV") AND ("exacerbation" OR "“reactivation" OR "flare" OR "recurrence") AND ("PD-1" OR "PD-L1" OR "CTLA-4" OR "Immune checkpoint inhibitor" OR "ipilimumab" OR "nivolumab" OR "pembrolizumab" OR "atezolizumab" OR "durvalumab" OR "avelumab" OR "tremelimumab" OR "toripalimab" OR "sintilimab" OR "cemiplimab" OR "camrelizumab" OR "tislelizumab"). We obtained 400 articles. In addition, three meeting abstracts were identified after reviewing annual meeting proceedings from the American Society of Clinical Oncology (ASCO), Society of Immunotherapy of Cancer, American Association for Cancer Research, American Society of Hematology, and American Association for the Study of Liver Diseases [8-10]. All publications were screened by two investigators independently.
Inclusion and exclusion criteria
The inclusion criteria were: (1) Patients with malignant tumors. (2) Patients treated with anti-PD-1/ PD-L1 or CTLA-4 monotherapy or in combination with other anti-tumour therapy. Studies were excluded if they were: (1) Reviews or letters. (2) Studies focused on the mechanism of HBV reactivation. (3) Studies without adequate information on HBV reactivation. Eventually, 22 publications were included (Fig. 1). We also screened related review articles to ensure comprehensive retrieval [1,11-15].
PRISMA Diagram Detailing Article Selection
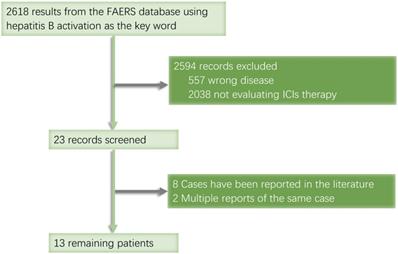
When searching the ASCO website, we found a conference report titled 'Hepatitis B reactivation with pembrolizumab, atezolizumab, and nivolumab: A pharmacovigilance study and literature review' in the Food and Drug Administration Adverse Event Reporting System (FAERS). Although the full text was not available, we used this open database to search for more records on HBV reactivation, from which we gathered a total of 2,618 records collected between January 2013 and September 2020. We identified 12 cancer patients treated with ICIs whose cases were not duplicated or published (Fig. 2).
FAERS database retrieval flowchart
Statistical analysis
We used descriptive statistics to summarize the study findings. Statistical calculations were performed with Excel (Microsoft Excel, Microsoft Office, Redmond, Washington).
Results
We included 633 patients with advanced cancer and HBV infection treated with ICIs in 22 studies (8 case reports [16-23], 10 retrospective case series [24-33], 2 prospective clinical trials, and 2 meeting abstracts [8,9]) (Table 1). Since one study included patients with HBV and hepatitis C virus infections (n = 16) in a composite cohort and two meeting abstracts lacked patient data (n = 79), 95 patients were excluded. The baseline characteristics of 538 patients are summarized in Table 2. Among these eligible patients, the most prevalent cancer type was hepatocellular carcinoma (HCC) (353/538 [65.6%]), followed by non-small cell lung cancer (NSCLC) (70/538 [13.0%]), and melanoma (46/538 [8.6%]). A total of 416 patients (77.3%) received antiviral prophylaxis, of whom 57.7% received entecavir, 15.6% received tenofovir, and 3.1% received lamivudine; data on the antiviral prophylaxis regimen was not available for 96 patients (23.1%).
Select Studies of Immune Checkpoint Inhibitor Therapy in Patients with HBV Infection and Advanced Cancer
| Reference | Year | Sample Size | Tumor Type | Number of HBV Infection | ICIs Therapy | Viral Load* | Antiviral Prophylaxis | Antiviral Therapy | HBV Reactivation Number of Events | Adverse Events | ORR (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective clinical trails (n=2) | ||||||||||||
| El-Khoueiry AB | 2017 | 226(66HBV) | HCCs | 15 in escalation phase/51 in expansion phase | Nivolumab | Undetectable (66) | Yes (All patients were required to be receiving effective antiviral therapy) | Nil (0/66) | (0/66) No patient had reactivation of HBV, no anti- HBs sero conversion | in the dose-expansion phase, HBV infected group Diarrhoea(G3/4):1(2%) | CR(1) PR(8) SD(39) PD(17) NR(1) 13.6% | |
| Zhu AX | 2018 | 104(22HBV) | HCCs | Hepatitis B positive (22) | Pembrolizumab | Undetectable (22) | Yes (All patients were required to be receiving effective antiviral therapy) | Nil (0/22) | (0/22) No cases of flares of hepatitis B virus occurred. | Not specifically reported for HBV positive patients. | PR(5) SD+PD(16) NR(1) 23.8% | |
| Retrospective case series (n=10) | ||||||||||||
| Ravi S | 2014 | 9(5HBV) | Melanoma (9) | 5 HBV positive (active/inactive 3/2) | Ipilimumab (9) | Undetectable/Detected (2/3) | Entecavir (2) Tenofovir(2) Nil (1) | Nil (0) | (0/5) No patient had reactivation of HBV, (3/5) HBV-DNA levels remained undetectable | Only ALT elevation reported. ALT elevation G1: 2 | SD(1) PD(4) | |
| Wen X | 2016 | 23(11HBV) | Melanoma (11) | 11 pre-existing hepatitis B virus (HBV) infection | Ipilimumab (3) Pembrolizumab (5) Concurrent Ipilimumab -pembrolizumab (3) | Undetectable/Detected (10/1) | Entecavir (3) Nil (8) | Nil (0) | (0/11) No patient had reactivation of HBV, (6/11) HBV-DNA levels remained undetectable | Any G: (8/11)73%, ALT/AST increaseG3:(1/11) | NR | |
| Kothapalli A | 2018 | 7(5HBV/2HCV) | NSCLC(4) Melanoma(1) | Chronic HBV (1), Possible Past HBV (1), Past HBV (3, 1 of these had HCV co-infection) | Nivolumab(4) Pembrolizumab(1) | Unknown(5) | Unknown(5) | Nil (0/5) | (0/14) No patient had reactivation of HBV | ALT rise G1:(3), Nil(2) | PR(1) SD(2) PD(2) | |
| Tio M | 2018 | 46(14HBV/14HCV/12HIV/6Organ transplant) | Melanoma (9) Mesothelioma (1)Glioblastoma (1) HCC (1)Gastric (1)Urothelial (1) | hepatitis B infection(14) | Pembrolizumab (9)Nivolumab (4)Sequentialpembrolizumab + Ipilimumab (1) | Undetectable/Detected (4/4) Unknown (6) | Tenofovir (3) Entecavir (5) Nil (6) | Nil (0/14) | (0/14) No patient had reactivation of HBV | hypothyroidism G2:(1), rashG1:(1) G2:(1) pneumonitis G2:(1) vitiligo G1:(1) | CR(1)/PR(2) /SD(8) (21.4%). | |
| Zhang X | 2019 | 114 | NSCLC (13) HCC (28) Melanoma (14) Nasopharyngeal carcinoma (35) Lymohoma(8) Others(16) | HBsAg positive (114) | Anti-PD-1/PDL-1 monotherapy (83) Combination therapy (31) Immunotherapy monotherapy(83): pembrolizumab, nivolumab, toripalimab, camrelizumab, sintilimab, atezolizumab. Combinations of immunotherapy and chemotherapy (22), targeted agent ((osimertinib [n = 1], bevacizumab [n = 1],regorafenib [n = 1], apatinib[n = 1], sunitinib [n = 1], nimotuzumab [n = 2], cetuximab [n = 1]) and ipilimumab (n = 2) | Undetectable/Detectable (79/35) | Tenofovir (5) Entecavir (68) Lamivudine (10) Telbivudine (1)Adefovir (1) Nil (29) | Entecavir (4) Entecavir + Tenofovir (1)Nil (1) | (6/114)These 6 patients had baseline HBV- DNA negative, only 1 patients receiving prophylactic entecavir | hepatitis G1-2: (35) G3-4: (10) | NR | |
| Shah NJ | 2019 | 34 (16 HBV/ 18 HCV | HCC (16) NSCLC (10) RCC (3)Gastric (1) SCLC (1)H&N (1)Anal SCC (2) | 16 HBV chronic viral infections (1 of these had HCV and 1 of these had HIV co-infection. 8 patients had positive HBsAg, 4 patients were HBsAg (-), HBsAb (-), and HBcAb (+), and 3 patients were HBsAg (-), HBsAb (+), and HBcAb (+). One patient's HBV status was unknown.) | Anti-PD-(L)1 monotherapy (30) Anti-PD-(L)1 plus chemotherapy (3)Concurrent Ipilimumab -Nivolumab (1) | Undetectable/Detectable (8/5) Unknown (3) | Tenofovir (6) Entecavir (3) Nil (7) | Nil(0) | No patient had reactivation of HBV | Any grade 15 (44%)Grade≥ 3: 10 (29%) | 6 PR (18%) | |
| Pertejo-Fernandez A | 2020 | 19(16HBV/3HCV) | NSCLC | HBV (16, 2 of these had HCV co-infection) | Anti-PD-1/PDL-1 monotherapy(14) Combination therapy(2)Immunotherapy monotherapy(14):nivolumab(3), pembrolizumab(7), atezolizumab(3), durvalumab(1)Combinations of immunotherapy and chemotherapy(2):pembrolizumab+chemotherapy(1), ipilimumab+nivolumab(1) | Undetectable/Detectable (11/4), one is N/A | Tenofovir (2) Entecavir (1) Nil(13) | Nil(0) | No patient (0) | Neutropenia G4:(1) Colitis G3:(1) Diabetes mellitus G3:(1) | SD(7) PD(4) PR(5) 31% | |
| Byeon S | 2020 | 61(32HBV) | NSCLC | HBV (32, 16 past HBV infections and 16 chronic HBV infections) | Nivolumab(15) Pembrolizumab (17) | Undetectable/Detectable (23/4), N/A(5) | Entecavir(10), Tenofovir(1), Lamivudine(3) Nil(18) | Entecavir(2), Tenofovir(1) | (3/32) 2 patients had baseline HBV- DNA undetectable, 1 patient had an HBV DNA level of 1553 IU/mL before treatment, then rose to 11 317 IU/mL after 1 month of pembrolizumab treatment | Fatigue G2:(1) DNA seroconversion:(1)Pneumonitis G2:(1),G4:(1)Hyperglycemia G3:(1),G2(1)AST elevation G3:(6),G2:(1),G1(2) ALT elevation G4(2), G3(3),G2(1),G1(3) | SD(9) PD(11) PR(6) Not evaluate(6) 18.8% | |
| He MK | 2021 | 202 (202 HBV) | HCC | HBsAg positive and HBV-DNA positive (202) | Nivolumab (50) Pembrolizumab (45) Toripalimab (59) Sintilimab (35) Camrelizumab (13) Combinations of immunotherapy and HAIC (84) | HBV-DNA Low group ( ≤ 500 IU/ml N = 94) High group ( > 500 IU/ml N = 108) | Entecavir (148) Tenofovir (51) Other (3) | Entecavir(5), Tenofovir(2) | 7 patients had HBV reactivation, 5 patients in the HBV-DNA low group and 2 patients in the HBV-DNA high group. | Hepatitis All grades (49), Grade 3/4 (14) | Not reported | |
| Zhong L | 2021 | 120(43HBV) | HCC (32) Lung cancer (2) Esophgeal cancer (1) Melanoma (4) Others (4) | HBsAg positive (43) | Anti-PD-1 monotherapy(13) Combinations of immunotherapy and chemotherapy/targeted agent (30) | Undetectable/Detectable (20/14), N/A(9) | All patients (n=43) with HBV received regular antiviral therapy | All patients (n=43) with HBV received regular antiviral therapy | No patient (0) | Not reported | CR (0) PR (5) SD(9) PD (12) 11.63% | |
| Case reports (n=8) | ||||||||||||
| Sharma A | 2013 | 2(1HBV/1HCV) | Melanoma | 1 HBeAb(+) | Ipilimumab | Undetectable | tenofovir | tenofovir | 0 | Not reported | PD | |
| Koksal AS | 2017 | 1 | Melanoma | 1 HBsAg(+) | Ipilimumab+Nivolumab | Unknown | No | tenofovir disoproxil fumarate | 1 | hepatitis (G3-4) | Not reported | |
| Pandey A | 2018 | 1 | NSCLC (ADC) | 1 HBsAg(+) | Pembrolizumab | Unknown | No | tenofovir disoproxil fumarate | 1 | hepatitis (G3-4) | Not reported | |
| Ragunathan K | 2017 | 1 | NSCLC (ADC) | 1 HBsAg(+) | Pembrolizumab | Unknown | No | Unknown | 1 | hepatitis (G3-4) | Not reported | |
| Lake AC | 2017 | 1 | NSCLC (ADC) | 1 HBsAg(+) HBeAg (+) /HIV | Nivolumab | Undetectable | No | tenofovir disoproxil fumarate | 1 | hepatitis (G3-4) | CR | |
| Liu Z | 2019 | 1 | HCC | Chronic HBV infection | Pembrolizumab | Detectable | Entecavir | Entecavir | 0 | Diarrhea G3, Thrombocytopenia G4 occurred after the administration of Lenvatinib | CR | |
| Akar E | 2019 | 1 | RCC(clear cell) | HBsAg(+), also HDV-DNA(+) | Nivolumab | Detectable | Entecavir | Entecavir | 0 | Nil | SD | |
| Duan X | 2020 | 1 | HCC | Chronic HBV infection | Sintilimab | Detectable | Entecavir | Entecavir | 0 | fatigue G1, thrombocytopenia G1, AST elevation G1, ALT elevation G2 | CR | |
| ASCO meeting abstract&Poster (n=2) | ||||||||||||
| Kennedy Ng | 2020 | 114(62HBV/13HCV/) | HCCs | 62 HBV positive | Most received ICI as monotherapy (62%),most commonly a PD-1(58.8%) | Undetectable/Detectable (15/40) | Unknown (Most HBV patients (57, 91.9%) were on antiviral therapy) | Unknown | 6 /62 (1 patient were on baseline anti-virals before ICI initiation, 5 were not. 1 required treatment discontinuation. ) | Any Grades(79 69.3%), Grade3 or 4(17, 14.9%) | PR(21, 18.4%) SD(37, 32.5%) PD(46, 40.4%) ORR(21, 18.4%) | |
| Chongrui Xu | 2020 | 17 | NSCLC | 17 HBsAg(+) | Nivolumab(4) Pembrolizumab(5) Nivolumab+chemo(2) Pembrolizumab+chemo(5) | Unknown | 8 patients received anti-virus therapy including entecavir and adfovir during the treatment | Nil | 0 | Transaminase or bilirubin elevation(G1):6, Transaminase elevation(G3):1, bilirubin elevation(G4):1 | NR | |
HBV=Hepatitis B virus, HCV=Hepatitis C virus, HIV=Human immunodeficiency virus; HCC=Hepatocellular Carcinoma; NSCLC=Non-Small cell lung cancer; ADC=adenocarcinoma; SCLC=Small cell lung cancer; H&N=Head and neck squamous cell carcinoma,RCC=Renal cell carcinoma; CR= complete response, PR=partial response, SD=stable disease, PD=progressive diseas; NR=Not Report;Nil=Zero; ALT=Alanine transaminase; AST=Aspartate aminotransferase; HBsAg=Hepatitis B surface antigen, HBsAb=Hepatitis B surface antibody, HBcAb=Hepatitis B core antibody, HBeAg=Hepatitis B e antigen, HBeAb=Hepatitis B e antibody HAIC=Hepatic arterial infusion chemotherapy.
*Viral Load (IU/ml) Detectable HBV-DNA≥100 IU/ml;Undetectable<100.
Characteristics of 538 patients with HBV infection and Advanced-Stage Cancer included in Review
| Characteristic | No | % |
|---|---|---|
| Age, mean(range) | 58.5(16-88) | |
| Sex | ||
| Male | 385 | 72% |
| Female | 103 | 19% |
| Unknown | 50 | 9% |
| Disease type (n=538) | ||
| NSCLC | 70 | 13.0% |
| Melanoma | 46 | 8.6% |
| HCC | 353 | 65.6% |
| Lymphoma | 9 | 1.7% |
| NPC | 35 | 6.5% |
| Other | 25 | 4.6% |
| ICI treatment (n=538) | ||
| Nivolumab | 144 | 80.4% |
| Pembrolizumab | 110 | 61.5% |
| Ipilimumab | 9 | 5.0% |
| Pembrolizumab+Ipilimumab | 4 | 2.2% |
| Atezolizumab | 3 | 1.7% |
| Durvalumab | 1 | 0.6% |
| Ipilimumab+Nivolumab | 2 | 1.1% |
| Sintilimab | 36 | 20.1% |
| Camrelizumab | 13 | 7.3% |
| Toripalimab | 59 | 33.0% |
| Anti-PD-1/PDL-1(not specified) | 114 | 21.2% |
| Anti-PD-1 (not specified) | 43 | 8.05 |
| Baseline viral load recorded (n=444) | ||
| Undetectable | 237 | 53.4% |
| Detectable | 176 | 39.6% |
| Unknown | 31 | 7.0% |
| Antiviral prophylaxis (n=416) | ||
| Entecavir | 240 | 57.7% |
| Tenofovir | 65 | 15.6% |
| Lamivudine | 13 | 3.1% |
| Telbivudine | 1 | 0.2% |
| Adefovir | 1 | 0.2% |
| Unknown | 96 | 23.1% |
The FAERS searches yielded 2,618 potentially relevant patients, of whom 12 had cancer and developed HBV reactivation during ICI treatment (Table 3). The first HBV reactivation was reported in 2015. The main cancer types were lung cancer (n = 3) and non-Hodgkin lymphoma (n = 2). With respect to specific medications, six patients were administrated pembrolizumab, three were administered nivolumab, two were administered atezolizumab, and one was administered durvalumab. Four patients received ICIs in combination with other drugs, including two with non-Hodgkin lymphoma who were treated with rituximab and brentuximab vedotin, which respectively target CD20 and CD30, one with NSCLC who was treated with glucocorticoids and pembrolizumab, and one with triple-negative breast cancer who received paclitaxel with atezolizumab. Four of these patients died, but their causes of death were not described in detail.
Safety
The main adverse events related to laboratory test results were elevated liver aminotransferase, including 18 cases of Common Terminology Criteria for Adverse Events (CTCAE) grade 3-4 aspartate aminotransferase (AST) elevation and 36 cases of CTCAE grade 3-4 alanine aminotransferase (ALT) elevation. Other severe adverse events (CTCAE grade 3 or 4) included hyperglycaemia (n = 3), thrombocytopenia (n = 1), and neutropenia (n = 1). Treatment-related CTCAE grade 3-4 adverse events included diarrhoea (n = 1), pneumonia (n = 1), and colitis (n = 1) (Table 4).
Adverse Events Reported in The Literature for Patients with Advanced Cancer and Hepatitis B Infection Treated with ICI
| Any grade | Grade1 | Grade2 | Grade3-4 | |
|---|---|---|---|---|
| Treatment-related Aes | ||||
| Diarrhoea | 1 | 0 | 0 | 1 |
| Rash | 2 | 1 | 1 | 0 |
| Fatigue | 2 | 1 | 1 | 0 |
| Hypothyroidism | 1 | 0 | 1 | 0 |
| Pneumonitis | 3 | 0 | 2 | 1 |
| Vitiligo | 1 | 1 | 0 | 0 |
| Colitis | 1 | 0 | 0 | 1 |
| Laboratory-treatment related Aes | ||||
| ALT elevation | 122 | 49 | 37 | 36 |
| AST elevation | 63 | 44 | 1 | 18 |
| Hyperglycemia | 4 | 0 | 1 | 3 |
| Thrombocytopenia | 2 | 1 | 0 | 1 |
| Neutropenia | 1 | 0 | 0 | 1 |
| Unspecified Aes | 27 | 0 | 0 | 27 |
ALT=Alanine transaminase; AST=Aspartate aminotransferase
HBV reactivation developed in 26 patients (4.1%), with a median onset of 11 weeks (range, 2-35 weeks) after ICI therapy, 6 of whom were excluded from our analysis because of missing baseline data (Table 5) [26]. The tumour types of the remaining 20 patients were HCC (n = 8), NSCLC (n = 5), nasopharyngeal carcinoma (n = 2), melanoma (n = 2), metastatic adenocarcinoma of the lung (n = 1), head and neck squamous cell cancer (n = 1), and soft tissue sarcoma (n = 1). Seven of these patients were treated with pembrolizumab, five with nivolumab, four with toripalimab, two with camrelizumab, and one with sintilimab. Notably, HBV reactivation occurred in one patient who underwent four cycles of ipilimumab and one follow-up cycle of nivolumab. Thirteen of these patients received ICI monotherapy, six received ICIs combined with hepatic arterial infusion chemotherapy (HAIC), and one received ICIs with whole brain radiotherapy.
Only five patients had a detectable HBV-DNA viral load before ICI treatment. At reactivation, the median HBV-DNA viral load was 3.92 × 103 copies/mL. Fifteen patients experienced hepatitis, with a median peak ALT (n = 15) of 281 U/L (range, 151-994 U/L) and a median peak AST (n = 5) of 520 U/L (range, CTCAE G1 to 845 U/L).
Eleven patients were treated with prophylactic antivirals (eight received entecavir and three received tenofovir) before ICI therapy. Two patients diagnosed with immune-related hepatitis received glucocorticoids before being diagnosed with HBV-related hepatitis [18,29]. After HBV reactivation, 11 patients were treated with entecavir, 6 with tenofovir, and 1 with entecavir combined with tenofovir; the type of treatment was not noted for 2 patients. HBV-DNA returned to undetectable status in 15 of 17 patients (88.2%) after a median 5.5 weeks (range, 1-14 weeks). One patient (Table 5, patient No. 3) had undetectable HBV-DNA before immunotherapy. After 28 weeks of pembrolizumab treatment, there was an increase in HBV-DNA but no increase in AST or ALT. However, the patient's HBV-DNA returned to undetectable status after 5 weeks without antiviral therapy [31]. One patient with both HIV and HBV infections before administration of ICIs, received anti-HIV treatment with dolutegravir and abacavir, and the HIV-RNA copies appeared to be normal (<20 copies/mL). Therefore, the antiviral regimen was adjusted to dolutegravir combined with tenofovir [20].
Fourteen patients experienced immunotherapy disruption due to HBV reactivation, including 2 who discontinued therapy and 12 who delayed treatment. There were no fatal HBV reactivations. Sixteen of 20 patients achieved undetectable HBV-DNA levels after a median of 4 weeks (range, 1-14 weeks), whereas the HBV-DNA levels in 3 patients were still detectable at 4-14 weeks after HBV reactivation (183 U/mL, 599 IU/mL, and 4743 IU/mL, respectively). In the 14 patients with HBV-related hepatitis, liver enzymes returned to normal after a median of 4.5 weeks (range, 2-11 weeks).
Efficacy
The safety of ICIs has been the major concern in most studies of ICIs in patients with advanced tumours and HBV infection, and data on the efficacy are conflicting. Among the 146 patients with known responses to ICI therapy, 14 of 88 patients with HCC (15.91%), 6 of 45 patients with NSCLC (13.33%), and 3 of 13 patients with melanoma (23.08%) had a therapeutic response (Table 6). Two prospective clinical studies reported details about the objective response rate (ORR) of ICIs in patients with advanced HCC. In the CheckMate 040 study, objective responses included one complete response (CR) and eight partial responses (PRs), for an ORR of 13.6%. Disease control was observed in 48 patients [4]. The KEYNOTE-224 study reported five patients who achieved PR (ORR = 23.8%) [5].
Of the 10 retrospective studies included in this review, 2 did not report the efficacy of ICIs [25,28], and 7 studies, limited by sample size and experimental design, assessed the best efficacy of patients in terms of number of cases. Only one study used propensity score matching (PSM) to compare the effects of ICI treatment in patients with and without HBV infection (HBV group, n = 15; non-HBV group, n = 24). There were no statistically significant differences in the ORR (55.6% and 36.8%, P = 0.35) or overall survival (OS) (P = 0.15) between the two groups. Of the eight case reports included in the review, three only recorded HBV reactivation and the corresponding treatment. The other cases indicated best responses of CR (n = 3), SD (n = 1), and progressive disease (PD) (n = 1) [33].
Only 3 of 12 patients in the FAERS database who experienced HBV reactivation showed responses to immunotherapy, including 1 patient with NSCLC who achieved PR to pembrolizumab, 1 patient with small cell lung cancer with PD, and 1 patient with HCC with PD.
Characteristics of 12 patients with HBV infection and Advanced-Stage Cancer included in the FDA Adverse Event Reporting System
| Patient | Age | Gender | Country | Cancer Type | ICIs | Combined treatment | Outcome | Received Date | Response |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | M | USA | Malignant Melanoma Stage Iv | Pembrolizumab | No | Other | 20-Apr-19 | - |
| 2 | Not Specified | F | USA | Neoplasm Malignant | Pembrolizumab | No | Other | 7-Jun-17 | - |
| 3 | 36 | F | France | Non-Hodgkin'S Lymphoma | Pembrolizumab | Adcetris | Other | 11-Sep-18 | - |
| 4 | 57 | M | Bulgaria | Non-Small Cell Lung Cancer Metastatic | Pembrolizumab | Methylprednisolone | Died | 22-Apr-19 | PR |
| 5 | Not Specified | M | Bulgaria | Small Cell Lung Cancer | Pembrolizumab | No | Other | 26-Feb-19 | PD |
| 6 | Not Specified | Not Specified | USA | - | Pembrolizumab | No | Other | 22-Mar-16 | - |
| 7 | 45 | M | Korea | Hepatocellular Carcinoma | Nivolumab | No | Died | 19-Jan-18 | PD |
| 8 | Not Specified | Not Specified | Japan | Malignant Melanoma | Nivolumab | No | Other | 14-Dec-15 | - |
| 9 | Not Specified | Not Specified | USA | Hodgkin'S Lymphoma | Nivolumab | Rituximab etc | Died | 23-Jan-19 | - |
| 10 | Not Specified | M | HK | Transitional Cell Carcinoma | Atezolizumab | No | Other | 30-Mar-18 | - |
| 11 | 83 | F | Belgium | Triple Negative Breast Cancer | Atezolizumab | Paclitaxel | Other | 15-Jun-20 | - |
| 12 | 67 | M | France | Lung Adenocarcinoma | Durvalumab | No | Died | 18-Nov-19 | - |
The Situation of 13 Patients with Advanced Cancer Who Had Hepatitis B Reactivation After Using ICIs
| Patients Characteristics | Baseline | At reactivation | Antiviral Treatment | Time for Achieving HBV-DNA Undetectable (Weeks) | Time for ALT Recovery (Weeks) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Patient | Age | Gender | Country | Cancer Type | ICIs | Combined Treatment | HBV-DNA | Antiviral Prophylaxis | Weeks From Start of ICI | HBV-DNA(copies/ml) | Peak AST/ALT(U/L) | Anti-PD-1/PD-L1 Therapy Disruption | |||
| Zhang X 2019(a) | 1 | 48 | M | China | NPC | Camrelizumab | No | Undetectable | No | 3 | 7.81×103 | ALT=191.4 | Delayed | Entecavir | 1 | 2 |
| 2 | 47 | M | China | NPC | Camrelizumab | No | Undetectable | No | 16 | 6.98 × 104 | ALT=203 | Delayed | Entecavir | 4 | 4 | |
| 3 | 39 | M | China | Melanoma | Pembrolizumab | No | Undetectable | No | 28 | 2.10 × 103 | ALT=27.6 | No | Not use | 5 | Not reported | |
| 4 | 36 | M | China | HCC | Nivolumab | No | Undetectable | Entecavir | 12 | 1.80 × 103 | ALT=298 | Discontinued | Entecavir+tenofovir | 1 | 3 | |
| 5 | 45 | M | China | H&NSCC | Toripalimab | No | Undetectable | No | 35 | 4.04 × 106 | ALT=281.2 | Delayed | Entecavir | 3 | 6 | |
| 6 | 41 | F | China | Sarcoma | Nivolumab | No | Undetectable | No | 20 | 6.00 × 107 | ALT=465.1 | Not reported | Entecavir | 8 | 4 | |
| Byeon S 2020 | 7 | 59 | M | Korea | NSCLC | Nivolumab | No | 70 IU/ml | Tenofovi | 3 | 2.81× 103 | AST G1 | Delayed | Tenofovi | 4 weeks PD die | Not reported |
| 8 | 60 | M | Korea | NSCLC(adenocarcinoma) | Pembrolizumab | No | Undetectable | Entecavir | 4 | 1.48 × 103 | Normal | Delayed | Entecavir | 4 | Not reported | |
| 9 | 45 | M | Korea | NSCLC(adenocarcinoma) | Pembrolizumab | No | 1553 IU/ml | Entecavir | 4 | 1.13 × 104 | Normal | No | Entecavir | 599 IU/ml | Not reported | |
| Koksal AS 2017 | 10 | 56 | M | Turkey | Melanoma | Ipilimumab*4 cycle,Nivolumab | No | Unknown | No | 14 | 2.45 × 105 | 845/888 | No | Tenofovir | 11week(183IU/ml) | 11 |
| Pandey A 2018 | 11 | 51 | M | USA | NSCLC(adenocarcinoma) | Pembrolizumab | Whole Brain radiation | Unknown | No | 4 | >1.70× 108 | 670/994 | No | Tenofovir | 10 | 10 |
| Ragunathan K 2017 | 12 | 51 | M | USA | metastatic adenocarcinoma of the lung | Pembrolizumab | No | Unknown | No | 2 | >170.0 IU/ml | 217/615 | Not reported | Not reported | Not reported | Not reported |
| Lake AC 2017 | 13 | 72 | M | USA | NSCLC(adenocarcinoma) | Nivolumab | No | Undetectable | Dolutegravir/Abacavir | 13 | >1.7 × 105 | 520/474 | Delayed | Dolutegravir /Tenofovir | 14 | 9 |
| He MK 2021 | 14 | Not reported | M | China | HCC | Pembrolizumab | No | 1.65e2 IU/mL | Tenofovir | 6 | 2.85×104 | ALT=198.2 | Discontinued | Entecavir | 7 | 5 |
| 15 | Not reported | M | China | HCC | Pembrolizumab | HAIC | 3.83e6 IU/ml | Tenofovir | 14 | 4.86×103 | ALT=271.6 | Delayed | Tenofovir | 9 | 6 | |
| 16 | Not reported | M | China | HCC | Toripalimab | HAIC | Undetectable | Entecavir | 6 | 1.31×103 | ALT=39.6 | No | Entecavir | 1 | Not reported | |
| 17 | Not reported | M | China | HCC | Nivolumab | HAIC | 5.86e3IU/ml | Entecavir | 12 | 1.58×103 | ALT=208.5 | Delayed | Entecavir | 3 | 3 | |
| 18 | Not reported | M | China | HCC | Sintilimab | HAIC | Undetectable | Entecavir | 4 | 2.98×103 | ALT=151 | Delayed | Entecavir | 4 | 3 | |
| 19 | Not reported | M | China | HCC | Toripalimab | HAIC | Undetectable | Entecavir | 16 | 1.21×103 | ALT=174.8 | Delayed | Tenofovir | 2 | 3 | |
| 20 | Not reported | M | China | HCC | Toripalimab | HAIC | Undetectable | Entecavir | 4 | 1.21×103 | ALT=378.8 | Delayed | Entecavir | 7 | 6 | |
Objective Response Rates Reported in The Literature for Patients with Advanced Cancer and Hepatitis B Infection Treated with ICI
| Response(No. of Patients) | ORR | |||||
|---|---|---|---|---|---|---|
| Tunor | Number of Patients With Known Response | CR | PR | SD | PD | |
| HCC | 88 | 6 | 8 | 50 | 24 | 15.91% |
| NSCLC | 45 | 0 | 6 | 18 | 16 | 13.33% |
| Melanoma | 13 | 1 | 2 | 4 | 6 | 23.08% |
CR= complete response, PR=partial response, SD=stable disease, PD=progressive diseas; ORR=Objective response rate.
Ongoing Clinical Trials of ICIs in Patients With HBV Infection and Advanced-Stage Cancer
| Trial | NCT Trial No | Location | ICI | Tumor | Phase | Study Type | Sample size | Primary Endpoint |
|---|---|---|---|---|---|---|---|---|
| P1101 and Anti-PD1 for After Curative Surgery of Hepatitis B-related Hepatocellular Carcinoma | NCT04233840 | Taiwan | Nivolumab | HCC | I/II | Interventional (Clinical Trial) | 72 | Dose-limiting Toxicity/Recurrence-free survival |
| Pembrolizumab in Hepatocellular Carcinoma | NCT03419481 | Hongkong | pembrolizumab | HCC | II | Interventional (Clinical Trial) | 30 | Response rate |
| Atezolizumab Plus Bevacizumab for Patients With Advanced Hepatocellular Carcinoma (HCC) and Chronic Hepatitis B Virus (HBV) Infection | NCT04180072 | Taiwan | Atezolizumab | HCC | II | Interventional (Clinical Trial) | 48 | Best overall response rate Best overall response rate |
| Durvalumab for Advanced Hepatocellular Carcinoma in Patients With Active Chronic Hepatitis B Virus Infection | NCT04294498 | Taiwan | Durvalumab | HCC | II | Interventional (Clinical Trial) | 43 | The rate of HBV reactivation |
| Safety and Immunotherapeutic Activity of Cemiplimab in Participants With HBV on Suppressive Antiviral Therapy | NCT04046107 | Los Angeles | Cemiplimab | Nil | I/II | Interventional (Clinical Trial) | 30 | Targeted safety events/Number of discontinue treatment |
HCC=Hepatocellular Carcinoma; HBsAg=Hepatitis B surface antigen.
Discussion
We screened all the published cases and meeting abstracts involving patients with advanced cancer and HBV infection receiving ICIs and identified a total of 22 publications including 633 patients. Of these, 26 (4.1%) developed HBV reactivation, including 6 cases among 79 patients in two meeting abstracts. Moreover, grade 3 or higher elevation of liver aminotransferases occurred frequently in those patients, consistent with the findings of most retrospective studies. Through the FAERS database search, we identified 12 patients with advanced cancer with HBV infections who received ICIs and developed HBV reactivation. Four of these patients died, and the data were rather limited with regard to HBV-DNA load and antiviral treatments. Hence, we should consider that HBV reactivation may not have been reported among these patients, and selective publication may underreport the risk of HBV reactivation.
As of April 2022, to the best of our knowledge, there were only two prospective clinical trials (CheckMate 040 and KEYNOTE-224) evaluating the safety and efficacy of ICIs in HCC patients with HBV infection, both of which highlighted that patients with HBV infection should receive antiviral therapy and exhibit a viral load < 100 IU/mL prior to immunotherapy [4,5]. In the CheckMate 040 study, 262 HCC patients were treated with nivolumab, 66 of whom were HBsAg-positive (15 recruited in the escalation phase and 51 in the expansion phase). The ORR in the HBV-infected group was 13.6%. The KEYNOTE-224 study recruited 104 patients with advanced HCC receiving sorafenib and pembrolizumab, 22 of whom had HBV infections. In this study, the ORR in the HBV-infected group was 23.8%. Intriguingly, HBV reactivation did not occur in the 88 HBsAg-positive patients in the two studies, which might be attributable to the strict enrolment conditions. However, we could not determine whether ICIs are safe for patients with HBV infection or whether there is a definite link between ICI administration and HBV reactivation.
We included 458 patients from 12 retrospective studies (including two meeting abstracts) with advanced cancer and HBV infection who received ICIs. The study by He et al. with 202 patients, which was the largest study, recorded 7 patients with HBV reactivation. The 202 patients were divided into a low HBV-DNA group (n = 94, HBV-DNA ≤500 IU/mL) and a high HBV-DNA group (n = 108, HBV-DNA >500 IU/mL) according to their baseline HBV-DNA level. However, there was no significant difference in the incidence of HBV-associated hepatitis (P = 0.56) or PD-1 inhibitor disruption (P = 0.82) between the two groups. Further, six patients who developed HBV reactivation were treated with hepatic arterial infusion chemotherapy (HAIC). Therefore, the authors divided patients into HAIC and non-HAIC groups and found that the HBV reactivation rate in the HAIC group was higher than that in the non-HAIC group (P = 0.04) [32]. The study by Zhang et al., which had the second largest sample size (n = 114), reported six patients with HBV reactivation, five of whom were not administered prophylactic antiviral treatment. They noted that the most significant risk factor for viral reactivation was lack of antiviral prophylaxis before immunotherapy (odds ratio [OR], 17.50 [95% CI, 1.95-157.07]; P = 0.004) [28]. The study by Ng et al. with 62 patients indicated that 5 of 6 patients with HBV reactivation were administered antiviral drugs before ICI therapy, which differs from the results reported by Zhang et al. [8]. Although their study was presented in the form of a meeting abstract lacking baseline information and treatment regimens, the authors noted that baseline HBV-DNA levels higher than 100 IU/mL were not associated with an increased risk of HBV reactivation, which was similar to the findings of Byeon et al. and He et al. [31,32]. The study by Byeon et al. included 16 patients with previous HBV infections and 16 with chronic HBV infections, and they showed that patients with chronic infections were more likely to develop HBV reactivation after ICI treatment. However, there was no HBV reactivation reported in seven of the studies included in this review. There was only one study that directly examined the impact of HBV infection on the outcome of patients with advanced cancer treated with PD-1 inhibitors. The researchers evaluated the ORR, disease control rate, OS, and progression-free survival in the HBV (n = 15) and non-HBV groups (n = 24) after PSM. They found that HBV infection status did not impact the therapeutic response (the ORR) or prognosis [33]. However, research about HBV reactivation caused by ICI administration among patients with advanced cancer mainly comes from retrospective studies, which provide low-level evidence and are subject to selection bias. Therefore, prospective clinical trials with HBV reactivation as the primary endpoint are needed.
We included eight case reports, of which four described patients seropositive for hepatitis B surface antigen with HBV reactivation. Three patients with NSCLC received PD-1 inhibitor and one with melanoma was treated with ipilimumab and nivolumab. Additionally, CTCAE grade 3-4 hepatitis and HBV reactivation occurred in four patients. Four of the patients did not experience HBV reactivation, three of whom were treated with prophylactic antiviral medication (entecavir) and had detectable HBV-DNA prior to ICI therapy [16,21-23]. The remaining patient had malignant melanoma, and tenofovir was administered for prophylactic antiviral therapy despite the baseline HBV-DNA being undetectable [16]. Although HBV reactivation in patients with advanced cancers receiving ICIs has been recorded, the lack of long-term observation data regarding ICI therapy efficacy is a limitation.
We observed three patterns of HBV reactivation in the studies included in this systematic review. The first occurred in patients with undetectable HBV-DNA and no antiviral prophylaxis before immunotherapy. In these patients, there were significant increases in HBV-DNA load and AST/ALT at virus reactivation after receiving immunotherapy. AST/ALT and HBV-DNA load returned to normal after delaying immunotherapy and administration of antiviral treatment. The second pattern involved HBV reactivation despite antiviral prophylaxis and undetectable HBV-DNA, and antiviral therapy was adjusted to control the viral infection in these patients. The third included patients undergoing antiviral prophylaxis in whom HBV-DNA was detectable. Although HBV was reactivated in these patients, AST/ALT and HBV-DNA returned to normal after antiviral treatment.
An interesting retrospective pharmacovigilance study from FAERS reported at the 2020 ASCO meeting examined HBV reactivation in patients treated with pembrolizumab, atezolizumab, or nivolumab between 2016 and 2019. This study included 15 cases of HBV reactivation related to the use of PD-1/PD-L1 inhibitors (ROR 1.2 [95% CI 0.72-1.99]). Intriguingly, only pembrolizumab was significantly correlated with HBV reactivation (ROR 2.93 [95% CI 1.57-5.46]). Unfortunately, the diagnostic criteria, treatment, and prognosis associated with HBV reactivation were not reported.
We also searched the clinical trial network, and five registered, single-arm clinical trials were identified (Table 7). However, only one study's primary endpoint was HBV reactivation, whereas the remaining studies assessed the efficacy of ICIs. Because there are few prospective clinical studies on whether ICIs cause HBV reactivation, the answer to this question may mainly come from retrospective analysis.
The mechanisms behind HBV reactivation
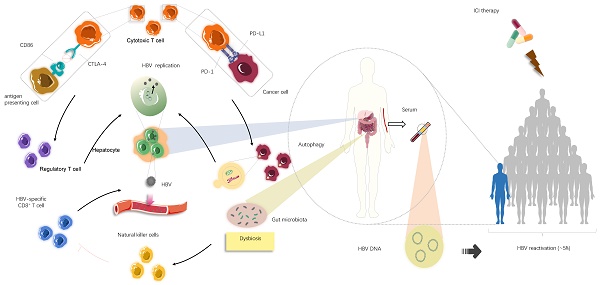
HBV reactivation, a serious complication in patients with cancer or receiving organ transplantation, is defined as the emergent reappearance or surge of HBV-DNA by at least 100-fold in the serum of individuals with resolved or inactive chronic HBV infection. Reactivation occurs in response to cytotoxic chemotherapeutics (e.g. vincristine), biological agents (e.g. anti-T and B lymphocyte monoclonal antibodies), or immunosuppressive drugs (e.g. glucocorticoids) [34-36]. Immunosuppressive therapy can make a patient susceptible to HBV reactivation; in particular, novel agents that modulate the immune system, such as ICIs, may induce HBV reactivation. HBV reactivation has been reported to occur in 4.1% of patients with advanced cancer receiving ICIs, which raises concerns about the safety of ICIs in patients ever exposed to HBV and the risk/benefit ratio of these newly developed therapeutic modalities [28,35,37,38]. Additionally, the PD-1/PD-L1 axis is a crucial suppressor of HBV-specific CD8+ T cell activities; therefore, PD-1/PD-L1 blockade could partially recover effective HBV-specific T-cell responses against viral proteins [39-42]. Therefore, HBV reactivation in this circumstance appears to be a paradoxical event with no rational explanation.
Although there is little information regarding the mechanism behind HBV reactivation during ICI treatment, examining virology, microbiology, and immunology in HBV carriers with advanced cancer may provide some answers (Fig. 3). Most importantly, it is difficult to thoroughly eradicate HBV to obtain long-lasting survival once infection occurs [36], and HBV persistence could be established by modifying host immune responses and sustained by low viral loads and dysbiosis of gut microbiota. Further, the presence of maternal hepatitis Be antigen (HBeAg) [42-45] might explain potential HBV reactivation among patients with a history of HBV infection to some extent. The innate immune system is unable to eliminate all infected hepatocytes harbouring low-level replicating HBV and/or persistent CCC HBV-DNA, which allows for a repository for HBV reactivation [28,46,47]. The PD-1/PD-L1 axis also plays a critical role in maintaining immune homeostasis. PD-1 protects the body from liver damage triggered by overactive immune response to infection [28,48-53]. Therefore, blocking PD-1/PD-L1 signalling might cause dysbiosis of immunoregulation, leading to the destruction of hepatocytes harbouring remaining HBV and the subsequent release of latent virus into circulation [54,55] (Fig. 3). Conversely, PD-1 expression is inversely correlated with proliferation and suppression capacity of regulatory T cells (Tregs) that help establish the equilibrium between immune pathology and protection, which suggests that clinical PD-1 blockade might partially impair protective immune responses, accompanied by elevated immunosuppression, eventually increasing the likelihood of HBV reactivation [55-57] (Fig. 3). Consistent with this idea, a recent study suggested that the frequency of Tregs is increased and levels of CTLA-4 are decreased in patients lacking CTLA-4, indicating that inhibition of CTLA-4, a crucial regulator responsible for immune homeostasis maladjustment, could promote HBV survival and expansion to some degree [58]. Moreover, other immune checkpoints, such as TIGIT, LAG-3, and TIM-3, can boost tumour immune evasion and motivate the exhaustion of virus-specific T cells. The resultant immunosuppressive effects may help reactivate HBV under certain conditions in a small cohort of patients after PD-1/PD-L1 inhibitor monotherapy or alternative single application of ICIs [7]. Given these questions, it is difficult to determine whether ICIs are beneficial or harmful due to a wide spectrum of potential relationships between immunotherapy, the immune system, and residual virus.
Aside from the direct effect of the immune system on virus reactivation, there may be other factors associated with the immune response affecting HBV reactivation. HBV reactivation is associated with gut microbiota, and alteration of bacterial microbiota profiles could affect the liver to determine viral fate through the gut-liver axis by several immunological signalling pathways [44,48,59,60]. Certain routine therapies cause perturbations to the gut microbiota, and immunotherapy is no exception [61-64]. Anti-CTLA-4 therapy can modulate the composition of gut-derived microbiota owing to the interplay between the immune response and intestinal microbiota [62]. In particular, a study using a mouse model deficient for PD-1 indicated a reduction of symbiotic flora, such as Bifidobacterium and Bacteroidaceae, and an increase in pathogenic flora, such as Enterobacteriaceae, in the colons of PD-1-/- mice compared with those of wild-type mice [65]. Further, there is ample evidence indicating that increased pathogenic intestinal bacteria can facilitate liver injury mediated by excessive immune responses owing to natural killer (NK) cell activation. This may allow HBV to escape from damaged hepatocytes and enter a reactive, replicating state in individuals infected with HBV [66,67] (Fig. 3). In addition, the activated NK cells promote the exhaustion of virus-specific T cells to limit antiviral responses, which could promote HBV activation [68,69]. Therefore, ICIs may promote considerable changes within the gut microbiota or the expansion of pathogenic bacteria, which in turn conduces construction of a distinct, supportive niche where HBV reactivation occurs. Despite the effects of immune factors on HBV reactivation, several factors independent of host immunity are implicated in this process, such as interconnected alterations in major cellular signalling and behaviours of the infected cells [70].
The underlying mechanism causing HBV reactivation induced by immune checkpoint inhibitors (ICIs). Overall, HBV reactivation has been reported to occur in approximately 4.1% of patients with advanced tumour undergoing ICIs therapies (i.e., anti-PD-1/PD-L1 and anti-CTLA-4 therapies), manifested by the re-detected HBV DNA levels in serum samples. In individuals ever infected with HBV, blockade of PD-1/PD-L1 axis could stimulate autophagic activities of cancer cells, which might be involved in further HBV replication. Apart from that, suppression of CTLA-4 may lead to increase in the frequency of regulatory T cells, thereby creating a context for HBV survival and expansion. In addition to these immediate impacts of immunological systems on HBV fate, most likely dysbiosis of gut microbiota, partly mediated by ICIs treatments, serves an immunological role by activating natural killer cells and in turn driving the exhaustion of HBV-specific T cells, consequently facilitating the release of latent HBV from damaged hepatocyte into circulation.
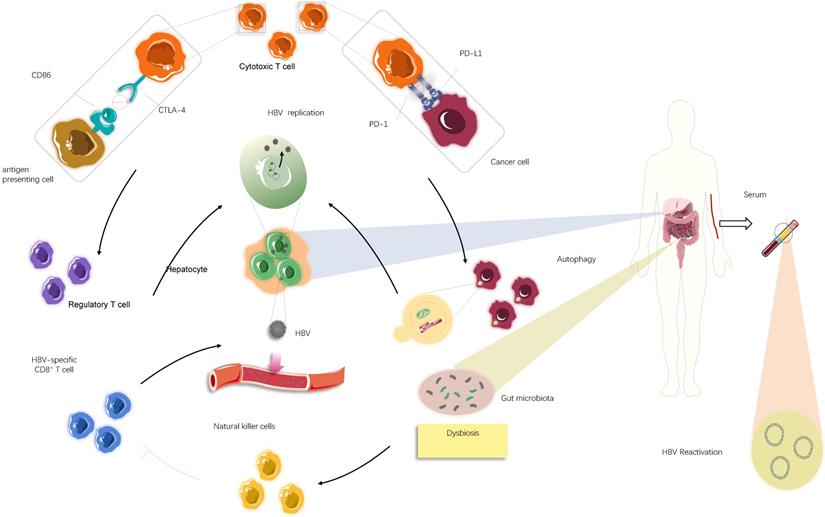
Emerging evidence indicates autophagy mediated by Akt/mTOR signalling occurs during HBV replication since HBV can hijack components of the autophagic pathway [71-73]. A study on the intrinsic functions of PD-1/PD-L1 suggested that PD-L1 blockade may cause PD-L1 attenuation and subsequently augment autophagy because of mTORC1 activation driven by tumour-expressed PD-L1 [74]. Therefore, it is plausible that autophagy enhanced by anti-PD-1/PD-L1 immunotherapy, if it occurs, will potentiate viral replication, which would lead to detectable serum HBV-DNA. In addition to the involvement of autophagy in stimulating HBV replication, inhibition of cell proliferation following cytotoxic chemotherapy appears to be advantageous for viral replication, with intracellular organelles and nutrients preferentially serving the virus rather than the cell [67]. Related to this observation, PD-L1 antibody therapy exhibits adverse impacts on tumour cell growth and remarkably reduces cell proliferation, which could explain why HBV reactivation can take place after ICI treatment [74]. Although counterintuitive, two therapies affecting immune functions in opposing directions may converge to contribute to the severe outcome represented by HBV reactivation in very similar manners.
Clinical implications
Because HBV reactivation in the context of ICIs remains unsolved, for patients with cancer and HBV infection receiving ICIs, we recommend regular monitoring of the HBV-DNA level, examination of serological characteristics, and antiviral prophylaxis, along with the optimization of monitoring, prevention, and management of HBV reactivation throughout the course of treatment. Based on the studies discussed above, we further suggest that all patients should be screened for HBV using HBsAg and HBcAb. Additionally, HBV-DNA testing is recommended in areas with a high incidence of hepatitis B. If the test result indicates a chronic HBV infection, initiation of prophylactic antiviral treatment is recommended, although it is still unclear how long prophylactic treatment should be continued. With regard to those with past HBV infection, regular monitoring of serum ALT and HBV-DNA is suggested. If HBV-positive patients display elevated levels of serum liver transaminases during ICI treatment, HBV-DNA testing should be considered. In addition to monitoring the levels of liver aminotransferases, it is critical to differentially diagnose whether the elevated aminotransferase is due to liver damage from hepatitis B reactivation or immune liver damage.
Strengths and limitations
This review comprehensively explores current studies examining the reactivation of HBV caused by ICIs. It is the first study to summarize important clinical information of 20 patients with advanced cancer and HBV infection who developed HBV reactivation after ICI treatment (Table 5). We also outline three patterns of HBV reactivation. In addition, we used the FAERS database to include previously unreported patient information. Because there are inconsistencies between basic research and clinical reports, we also examined the mechanism of HBV reactivation, including mechanisms associated with intestinal microorganisms, viruses, and the immune system.
There were several limitations to our systematic review. First, the data were mainly obtained from retrospective studies (n = 10, 45.5%) and case reports (n = 8, 36.4%), with two prospective clinical trials (9.1%) and two meeting abstracts (9.1%). Inevitably, the data extracted from studies may lead to selection bias and heterogeneity in treatment response and adverse events. Even the representativeness of prospective studies may be affected by strict inclusion criteria. Second, there were few published cases, and those detailing HBV reactivation were not comprehensive. For instance, the cases were mainly distributed in East Asia, which may restrict the application of our findings to other regions. Finally, the LAG-3 immune checkpoint inhibitor relatlimab was approved by the FDA on March 18, 2022, and currently, there are an unprecedented number of clinical trials of novel immune checkpoint inhibitors. (The data on ongoing clinical trials of relatlimab and TIM3 ICIs are detailed in Table S1 and Table S2). However, limited by the current stage of research, there are no reports of HBV reactivation caused by these novel immune checkpoint inhibitors. Future studies will be important for understanding the relationship between HBV reactivation and other ICIs.
Conclusion
HBV reactivation might occur in HBV-positive cancer patients receiving ICI therapy. As HBV reactivation can be effectively controlled with current antiviral drugs, ICIs seem to be a safe and effective treatment option in this patient population. However, there is still a need for clinical monitoring of liver enzymes and HBV-DNA levels. Ongoing prospective clinical trials will shed further light on the safety and efficacy of ICI therapy in cancer patients with HBV infections.
Abbreviations
ALT: alanine aminotransferase; ASCO: American Society of Clinical Oncology; AST: aspartate aminotransferase; CI: confidence interval; CR: complete response; CTCAE: Common Terminology Criteria for Adverse Events; CTLA-4: cytotoxic T lymphocyte antigen 4; FAERS: Food and Drug Administration Adverse Event Reporting System; HAIC: hepatic arterial infusion chemotherapy; HBeAg: hepatitis B e antigen; HBsAg: hepatitis B surface antigen; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HIV: human immunodeficiency virus; ICI: immune checkpoint inhibitor; NK: natural killer; NSCLC: non-small cell lung cancer; OR: odds ratio; ORR: overall response rate; OS: overall survival; PD: progressive disease; PD-1: programmed cell death 1; PD-L1: programmed cell death ligand 1; PR: partial response; PSM: propensity score matching; ROR: reporting odds ratio; SIT: superinfection therapy; Treg: regulatory T cell.
Supplementary Material
Supplementary tables.
Acknowledgements
This work was supported by the Chinese NSFC under Grant 81872489.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations: Immune Therapy in Difficult Populations. Cancer. 2017;123:1904-11
2. World Health Organization. Hepatitis B. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
3. Ramsey SD, Unger JM, Baker LH, Little RF, Loomba R, Hwang JP. et al. Prevalence of Hepatitis B Virus, Hepatitis C Virus, and HIV Infection among Patients with Newly Diagnosed Cancer from Academic and Community Oncology Practices. JAMA Oncology. 2019;5:497-505
4. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. The Lancet. 2017;389(10088):2492-2502
5. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D. et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. The Lancet Oncology. 2018;19(7):940-952
6. Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell death & disease. 2015;6:e1694-e1694
7. Raziorrouh B, Schraut W, Gerlach T, Nowack D, Grüner NH, Ulsenheimer A. et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology. 2010;52:1934-47
8. Ng KYY, Wong LWJ, Ang AJS, Tan SH, Choo SP, Tai DW. et al. Real-world efficacy and safety of immune checkpoint inhibitors in advanced hepatocellular carcinoma: Experience of a tertiary Asian Center. Asia-Pacific Journal of Clinical Oncology. 2020;17(5):e249-e261
9. Chongrui Xu, Hai-Yan Tu, Yue-Li Sun, Yang-Si Li, Bing-Fei Xu, Bin-Chao Wang. et al. Safety of nivolumab/pembrolizumab in hepatitis B surface antigen-positive non-small cell lung cancer (NSCLC) patients. Journal of Clinical Oncology. 2020;38:e21670-e21670
10. Muhsen I, Burns E, Umoru G, Arain A, Xu J, Abdelrahim M. Hepatitis B reactivation with pembrolizumab, atezolizumab, and nivolumab: A pharmacovigilance study and literature review. Journal of Clinical Oncology. 2020;38:e15127-e15127
11. Pu D, Yin L, Zhou Y, Li W, Huang L, Cai L, Zhou Q. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: A systematic review. Medicine. 2020;99:e19013-e19013
12. Escoin-Perez C, Blasco S, Juan-Vidal O. Immune checkpoint inhibitors in special populations. A focus on advanced lung cancer patients. Lung Cancer. 2020;144:1-9
13. Tapia Rico G, Chan MM, Loo KF. The safety and efficacy of immune checkpoint inhibitors in patients with advanced cancers and pre-existing chronic viral infections (Hepatitis B/C, HIV): A review of the available evidence. Cancer Treatment Reviews. 2020;86:102011
14. Jácome AA, Castro ACG, Vasconcelos JPS, Silva MHCR, Lessa MAO, Moraes ED. et al. Efficacy and Safety Associated With Immune Checkpoint Inhibitors in Unresectable Hepatocellular Carcinoma: A Meta-analysis. JAMA Netw Open. 2021;4:e2136128
15. Li B, Yan C, Zhu J, Chen X, Fu Q, Zhang H. et al. Anti-PD-1/PD-L1 Blockade Immunotherapy Employed in Treating Hepatitis B Virus Infection-Related Advanced Hepatocellular Carcinoma: A Literature Review. Front Immunol. 2020;11:1037
16. Sharma A, Thompson JA, Repaka A, Mehnert JM. Ipilimumab administration in patients with advanced melanoma and hepatitis B and C. Journal of Clinical Oncology. 2013;31(21):e370-2
17. Koksal AS, Toka B, Eminler AT, Hacibekiroglu I, Uslan MI, Parlak E. HBV-related acute hepatitis due to immune checkpoint inhibitors in a patient with malignant melanoma. Annals of Oncology. 2017;28(12):3103-3104
18. Pandey A, Ezemenari S, Liaukovich M, Richard I, Boris A. A Rare Case of Pembrolizumab-Induced Reactivation of Hepatitis B. Case Reports in Oncological Medicine. 2018;2018:5985131
19. Ragunathan K, Dadana S, Huang C-H. Hepatitis B Reactivation After Administration of Pembrolizumab (KEYTRUDA): A Unique Case Report:2145. American Journal of Gastroenterology. 2017;112:S1187-1188
20. Lake AC. Hepatitis B reactivation in a long-term nonprogressor due to nivolumab therapy. AIDS. 2017;31(15):2115-2118
21. Liu Z, Li X, He X, Xu Y, Wang X. Complete response to the combination of Lenvatinib and Pembrolizumab in an advanced hepatocellular carcinoma patient: a case report. BMC Cancer. 2019;19(1):1062
22. Akar E, Baytekin HF, Deniz H, Tural D. Safe use of nivolumab in a patient with renal cell carcinoma and hepatitis B. Journal of Oncology Pharmacy Practice. 2020;26(4):1022-1024
23. Duan X, Zhang H, Zhou L, Jiang B, Mao X. Complete response to the combination of sintilimab and IBI305 for a patient with HBV-associated hepatocellular carcinoma with multiple lung metastasis. Digestive and Liver Disease. 2020;52(7):794-796
24. Ravi S, Spencer K, Ruisi M, Ibrahim N, Luke JJ, Thompson JA. et al. Ipilimumab administration for advanced melanoma in patients with pre-existing Hepatitis B or C infection: A multicenter, retrospective case series. Journal for ImmunoTherapy of Cancer. 2014;2(1):33
25. Wen X, Wang Y, Ding Y, Li D, Li J, Guo Y. et al. Safety of immune checkpoint inhibitors in Chinese patients with melanoma. Melanoma Research. 2016;26(3):284-9
26. Kothapalli A, Khattak MA. Safety and efficacy of anti-PD-1 therapy for metastatic melanoma and non-small-cell lung cancer in patients with viral hepatitis: A case series. Melanoma Research. 2018;28(2):155-158
27. Tio M, Rai R, Ezeoke OM, McQuade JL, Zimmer L, Khoo C. et al. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. European Journal of Cancer. 2018;104:137-44
28. Zhang X, Zhou Y, Chen C, Fang W, Cai X, Zhang X. et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. Journal for ImmunoTherapy of Cancer. 2019;7(1):322
29. Shah NJ, Al-Shbool G, Blackburn M, Cook M, Belouali A, Liu SV. et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. j immunotherapy cancer. 2019;7:353
30. Pertejo-Fernandez A, Ricciuti B, Hammond SP, Marty FM, Recondo G, Rangachari D. et al. Safety and efficacy of immune checkpoint inhibitors in patients with non-small cell lung cancer and hepatitis B or hepatitis C infection. Lung Cancer. 2020;145:181-5
31. Byeon S, Cho JH, Jung HA, Sun JM, Lee SH, Ahn JS. et al. PD-1 inhibitors for non-small cell lung cancer patients with special issues: Real-world evidence. Cancer Medicine. 2020;9(7):2352-2362
32. He MK, Peng C, Zhao Y, Liang RB, Lai ZC, Kan A. et al. Comparison of HBV reactivation between patients with high HBV-DNA and low HBV-DNA loads undergoing PD-1 inhibitor and concurrent antiviral prophylaxis. Cancer immunology, immunotherapy : CII. 2021;70:3207-16
33. Zhong L, Zhong P, Liu H, Li Z, Nie Q, Peng W. Hepatitis B virus infection does not affect the clinical outcome of anti-programmed death receptor-1 therapy in advanced solid malignancies: Real-world evidence from a retrospective study using propensity score matching. Medicine. 2021;100(49):e28113
34. Guo L, Wang D, Ouyang X, Tang N, Chen X, Zhang Y. et al. Recent Advances in HBV Reactivation Research. BioMed research international. 2018;2018:2931402
35. Lee PC, Chao Y, Chen MH, Lan KH, Lee IC, Hou MC. et al. Risk of HBV reactivation in patients with immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Journal for immunotherapy of cancer. 2020;8(2):e001072
36. Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology. 2017;152:1297-309
37. Kudo M. Immune Checkpoint Blockade in Hepatocellular Carcinoma: 2017 Update. Liver cancer. 2016;6:13-5
38. Qin S, Jiang J, Lu Y, Nice EC, Huang C, Zhang J. et al. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduction and Targeted Therapy. 2020;5(1):228
39. Tzeng HT, Tsai HF, Liao HJ, Lin YJ, Chen L, Chen PJ. et al. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PLoS ONE. 2012;7(6):e39179
40. Tsai KN, Kuo CF, Ou JJ. Mechanisms of Hepatitis B Virus Persistence. Trends in microbiology. 2018;26:33-42
41. Isogawa M, Chung J, Murata Y, Kakimi K, Chisari FV. CD40 activation rescues antiviral CD8+ T cells from PD-1-mediated exhaustion. PLoS pathogens. 2013;9(7):e1003490
42. Tian Y, Kuo CF, Akbari O. Maternal-Derived Hepatitis B Virus e Antigen Alters Macrophage Function in Offspring to Drive Viral Persistence after Vertical Transmission. Immunity. 2016;44:1204-14
43. Tian Y, Chen WL, Kuo CF. Viral-load-dependent effects of liver injury and regeneration on hepatitis B virus replication in mice. Journal of virology. 2012;86:9599-605
44. Chen YF, Chong CL, Wu YC, Wang YL, Tsai KN, Kuo TM. et al. Doxorubicin Activates Hepatitis B Virus Replication by Elevation of p21 (Waf1/Cip1) and C/EBPα Expression. PloS one. 2015;10(6):e0131743
45. Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. E antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. The New England journal of medicine. 1976;294:746-9
46. Rehermann B, Ferrari C, Pasquinelli C. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nature medicine. 1996;2:1104-8
47. Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. Journal of hepatology. 2016;64:S60-70
48. Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Frontiers in oncology. 2015;4:385
49. Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nature immunology. 2013;14:1212-8
50. Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends in immunology. 2015;36:265-76
51. Muenst S, Soysal SD, Tzankov A, Hoeller S. The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert opinion on therapeutic targets. 2015;19:201-11
52. Kamphorst AO, Ahmed R. Manipulating the PD-1 pathway to improve immunity. Current opinion in immunology. 2013;25:381-8
53. Kulpa DA, Lawani M, Cooper A, Peretz Y, Ahlers J, Sékaly RP. PD-1 coinhibitory signals: the link between pathogenesis and protection. Seminars in immunology. 2013;25:219-27
54. Cho H, Kang H, Kim CW, Kim HY, Jang JW, Yoon SK. et al. Phenotypic Characteristics of PD-1 and CTLA-4 Expression in Symptomatic Acute Hepatitis A. Gut and liver. 2016;10:288-94
55. Knolle PA, Thimme R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology. 2014;146:1193-207
56. Takeru Asano, Yuriko Kishi, Yusuke Meguri, Takanori Yoshioka, Miki Iwamoto, Yoshinobu Maeda. et al. PD-1 Signaling Has a Critical Role in Maintaining Regulatory T Cell Homeostasis; Implication for Treg Depletion Therapy By PD-1 Blockade. Blood. 2015;126:848-848
57. Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G. et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. The Journal of clinical investigation. 2009;119:551-64
58. Mitsuiki N, Schwab C, Grimbacher B. What did we learn from CTLA-4 insufficiency on the human immune system? Immunological reviews. 2019; 287: 33-49
59. Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Digestive diseases (Basel, Switzerland). 2010;28:737-44
60. Miyake Y, Yamamoto K. Role of gut microbiota in liver diseases. Hepatology research : the official journal of the Japan Society of Hepatology. 2013;43:139-46
61. Botticelli A, Zizzari I, Mazzuca F, Ascierto PA, Putignani L, Marchetti L. et al. Cross-talk between microbiota and immune fitness to steer and control response to anti PD-1/PDL-1 treatment. Oncotarget. 2017;8:8890-9
62. Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (New York, NY). 2015;350:1079-84
63. Deleemans JM, Chleilat F, Reimer RA, Henning JW, Baydoun M, Piedalue KA. et al. The chemo-gut study: investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC cancer. 2019;19(1):1243
64. Nam YD, Kim HJ, Seo JG, Kang SW, Bae JW. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PloS one. 2013;8(12):e82659
65. Maruya M, Kawamoto S, Kato LM. Impaired selection of IgA and intestinal dysbiosis associated with PD-1-deficiency. Gut microbes. 2013;4:165-71
66. Chen J, Wei Y, He J, Cui G, Zhu Y, Lu C. et al. Natural killer T cells play a necessary role in modulating of immune-mediated liver injury by gut microbiota. Scientific reports. 2014;4:7259
67. Xu L, Tu Z, Xu G, Hu JL, Cai XF, Zhan XX. et al. S-phase arrest after vincristine treatment may promote hepatitis B virus replication. World journal of gastroenterology. 2015;21:1498-509
68. Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481:394-8
69. Waggoner SN, Daniels KA, Welsh RM. Therapeutic depletion of natural killer cells controls persistent infection. Journal of virology. 2014;88:1953-60
70. Choi J, Lim YS. Characteristics, Prevention, and Management of Hepatitis B Virus (HBV) Reactivation in HBV-Infected Patients Who Require Immunosuppressive Therapy. The Journal of infectious diseases. 2017;216:S778-84
71. Chen X, Hu Y, Zhang W, Chen K, Hu J, Li X. et al. Cisplatin induces autophagy to enhance hepatitis B virus replication via activation of ROS/JNK and inhibition of the Akt/mTOR pathway. Free radical biology & medicine. 2019;131:225-36
72. Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nature reviews Molecular cell biology. 2018;19:349-64
73. Peng Y, Qiu L, Xu D, Zhang L, Yu H, Ding Y. et al. M4IDP, a zoledronic acid derivative, induces G1 arrest, apoptosis and autophagy in HCT116 colon carcinoma cells via blocking PI3K/Akt/mTOR pathway. Life sciences. 2017;185:63-72
74. Clark CA, Gupta HB, Sareddy G, Pandeswara S, Lao S, Yuan B. et al. Tumor-Intrinsic PD-L1 Signals Regulate Cell Growth, Pathogenesis, and Autophagy in Ovarian Cancer and Melanoma. Cancer research. 2016;76:6964-74
Author contact
![]() Corresponding authors: Yongsheng Wang, MD, PhD, Phone: +86 18980602258, E-mail: wangyscn. Bingwen Zou, MD, PhD, Phone: +86 18980606691, Email: zoubingwen81com.
Corresponding authors: Yongsheng Wang, MD, PhD, Phone: +86 18980602258, E-mail: wangyscn. Bingwen Zou, MD, PhD, Phone: +86 18980606691, Email: zoubingwen81com.

 Global reach, higher impact
Global reach, higher impact