3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(4):1363-1369. doi:10.7150/jca.70385 This issue Cite
Research Paper
A phase II study of chemotherapy in combination with telomerase peptide vaccine (GV1001) as second-line treatment in patients with metastatic colorectal cancer
1. Division of Hematology-Oncology, Department of Internal Medicine, Hallym University Medical Center, Hallym University College of Medicine, Anyang-si, Republic of Korea.
2. Division of Oncology, Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Republic of Korea.
3. Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Republic of Korea.
4. Division of Hematology and Oncology, Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University College of Medicine, Daejeon, Republic of Korea.
5. Department of Internal Medicine, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Republic of Korea.
*These authors contributed equally as first authors.
Abstract
Background: GV1001 is a human telomerase peptide vaccine that induces a CD4/CD8 T-cell response against cancer cells, thereby affording an immunological anti-tumor effect. Here, we evaluated the efficacy and safety of GV1001 in combination with chemotherapy in patients with metastatic colorectal cancer who had failed first-line chemotherapy.
Methods: This multicenter, non-randomized, single-arm phase II study recruited recurrent or metastatic colorectal cancer patients with measurable disease who had failed first-line chemotherapy. Patients received GV1001 and chemotherapy concomitantly based on a pre-established schedule. Cytotoxic chemotherapy and targeted agents (bevacizumab, cetuximab, or aflibercept) were allowed to be used at the discretion of the investigator. The primary endpoint was the disease control rate; secondary endpoints were the objective response rate, progression-free survival, overall survival, and safety outcomes. The baseline serum eotaxin level (a potential predictive biomarker of GV1001) was analyzed. To determine whether an adequate immune response had been induced, a delayed-type hypersensitivity test and a T-cell proliferation test were performed.
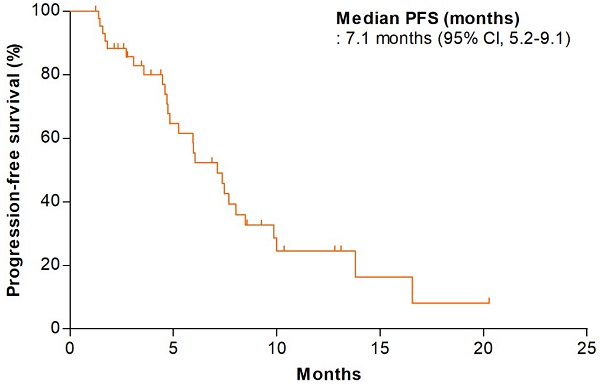
Results: From May 13, 2015 to October 13, 2020, 56 patients with recurrent or metastatic colorectal cancer treated in seven hospitals of South Korea were enrolled. The median patient age was 64 years (range, 29-82 years); 67.9% were men. Of all patients, 66.1% had left-side colorectal cancer and the RAS mutation was present in 25%. The disease control rate and the objective response rates were 90.9% (95% confidence interval [CI]: 82.4-99.4%) and 34.1% (95% CI, 20.1-48.1%), respectively. The median progression-free survival was 7.1 months (95% CI, 5.2-9.1 months) and the median overall survival was 12.8 months (95% CI, 9.9-15.8 months). The most common all-grade adverse events were neutropenia (48.2%), nausea (26.8%), neuropathy (25.0%), stomatitis (21.4%), and diarrhea (21.4%). Immune response analysis showed that no patient had positive delayed-type hypersensitivity test results; antigen-specific T-cell proliferation was observed in only 28% of patients. The baseline eotaxin level was not associated with any efficacy outcome.
Conclusion: Although no clear GV1001-specific immune response was observed, the addition of GV1001 vaccination to chemotherapy was tolerable and associated with modest efficacy outcomes.
Keywords: Colorectal neoplasm, cancer vaccines, GV1001

 Global reach, higher impact
Global reach, higher impact