Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(2):602-609. doi:10.7150/jca.64335 This issue Cite
Research Paper
Upfront brain radiotherapy improves intracranial progression-free survival but not overall survival in lung adenocarcinoma patients with brain metastases: a retrospective, single-institutional analysis from China
1. Department of Radiation Oncology, Shandong Cancer Hospital, Shandong University, Jinan, 250117, Shandong, China.
2. Department of Radiation Oncology, Qingdao University Medical College Affiliated Yantai Yuhuangding Hospital, Yantai, 264000, Shandong, China.
3. Department of Radiation Oncology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, 221002, Jiangsu, China.
Received 2021-6-28; Accepted 2021-12-2; Published 2022-1-1
Abstract
Aims: The optimal timing of brain radiotherapy (BRT) for lung adenocarcinoma patients with brain metastases (BM) remains controversial. In this retrospective study, we performed a retrospective review to investigate the differential benefit of upfront versus deferred BRT for lung adenocarcinoma patients with BM.
Methods: A total of 354 lung adenocarcinoma patients with BM treated in the Affiliated Cancer Hospital of Shandong University met the inclusion criteria for the study. Patients were divided into two groups: upfront BRT and deferred BRT. Intracranial progression-free survival (PFS) and overall survival (OS) were measured from the date of brain metastases. Subgroup analyses according to gene mutation status were also performed.
Results: Among the entire cohort, the median intracranial PFS with upfront BRT (16.3 months) was longer than that with deferred BRT (11.3 months, p=0.001). However, the median OS did not differ significantly between patients who received upfront BRT and deferred BRT (27.6 and 31.5 months, respectively, p=0.813). Subgroup analyses indicated that upfront BRT yielded a significantly longer intracranial PFS than deferred BRT (p=0.003) for patients without EGFR (19 or 21) mutation. In both subgroups, the median OS showed no significant difference between upfront BRT and deferred BRT.
Conclusion: This single-institutional retrospective study showed that in lung adenocarcinoma patients with brain metastases, upfront BRT was associated with a significantly longer intracranial PFS but not improvement in OS compared with deferred BRT. Considering the neurocognitive toxicities of BRT previously reported in the literature, deferred BRT might be considered as an acceptable therapeutic option for the treatment of patients with lung adenocarcinoma and BM.
Keywords: brain metastases, lung adenocarcinoma, epidermal growth factor receptor, tyrosine kinase inhibitor, brain radiotherapy
Introduction
Non-small cell lung cancer (NSCLC) accounts for about 87% of all lung cancer cases [1] and 20-40% of NSCLC patients will develop brain metastases (BM) [2, 3]. The proportion of adenocarcinoma among NSCLC cases has continued to increase in recent years [1]. In Asians, ~50% of lung adenocarcinomas harbor epidermal growth factor receptor (EGFR) mutations [4]. EGFR mutation status is highly concordant between primary NSCLC and corresponding BM [5]. Chemotherapy for such BM is not an effective treatment because of the existence of the blood-brain-barrier (BBB) [6]. For decades whole brain radiotherapy (WBRT) has been considered the standard care for patients with BM, which is associated with an overall response rate of 60%, a 6-month disease control rate of 50% and a median overall survival (OS) of 4-6 months [7-9].
During the past decade, the advancement of EGFR tyrosine kinase inhibitors (EGFR-TKIs) has revolutionarily transformed the landscape of treatment and prognosis of advanced lung adenocarcinoma patients [10]. EGFR-TKIs were demonstrated to be safe and significantly efficacious in EGFR mutated NSCLC patients with BM, leading to a median progression-free survival of 14.5 months, a median OS of 21.9 months [11] and intracranial disease response rates of 75-88% [12-14]. Among patients with previously untreated advanced NSCLC with EGFR mutation, osimertinib yielded a longer OS of 38.6 months than a comparator EGFR-TKI (31.8 months) [15]. EGFR TKIs are recommended as first line systemic therapy for patients with metastatic NSCLC harboring EGFR activating mutations [16, 17].
Interestingly, a recent study demonstrated that patients with EGFR-mutated NSCLC are more sensitive to RT [18]. It also has been reported that combination treatment with brain radiotherapy (BRT) and EGFR-TKIs presents superior response rate and disease control rate compared with BRT or TKI alone [19, 20]. However, the retrospective study by Magnuson et al [21] demonstrated inferior OS with deferral of BRT. Another study reported that upfront BRT followed by TKI therapy may be an appropriate initial management approach for EGFR-mutant NSCLC patients with BM [22].
However, in contrast to the conclusions of Magnuson et al, a retrospective analysis demonstrated that the addition of WBRT to EGFR-TKIs, compared with TKIs alone, did not improve the intracranial progression-free survival (PFS) and led to a worse OS in NSCLC with EGFR mutation and BM [23]. A study in China also suggested that upfront WBRT can be safely delayed in EGFR-mutant lung cancer with BM [24]. In the European Society of Medical Oncology guidelines for metastatic NSCLC, delay of upfront WBRT is considered in NSCLC patients with minimal and asymptomatic BM.
Thus, there is no consensus on the management of patients with lung adenocarcinoma and BM, and specifically, the optimal timing of BRT has yet to be determined. In the present retrospective study, we investigated the differential benefit of upfront versus deferred BRT for lung adenocarcinoma patients with BM. We also performed subgroup analyses according to gene mutation status.
Materials and methods
Study design and patient selection
This study was conducted in compliance with the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Cancer Hospital of Shandong University (SDTHEC: 201611004). Through a review of hospital records, we identified 776 lung adenocarcinoma patients with BM treated at the Affiliated Cancer Hospital of Shandong University between June 1, 2013 and October 31, 2016. The eligibility criteria for this study were: (1) lung adenocarcinoma with BM, and (2) treatment with linear accelerator-based BRT. The exclusion criteria were: (1) upfront use of a TKI before BM diagnosis; (2) failure to complete the BRT plan; (3) meningeal metastases; (4) surgical resection or stereotactic radiosurgery (SRS); (5) presence of other primary tumors; (6) history of BRT; and (7) < 3 months of loss to follow-up. The included patients were divided into two groups: upfront BRT and deferred BRT. Upfront BRT was defined as BRT within 2 months after diagnosis of BM. Tumor response was assessed every 2-3 months according to response evaluation criteria in solid tumors (RECIST) 1.1 [25] by contrast-enhanced magnetic resonance imaging (MRI) or computed tomography (CT).
The last date of follow-up was March 31, 2018. The following variables were collected at BM diagnosis: (1) age; (2) gender; (3) smoking status; (4) Karnofsky performance status (KPS) ; (5) histology; (6) gene mutation status; (7) extracranial metastases; (8) size of largest BM; (9) number of BMs; (10) symptoms of BMs; (11) radiotherapy mode; and (12) treatments. A disease-specific Graded Prognostic Assessment (DS-GPA) was calculated. The radiotherapy modes were divided into three groups: WBRT, LBRT (local brain radiotherapy) and WBRT + LBRT. The gross tumor area radiotherapy was named as LBRT. The equivalent dose in 2 Gy/fraction (EQD2) of WBRT was calculated to be 32.5-50 Gy based on the LQ model. The accumulated LBRT dose was 50-60Gy with 2-4Gy per fraction and one fraction per day. When subgroup analyses, patients with ALK, ROS-1, or c-MET mutation were excluded. The dates of initial cancer diagnosis, BM diagnosis, BRT, systemic treatments, intracranial progression, most recent follow-up, and death were recorded. Intracranial PFS was defined as the time from BM diagnosis to intracranial progression, death, or last follow-up. OS was defined as the time from BM diagnosis to death or last follow-up.
Statistical analysis
Statistical comparisons between two groups were performed in SPSS, version 21.0 (SPSS Inc., Chicago, IL, USA). Comparisons of clinical characteristics between the upfront and deferred BRT groups were performed using the chi-square test. Kaplan-Meier analysis was used to estimate intracranial PFS and OS and generate surviva1 curves, and log-rank testing was used to assess differences between the groups. Multivariable Cox proportional hazards analysis were performed according to backward stepwise regression. Differences were considered to be statistically significant if p<0.05.
Results
Patient characteristics
After application of the inclusion and exclusion criteria, a total of 354 patients were identified for survival analyses (273 who received upfront BRT and 81 who received deferred BRT). The patients' demographic and clinical characteristics are summarized in Table 1. The median follow-up for all patients was 23.0 months (range, 0.6-113.6 months). The median age at BM diagnosis for all patients was 57 years (range, 27-78 years). The median ages at BM diagnosis for the upfront BRT and deferred BRT groups were 57 and 55 years, respectively. Additionally, 188 (53%) patients were male, and 89 patients (25%) were smokers. In total, 91 patients (25.7%) displayed EGFR (19 or 21) mutation, 5 patients (1.4%) displayed ALK+ mutation, 77 patients (21.8%) displayed no EGFR (19 or 21) or ALK gene mutation, and 181 patients (51.1%) did not undergo genetic testing. Among the patients with a positive gene mutation, 92.7% patients (89/96) received targeted therapy. However, 42.0% patients (76/181) with unknown gene mutation status and 35.1% patients (27/77) without EGFR gene mutation also received targeted therapy.
Among all 354 patients, 168 patients (47.5%) received WBRT, 54 (15.3%) received LBRT, and 132 (37.3%) received both WBRT and LBRT. The median maximum diameter of the BMs was 15.5 mm. Overall, 52.5% of patients (186/354) had BM at primary diagnosis. Patients who received upfront BRT were more likely to have symptomatic BM (64.1% vs. 37%; p<0.001) and were more likely to have BMs >1 cm (72.9% vs. 44.4%; p<0.001). Patients who received upfront BRT were less likely to have extracranial metastases (56% vs. 72.8%; p=0.007) and more likely to receive LBRT and WBRT (41.8% vs. 22.2%; p=0.006). No differences were observed between the two groups with respect to age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, smoking status, number of BMs, gene mutation, or DS-GPA at the time of BM diagnosis.
Survival outcomes for the entire cohort
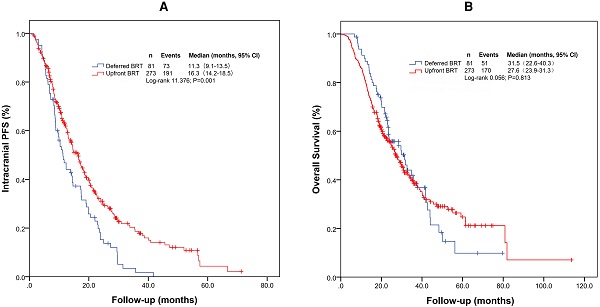
For the entire cohort, the median intracranial PFS and OS were 14.6 months (95% confidence interval [CI]: 12.4-16.7 months) and 28.2 months (95% CI: 24.7-31.7 months), respectively. The median intracranial PFS durations with upfront BRT and deferred BRT were 16.3 months (95% CI: 14.2-18.5 months) and 11.3 months (95% CI: 9.1-13.5 months), respectively (Figure 1A and 1B). The median intracranial PFS with upfront BRT was significantly longer than that with deferred BRT (log-rank p=0.001; Table 2). The impact of upfront BRT on intracranial PFS remained significant on multivariable analysis (p=0.003, hazard ratio [HR]=0.662[0.503-0.871]). Interestingly, the median OS with upfront BRT (27.6 months, 95% CI: 23.9-31.3 months) appeared to be shorter than that with deferred BRT (31.5 months, 95% CI: 22.6-40.3 months), but the difference was not significant (log rank p=0.813; Table 3).
Characteristics of 354 lung adenocarcinoma patients with BM.
| Upfront BRT(n=273) | Deferred BRT(n=81) | |||||
|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | χ2 | p |
| Age | 0.133 | 0.716 | ||||
| ≤60 years | 176 | 64.5 | 54 | 66.7 | ||
| >60 years | 97 | 35.5 | 27 | 33.3 | ||
| Gender | 2.327 | 0.127 | ||||
| Male | 151 | 55.3 | 37 | 45.7 | ||
| Female | 122 | 44.7 | 44 | 54.3 | ||
| Smoking status | 0.295 | 0.587 | ||||
| Current/former | 90 | 33.0 | 24 | 29.6 | ||
| Never | 183 | 67.0 | 57 | 70.4 | ||
| Symptomatic BM | 18.773 | 0.000 | ||||
| Yes | 175 | 64.1 | 30 | 37.0 | ||
| No | 98 | 35.9 | 51 | 63.0 | ||
| No. of BM | 0.756 | 0.385 | ||||
| 1-3 | 164 | 60.1 | 53 | 65.4 | ||
| >3 | 109 | 39.9 | 28 | 34.6 | ||
| Size of largest BM | 22.656 | 0.000 | ||||
| ≤1cm | 74 | 27.1 | 45 | 55.6 | ||
| >1cm | 199 | 72.9 | 36 | 44.4 | ||
| Gene mutation | 2.673 | 0.263 | ||||
| Positive | 71 | 26.0 | 25 | 30.9 | ||
| Negative | 56 | 20.5 | 21 | 25.9 | ||
| Unknown | 146 | 53.5 | 35 | 43.2 | ||
| Extracranial metastases | ||||||
| Yes | 153 | 56.0 | 59 | 72.8 | 7.335 | 0.007 |
| No | 120 | 44.0 | 22 | 27.2 | ||
| DS-GPA | 0.123 | 0.726 | ||||
| 0-2 | 166 | 60.8 | 51 | 63.0 | ||
| 2.5-4 | 107 | 39.2 | 30 | 37.0 | ||
| BRT mode | 10.210 | 0.006 | ||||
| WBRT | 120 | 44.0 | 48 | 59.3 | ||
| LBRT | 39 | 14.2 | 15 | 18.5 | ||
| WBRT + LBRT | 114 | 41.8 | 18 | 22.2 | ||
Abbreviations: BM: brain metastases; DS-GPA: Diagnosis-Specific Graded Prognostic Assessment; BRT: brain radiotherapy; WBRT: whole brain radiotherapy; LBRT: local brain radiotherapy.
Kaplan-Meier survival curves of intracranial PFS (A) and OS (B) for the entire cohort. Abbreviations: BRT: brain radiotherapy; PFS: progression-free survival; OS: overall survival.
Univariable and multivariable analyses of covariables associated with intracranial PFS in lung adenocarcinoma patients with BM.
| Univariable | Mutivariable | ||||
|---|---|---|---|---|---|
| Variable | χ2 | p | HR | 95% CI | p |
| Upfront BRT vs deferred BRT | 11.376 | 0.001 | 0.662 | 0.503-0.871 | 0.003 |
| Age (≤60 vs >60 years) | 0.865 | 0.352 | |||
| Gender (male vs female) | 2.244 | 0.134 | |||
| Smoking status (never vs current/former) | 1.313 | 0.252 | |||
| Symptom (yes vs no) | 1.475 | 0.225 | |||
| No. of BM (1-3 vs >3) | 1.435 | 0.231 | |||
| Size of largest BM (≤1 vs >1cm) | 0.229 | 0.632 | |||
| Gene mutation | |||||
| Positive vs negative | 4.051 | 0.044 | |||
| Positive vs unknown | 0.003 | 0.958 | |||
| Negative vs unknown | 4.018 | 0.045 | |||
| Extracranial metastases (yes vs no) | 12.466 | 0.000 | |||
| DS-GPA (2.5-4 vs 0-2) | 13.380 | 0.000 | 0.659 | 0.510-0.851 | 0.001 |
| BRT mode | |||||
| LBRT vs WBRT | 0.224 | 0.636 | |||
| LBRT + WBRT vs WBRT | 3.885 | 0.049 | |||
| LBRT + WBRT vs LBRT | 1.070 | 0.301 | |||
Abbreviations: BM: brain metastases; DS-GPA: Diagnosis-Specific Graded Prognostic Assessment; BRT: brain radiotherapy; WBRT: whole brain radiotherapy; LBRT: local brain radiotherapy.
Subgroup analyses of patients with EGFR (19 or 21) mutation
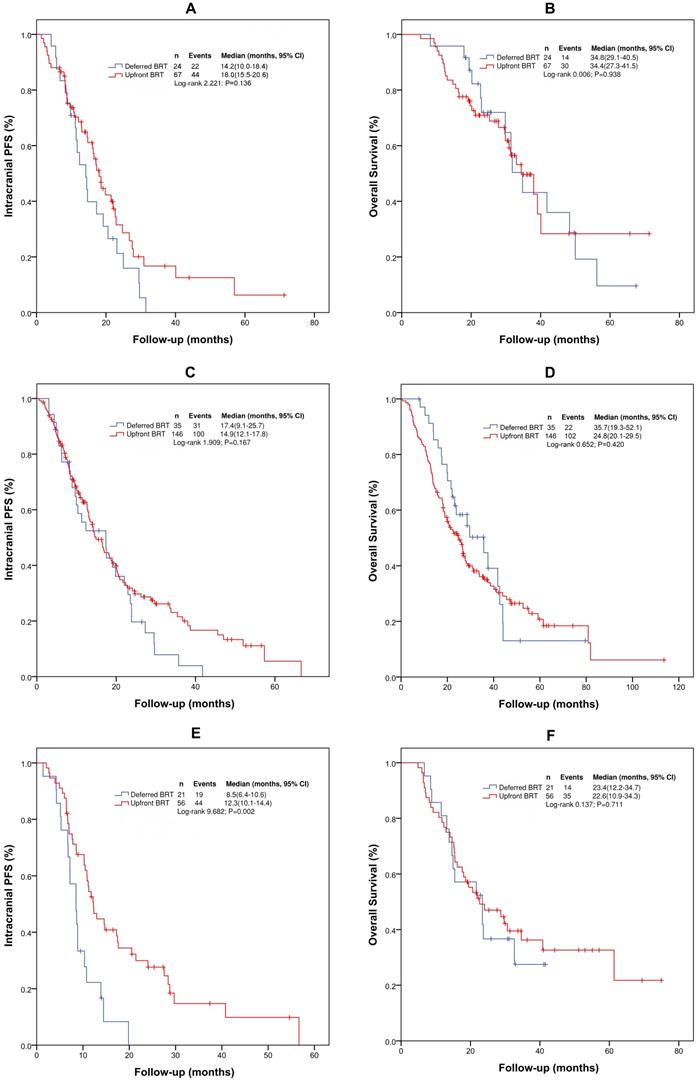
According to the gene mutation status, we performed further analyses by dividing the patients into three groups: EGFR (19 or 21) mutation group (positive), no EGFR (19 or 21) mutation group (negative), and no genetic testing group (unknown). Patients with ALK (5 patients) were excluded. For patients with EGFR (19 or 21) mutation, the median intracranial PFS and OS were 17.1 months (95% CI: 13.7-20.5 months) and 34.4 months (95% CI: 28.0-40.9 months), respectively. With upfront BRT and deferred BRT, the median intracranial PFS and OS were 18.0 vs 14.2 months (χ2= 2.222, p=0.136) and 34.4 vs 34.8 months (χ2=0.006, p=0.938), respectively. Univariate analysis indicated that upfront BRT and WBRT+LBRT were associated with intracranial PFS, but the differences were not significant. Upfront BRT was not significantly associated with OS according to the univariate and multivariate analyses (Supplementary Table 4 and 5).
Univariable and multivariable analyses of covariables associated with OS in lung adenocarcinoma patients with BM.
| Univariable | Mutivariable | ||||
|---|---|---|---|---|---|
| Variable | χ2 | p | HR | 95% CI | p |
| upfront BRT vs deferred BRT | 0.056 | 0.813 | |||
| Age (≤60 vs >60 years) | 0.027 | 0.869 | |||
| Gender (male vs female) | 4.259 | 0.039 | |||
| Smoking status (never vs current/former) | 4.517 | 0.034 | 0.651 | 0.489-0.865 | 0.003 |
| Symptom (yes vs no) | 0.532 | 0.466 | |||
| No. of BM (1-3 vs >3) | 7.141 | 0.008 | 0.678 | 0.515-0.892 | 0.006 |
| Size of largest BM (≤1 vs >1cm) | 3.318 | 0.069 | 0.730 | 0.546-0.976 | 0.034 |
| Gene mutation | |||||
| Positive vs negative | 3.869 | 0.049 | 0.660 | 0.442-0.985 | 0.042 |
| Positive vs unknown | 4.300 | 0.038 | 0.637 | 0.455-0.893 | 0.009 |
| Negative vs unknown | 0.012 | 0.914 | |||
| Extracranial metastases (yes vs no) | 14.194 | 0.000 | 1.749 | 1.314-2.330 | 0.000 |
| DS-GPA (2.5-4 vs 0-2) | 9.740 | 0.002 | |||
| BRT mode | |||||
| LBRT vs WBRT | 0.679 | 0.410 | |||
| LBRT + WBRT vs WBRT | 3.198 | 0.074 | |||
| LBRT + WBRT vs LBRT | 0.185 | 0.667 | |||
Abbreviations: BM: brain metastases; DS-GPA: Diagnosis-Specific Graded Prognostic Assessment; BRT: brain radiotherapy; WBRT: whole brain radiotherapy; LBRT: local brain radiotherapy.
Kaplan-Meier survival curves for intracranial PFS and OS in patient subgroups. (A) Intracranial PFS for patients with EGFR (19 or 21) mutation; (B) OS for patients with EGFR (19 or 21) mutation; (C) Intracranial PFS for patients without genetic testing results; (D) OS for patients without genetic testing results; (E) Intracranial PFS for patients without EGFR (19 or 21) mutation; (F) OS for patients without EGFR (19 or 21) mutation. Abbreviations: BRT: brain radiotherapy; PFS: progression-free survival; OS: overall survival.
Subgroup analyses for patients without EGFR (19 or 21) mutation
For patients without EGFR (19 or 21) mutation, the median intracranial PFS and OS were 10.8 (95% CI: 9.3-12.4) and 23.4 months (95% CI: 19.0-27.9), respectively. Upfront BRT in these patients provided a longer median intracranial PFS than deferred BRT (12.3 vs 8.5 months, χ2=9.682, p=0.002). However, no difference in the median OS (23.4 vs. 23.4 months, χ2=0.137, p=0.711) was observed between the patients who received upfront BRT and deferred BRT. On multivariate analysis, only upfront BRT yielded a longer intracranial PFS than deferred BRT (p=0.003, HR=0.412[0.231-0.733]). However, no correlation between upfront BRT and OS was found on univariate and multivariate analyses. Multivariate analysis identified only the number of BMs as significantly associated with OS (p=0.051, HR=0.570[0.324-1.002]) (Supplementary Table 6 and 7).
Discussion
Among NSCLC subtypes, adenocarcinoma is more prone to BM, and the prognosis and treatment strategy for lung adenocarcinoma patients are different from those of patients with other lung cancer types, especially for patients with EGFR gene mutations. The upfront BRT strategy has advantages and disadvantages. Although previous studies indicated that upfront BRT may be associated with better local control and longer OS, use of TKIs alone initially avoids the side effects of BRT. Two small randomized studies reported that the timing of WBRT did not influence OS in unselected NSCLC patients with BM receiving chemotherapy [26, 27]. Upfront SRS did not improve OS in oligo-brain metastases NSCLC patients compared with upfront chemotherapy [28]. The QUARTZ study [29] indicated that WBRT provided little additional clinically significant benefit for BM patients with a KPS of <70. Use of an EGFR-TKI alone for BM in EGFR-mutant lung cancer patients showed outcomes comparable to those who received upfront RT followed by EGFR-TKI therapy [30]. The BRAIN study also suggested that upfront BRT can be safely delayed in EGFR-mutant lung cancer patients with BM [24]. Thus, to date, the value and timing of BRT for patients with BM from lung adenocarcinoma have remained controversial, especially for EGFR-mutant patients.
To the best of our knowledge, this study was the first retrospective analysis from China to compare survival outcomes with upfront and deferred BRT in patients with BM from lung adenocarcinoma. The targeted population in this study was lung adenocarcinoma patients with BM regardless of number of BMs, which was different from the population of the BRAIN study [24]. Among the entire cohort, the median intracranial PFS was longer in patients who received upfront BRT than in those who received deferred BRT. However, in our subgroup analysis according to gene mutation status, intracranial PFS no longer significantly differed with upfront versus deferred BRT for patients with EGFR (19 or 21) mutation. Additionally, no significant differences in OS were observed between upfront and deferred BRT in both subgroups. Therefore, upfront BRT may offer an intracranial PFS benefit in patients with BM from lung adenocarcinoma, especially in patients without EGFR (19 or 21) mutation, but cannot provide an additional OS benefit.
Several qualitative reviews have suggested that TKI therapy may be used first before BRT in EGFR+ NSCLC patients with asymptomatic BM [31-34]. However, Magnuson et al [21] reported that the use of upfront EGFR-TKI and deferral of RT was associated with inferior OS in patients with EGFR-mutant NSCLC who developed BM. For EGFR-mutant BM patients, our subgroup results showed that upfront BRT may provide a longer intracranial PFS compared to deferred BRT, but the difference was not significant. On the other hand, upfront BRT was not significantly associated with OS according to the univariate and multivariate analysis.
In the present study, upfront BRT was defined as BRT within 2 months after diagnosis of BM, which was not consistent with the definition used in several previous studies [21, 23, 30]. Although SRS is recommended by guidelines as a standard radiotherapy method for patients with 1-3 BMs, it is not available for most RT units in China. Therefore, patients treated with SRS were excluded from the present study. Due to the lack of randomization, the presence of bias was inevitable. In upfront BRT group, patients were more likely to have symptomatic BM, BMs >1 cm and no extracranial metastases. Patients who received deferred BRT were less likely to receive WBRT + LBRT. All of these may affect the intracranial PFS and OS of the patients. Furthermore, the data from the subgroup analysis comparing the two treatment groups were insufficient due to the small number of patients treated with deferred BRT. The quality of evidence supporting these findings is low. In addition, the adverse events, quality of life and cognitive function could not be assessed due to the lack of clinical data. Moreover, the patients involved in the present study were all Chinese; hence, further investigations in patients of other races are required.
Conclusions
In summary, this single-institutional, retrospective study showed that in patients with lung adenocarcinoma and BM, upfront BRT was associated with a significantly longer intracranial PFS, especially in patients without EGFR (19 or 21) mutation. No difference in OS was observed between patients treated with upfront BRT versus deferred BRT. Considering the neurocognitive toxicities previously reported with upfront BRT, deferred BRT might be considered as an acceptable therapeutic option for the treatment of patients with lung adenocarcinoma and BMs.
Supplementary Material
Supplementary tables 4-13 (numbering continued from tables within the paper).
Acknowledgements
This study was partially funded by Natural Science Foundation of China (NSFC 81872475 and NSFC 82073345).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lee DS, Kim YS, Kay CS. et al. Distinctive Patterns of Initially Presenting Metastases and Clinical Outcomes According to the Histological Subtypes in Stage IV Non-Small Cell Lung Cancer. Medicine (Baltimore). 2016;95(6):e2795
2. Zhao J, Chen M, Zhong W. et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer. 2013;14:188-93
3. Brower JV, Robins HI. Erlotinib for the treatment of brain metastases in non-small cell lung cancer. Expert Opin Pharmacother. 2016;17(7):1013-21
4. Suda K, Mitsudomi T, Shintani Y. et al. Clinical impacts of EGFR mutation status: analysis of 5,780 surgically resected lung cancer cases. Ann Thorac Surg. 2020;29(6):S0003-4975 (20)30982-6
5. Luo D, Ye X, Hu Z. et al. EGFR mutation status and its impact on survival of Chinese non-small cell lung cancer patients with brain metastases. Tumour Biol. 2014;35(3):2437-44
6. Dagogo-Jack I, Gill CM, Cahill DP. et al. Treatment of brain metastases in the modern genomic era. Pharmacol Ther. 2017;170:64-72
7. Arrieta O, Villarreal-Garza C, Zamora J. et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol. 2011;6:166
8. Gaspar LE, Mehta MP, Patchell RA. et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. Journa1 of neuro-oncology. 2010;96(1):1732
9. Khuntia D, Brown P, Li J. et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295-304
10. Tang N, Guo J, Zhang Q. et al. Greater efficacy of chemotherapy plus bevacizumab compared to chemo- and targeted therapy alone on non-small cell lung cancer patients with brain metastasis. Oncotarget. 2015;7:3635-44
11. Iuchi T, Shingyoji M, Sakaida T. et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;82:282-7
12. Park SJ, Kim HT, Lee DH. et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77(3):556-60
13. Wu YL, Zhou C, Cheng Y. et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol. 2013;24(4):993-9
14. Mamon HJ, Yeap BY, Jänne PA. et al. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol. 2005;23(7):1530-7
15. Ramalingam SS, Vansteenkiste J, Planchard D. et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382(1):41-50
16. Planchard D, Popat S, Kerr K. et al. Correction to: "Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Ann Oncol. 2019;30(5):863-70
17. Tsakonas G, De Petris L, Ekman S. Management of brain metastasized non-small cell lung cancer (NSCLC) - From local treatment to new systemic therapies. Cancer Treat Rev. 2017;54:122-31
18. Johung KL, Yao X, Li F. et al. A clinical model for identifying radiosensitive tumor genotypes in non-small cell lung cancer. Clin Cancer Res. 2013;19:5523-32
19. Jiang T, Min W, Li Y. et al. Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: an update meta-analysis. Cancer Med. 2016;5:1055-65
20. Chen CH, Lee HH, Chuang HY. et al. Combination of Whole-Brain Radiotherapy with Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors Improves Overall Survival in EGFR-Mutated Non-Small Cell Lung Cancer Patients with Brain Metastases. Cancers (Basel). 2019;11(8):1092
21. Magnuson WJ, Lester-Coll NH. et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol. 2017;35(10):1070-7
22. Saruwatari K, Ikeda T, Saeki S. et al. Upfront Cranial Radiotherapy Followed by Erlotinib Positively Affects Clinical Outcomes of Epidermal Growth Factor Receptor-mutant Non-small Cell Lung Cancer With Brain Metastases. Anticancer Res. 2019;39(2):923-31
23. Jiang T, Su C, Li X. et al. EGFR TKIs plus WBRT Demonstrated No Survival Benefit Other Than That of TKIs Alone in Patients with NSCLC and EGFR Mutation and Brain Metastases. J Thorac Oncol. 2016;11(10):1718-28
24. Yang JJ, Zhou C, Huang Y. et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. 2017;5(9):707-16
25. Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-47
26. Robinet G, Thomas P, Breton JL. et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small cell lung cancer: Groupe Francais de Pneumo-Cancerologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12:59-67
27. Lee DH, Han JY, Kim HT. et al. Primary chemotherapy for newly diagnosed non-small cell lung cancer patients with synchronous brain metastases compared with whole-brain radiotherapy administered first. Cancer. 2008;113:143-9
28. Lim SH, Lee JY, Lee MY. et al. A randomized phase III trial of stereotactic radiosurgery (SRS) versus observation for patients with asymptomatic cerebral oligo-metastases in non-small-cell lung cancer. Ann Oncol. 2015;26(4):762-8
29. Mulvenna P, Nankivell M, Barton R. et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004-14
30. Hyun DG, Choi CM, Lee DH. et al. Outcomes according to initial and subsequent therapies following intracranial progression in patients with EGFR-mutant lung cancer and brain metastasis. PLoS One. 2020;15(4):e0231546
31. Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 2012;18(4):938-44
32. Bartolotti M, Franceschi E, Brandes AA. EGF receptor tyrosine kinase inhibitors in the treatment of brain metastases from non-small-cell lung cancer. Expert Rev Anticancer Ther. 2012;12(11):1429-35
33. Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014;40(6):716-22
34. Soon YY, Leong CN, Koh WY. et al. EGFR tyrosine kinase inhibitors versus cranial radiation therapy for EGFR mutant non-small cell lung cancer with brain metastases: a systematic review and meta-analysis. Radiother Oncol. 2015;114(2):167-72
Author contact
![]() Corresponding authors: Longzhen Zhang, M.D., Ph.D. Department of Radiation Oncology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, 221002, Jiangsu, China. Tel/Fax: +86-516-85803309. Email: jsxzzlzcom. Shuanghu Yuan, M.D., Ph.D. Department of Radiation Oncology, Shandong Cancer Hospital Affiliated to Shandong University, 440 Jiyan Road, Jinan 250117, Shandong, China. Tel/Fax: +86-531-67626931. Email: officeorg.
Corresponding authors: Longzhen Zhang, M.D., Ph.D. Department of Radiation Oncology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, 221002, Jiangsu, China. Tel/Fax: +86-516-85803309. Email: jsxzzlzcom. Shuanghu Yuan, M.D., Ph.D. Department of Radiation Oncology, Shandong Cancer Hospital Affiliated to Shandong University, 440 Jiyan Road, Jinan 250117, Shandong, China. Tel/Fax: +86-531-67626931. Email: officeorg.

 Global reach, higher impact
Global reach, higher impact