3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(2):426-435. doi:10.7150/jca.65315 This issue Cite
Research Paper
Optimize the number of cycles of induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma: a propensity score matching analysis
1. Department of Radiation Oncology, Guangxi Medical University Cancer Hospital, Nanning, China.
2. Department of Oncology, Affiliated Wuming Hospital of Guangxi Medical University, Nanning, China.
* Yu-Ting Jiang and Kai-Hua Chen contributed equally to this work
Received 2021-7-24; Accepted 2021-11-23; Published 2022-1-1
Abstract
Background: There is no conclusive on the optimal number of cycles of induction chemotherapy (IC) with the greatest benefit to patient survival. This study aimed to assess the efficiency and acute toxicities of different cycles of IC for patients with locoregionally advanced nasopharyngeal carcinoma (LA-NPC).
Methods: We reviewed data from patients with LA-NPC treated with IC plus concurrent chemoradiation (CCRT). Propensity score matching (PSM) was applied to match paired patients. After PSM, survival outcomes of matched patients were compared between two and three cycles of IC groups. Univariate and multivariate Cox regression analysis were carried out to identify potentially independent predictors. Treatment-related acute toxicities between the two groups were compared by Pearson X2 test or Fisher's exact test.
Results: In total, 189 pairs were selected. The median follow-up time was 60 months (range 5 to 126 months). There was no difference between two and three cycles of IC in terms of 5-year overall survival (87.0% vs. 89.7%, p = 0.991), distant metastasis-free survival (90.1% vs. 86.8%, p = 0.587), locoregional recurrence-free survival (97.0% vs. 93.8%, p = 0.488), or progression-free survival (79.4% vs. 79.3%, p = 0.896). Multivariate Cox analysis showed that T stage, N stage, and clinical stage were independent prognostic factors. Three cycles of IC were associated with a higher incidence of Grade 1-2 acute toxicity than two cycles during IC period.
Conclusion: The efficacy of two cycles of IC achieved similar survival outcomes as three cycles and has a lower incidence of treatment-related acute toxicity.
Keywords: Nasopharyngeal carcinoma, Induction chemotherapy, Cycle number, Survival
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant tumor with unbalanced geographic distribution, and It is often diagnosed in Southern China, Southeast Asia and North Africa [1]. Unlike other head and neck cancers, radiation therapy (RT) is the mainstay of treatment for non-metastatic NPC given its deep-seated anatomical location and high sensitivity to radiation. Among them, more than 70% of patients are classified as locoregionally advanced disease (LA-NPC) and concurrent chemoradiation (CCRT) has been recommended as the standard treatment for them [2-4]. The addition of adjuvant chemotherapy (AC) or induction chemotherapy (IC) to CCRT may decrease the risk of distant metastasis and local recurrence in patients, which contribute to a survival benefit. However, AC often has low compliance for three cycles (about 60%) due to a high incidence of adverse events [5]. Compared with AC, the addition of IC before radiotherapy is better tolerated and offers advantages for shrinkage of tumor volume and early eradication of micrometastases [5, 6]. In the past decade, IC has gained extensive research. Previous studies [6-10] have reported that LA-NPC patients benefited from two, three, and even four cycles of IC. Thus, the latest National Comprehensive Cancer Network (NCCN) Guidelines recommends IC plus CCRT as one of the most appropriate treatments for LA-NPC patients (category IA), which is superior to CCRT (category IIA) and CCRT+AC (category IB).
However, the survival improvement of this aggressive treatment is often accompanied by more toxicities, which may have negative influence on patients' tolerance to subsequent CCRT. Some studies have reported that as compared to CCRT alone, the addition of IC to CCRT increase the incidence of Grade 3 or 4 side events [6, 11]. As IC plus CCRT has been recommended as an effective treatment modality, more detailed and effective IC decisions (number of cycles, regimen, dose, etc.) with less adverse events are needed. Although most randomized controlled trials use three cycles of IC, about 3%-25% of patients do not successfully complete three cycles due to toxicity and treatment costs [6, 12-14]. There is no conclusive on the number of IC cycles with the greatest benefit to patient survival. Previous studies have shown that additional cycles of IC after two failed to increase survival rate for patients with LA-NPC compared with two cycles of IC, but increase a higher incidence of treatment-related toxicity [15-17]. It is important to identify optimal number of IC cycles in daily clinical practice. The current study aimed to explore the optimal number of cycles of IC in LA-NPC patients receiving IC and CCRT.
Materials and methods
Patients
From January 2010 to June 2018, a total of 655 patients in our hospital were enrolled in this retrospective study. The inclusion criteria of this study included: (1) newly histologically proved stage III-IVa NPC (restage based on 8th edition of the AJCC/UICC staging system); (2) treated with two or three cycles of IC plus CCRT; (3) no history of anti-tumor treatment before our study; (4) available clinical data, examination information and follow-up data; (5) no serious diseases or secondary malignancy when diagnosed as NPC. Of 655 patients, 189 pairs (57.7%) were matched for the present study. This retrospective study was approved by the Medical Ethics Committee of our hospital. The need to obtain informed consent was not required as this was a retrospective study.
Quantification of plasma EBV DNA level
The detailed measurement of EBV DNA level was reported by our previous study [18], The cutoff value for pretreatment EBV DNA level (pre-EBV DNA) was set at 5000 copies/mL, which was calculated by receiver operator characteristic (ROC) curve analysis.
Treatment
All patients received treatment based on the institutional guidelines' recommendation. The regimens of IC consisted of TPF, TP, PF, and GP regime, which were administered every three weeks for 2 to 4 cycles before CCRT. Concurrent chemotherapy was cisplatin regimen (80-100 mg/m2) for 1 to 3 cycles. Treatment-related acute toxicities were classified by the Common Toxicity Criteria for Adverse Events version 4.0 (CTCAE 4.0).
All patients underwent radical IMRT. Detailed chemotherapy and radiotherapy treatment information is in accordance with the principles of our previous studies and shown in Supplementary Materials [18, 19].
Follow-up
Patients received outpatient examination or telephone to conduct follow-up after treatment. All patients were regularly screened with physical examination, nasopharyngoscopy, and imaging every three months in first two years after RT, six months in the next three years, and annually thereafter until death. The overall survival (OS) was the main endpoint. The secondary endpoints included distant metastasis-free survival (DMFS), locoregional relapse-free survival (LRRFS), and progression-free survival (PFS). OS was defined as the date from histological diagnosis to death or last follow-up. DMFS was defined as the date from histological diagnosis to first distant metastasis or last follow-up visit. LRRFS was defined as the date from histological diagnosis to first locoregional relapse or last follow-up visit. PFS was defined as the date from histological diagnosis to first treatment failure, death, or last follow-up visit.
Statistical analysis
The statistical analyses were executed using SPSS (version 25.0) and R software (version 3.6.3). To minimize the influence of selection bias by potential confounding factors, 1 : 1 propensity score matching (PSM) was applied to compare baseline clinicopathological characteristics between IC = 2 and IC = 3 groups, with the nearest neighbor-matching method and a caliper of 0.05 (by the “Matchlt” package in R). Categorical variables were classified according to clinical knowledge, and numerical variables (age and cumulative cisplatin dose) were transformed to categorical variables based on the findings reported in previous studies [19-21]. Categorical variables were presented as whole numbers and proportion. The Pearson X2 test or Fisher's exact test was carried out to evaluate the differences in proportions of patients' baseline characteristics and acute toxicity between the two groups. Kaplan-Meier analysis and the log-rank test were carried out to calculate survival rates and compare the differences between the two groups (by the “survival” package in R). Univariate and multivariate Cox regression analysis were used to identify potentially independent prognostic factors by a backward stepwise algorithm. Variables selected by the univariate Cox analyses (p < 0.05) were included into the multivariable Cox analyses. Hazard ratios (HRs) and 95% confidence intervals (CIs) were recorded to indicate the prognostic value of risk factors.
Two-sided P-values < 0.05 was considered to be statistically significant.
Results
Patients characteristics
A total of 655 LA-NPC patients who met the eligibility criteria were enrolled into this study. Of those, there were 466 cases in IC = 3 group and 189 in IC = 2 group. Although not all patients in this study had pre-EBV DNA data, we still included it in propensity score matching (PSM) analysis because it might be a confounding factor. Overall, 189 pairs were selected according to the matching analysis. Table 1 summarized the patients' clinicopathological characteristics. The distribution of age, sex, smoking history, pathology, pre-EBV DNA, T stage, N stage, clinical stage, and cumulative cisplatin dose between the two groups were not statistically significant different (all P > 0.05). Compared with the IC = 2 group, the IC = 3 group had more patients accepted TPF or GP regime and less patients treated with PF regime (78.8% vs. 66.7%, 10.6% vs. 1.6%, and 5.3% vs. 27.5%, respectively, P < 0.001).
Survival outcome
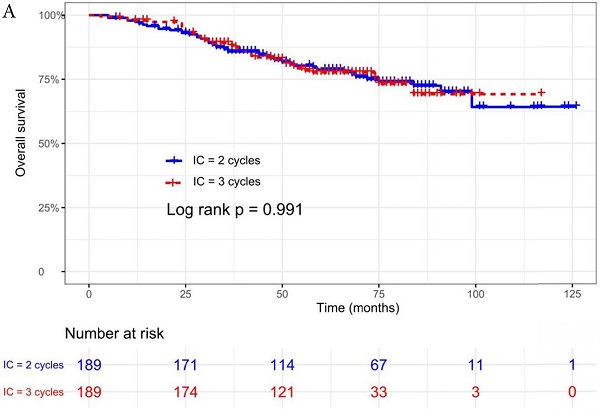
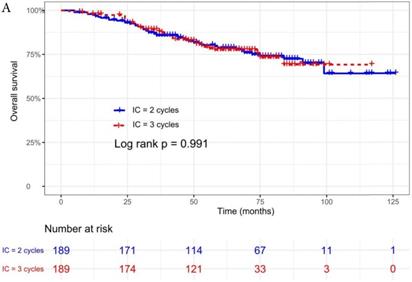
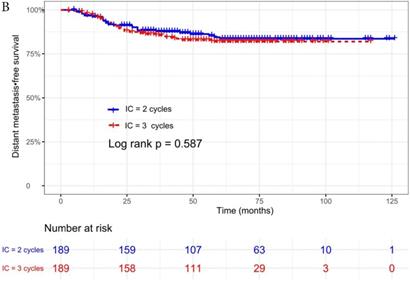
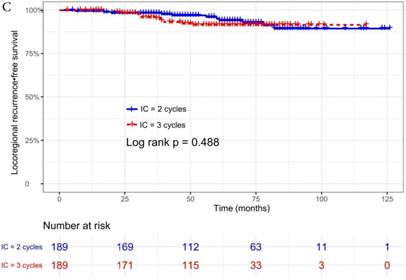
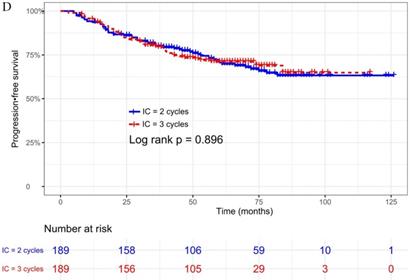
The median follow-up time was 60 months (range 5 to 126 months). The 5-year OS, DMFS, LRFS, and PFS in IC = 2 group and IC = 3 group were 83.9%, 87.7%, 97.0%, 79.4% and 83.5%, 85.6%, 93.8%, 79.3% respectively. Up to the last follow-up, 44 patients (23.3%) in IC = 2 group and 40 patients (21.1%) in IC = 3 group died; 27 patients (14.3%) in IC = 2 group and 31 patients (16.4%) in IC = 3 group experienced distant metastasis; 11 patients (5.8%) in IC = 2 group and 13 patients (6.9%) in IC = 3 group developed locoregional recurrence; 57 patients (30.2%) in IC = 2 group and 52 patients (27.5%) in IC = 3 group developed treatment failure. No statistically significant difference in OS, DMFS, LRFS, and PFS was detected between two IC cycles groups (all P > 0.05; Fig. 1 and Table 2).
Baseline clinical characteristics.
| Characteristics | n (%) | p-value | |
|---|---|---|---|
| IC = 2 (n = 189) | IC = 3 (n = 189) | ||
| Age (year) | 0.742 | ||
| < 50 | 60 (31.7) | 63 (33.3) | |
| ≥ 50 | 129 (68.3) | 126 (66.7) | |
| Sex | 0.737 | ||
| Female | 59 (31.2) | 56 (29.6) | |
| Male | 130 (68.8) | 133 (70.4) | |
| Smoking | |||
| No | 137 (72.5) | 140 (74.10 | 0.727 |
| Yes | 52 (27.5) | 49 (25.9) | |
| Pathology | |||
| WHO I/II | 20 (11.8) | 20 (11.8) | 1.000 |
| WHO III | 169 (88.2) | 169 (88.2) | |
| T stage | 0.261 | ||
| 1 | 1 (0.5) | 5 (2.6) | |
| 2 | 53 (28.1) | 43 (22.8) | |
| 3 | 83 (43.9) | 90 (47.6) | |
| 4 | 52 (27.5) | 51 (27.0) | |
| N stage | |||
| 0 | 1 (0.5) | 1 (0.5) | 0.629 |
| 1 | 44 (23.3) | 40 (21.2) | |
| 2 | 103 (54.5) | 96 (50.8) | |
| 3 | 41 (21.7) | 52 (27.5) | |
| Clinical stage | 0.216 | ||
| III | 106 (56.1) | 94 (49.7) | |
| IVa | 83 (43.9) | 95 (50.3) | |
| pre-EBV DNA (copies/ml) | 0.096 | ||
| NA | 27 (14.3) | 5 (2.6) | |
| < 5000 | 92 (48.7) | 88 (46.6) | |
| ≥ 5000 | 70 (37.0) | 96 (50.8) | |
| IC regimen | |||
| TPF | 126 (66.7) | 149 (78.8) | <0.001 |
| TP | 8 (4.2) | 10 (5.3) | |
| PF | 52 (27.5) | 10 (5.3) | |
| GP | 3 (1.6) | 20 (10.6) | |
| cumulative cisplatin dose (mg/m2) | 0.700 | ||
| ≥ 200 | 149 (78.8) | 153 (81.0) | |
| < 200 | 40 (21.2) | 36 (19.0) | |
Note: Data are shown as number of patients (%) or median (IQR). Abbreviations: WHO, World Health Organization; pre-EBV DNA, pretreatment Epstein-Barr virus DNA; TPF, docetaxel plus cisplatin plus 5-fluorouracil; TP, docetaxel plus cisplatin; GP, gemcitabine plus cisplatin; PF, cisplatin plus 5-fluorouracil. NA, these patients had not pre-EBV DNA data.
Identification of prognostic factors
We did univariate analysis about the candidate variables that might be predictors based on basic theoretical knowledge, clinical significance, and predictors confirmed by previous studies, the variables included gender (male vs. female), age (≥50 years vs. <50 years), smoking history (yes vs. no), pathology (WHO III vs. WHO I/II), T stage (T3, T4 vs. T1-2), N stage (N2, N3 vs. N0-1), clinical stage (Stage IVa vs. Stage III), pre-EBV DNA (≥ 5000 copies/ml vs. < 5000 copies/ml), IC regimen (TP, PF, GP vs. TPF), and cumulative cisplatin dose (≥ 200 mg/m2 vs. < 200 mg/m2). Concrete Results of univariate analysis are presented in Table 3. According to univariate analysis, the variables associated to lower OS were gender (male); advanced age, T stage, N stage, clinical stage; and higher pre-EBV DNA (≥ 5000copies/ml) (all P < 0.05). The following variables were not correlated with treatment failure: smoking history; pathology; IC regime, cumulative cisplatin dose, and number of IC cycles. Compared to 2 cycles of IC, additional more cycles (IC = 3) could not significantly improve OS (HR, 0.997; 95% CI, 0.648-1.536; P = 0.991), DMFS (HR, 1.153; 95% CI, 0.688-1.932; P = 0.588), LRRFS (HR, 1.329; 95% CI, 0.592-2.983; P = 0.490) and PFS (HR, 0.975; 95% CI, 0.669-1.422; P = 0.897; Table 3 and Fig. 1).
Efficacy of Study Treatment.
| Survival outcomes | IC = 2 (n = 189) | IC = 3 (n = 189) | p-value |
|---|---|---|---|
| Overall survival | 0.991 | ||
| Deaths | 44 (23.3) | 40 (21.2) | |
| 3 year OS rate (%) | 87.0 | 89.7 | |
| 5 year OS rate (%) | 83.9 | 83.5 | |
| Distant metastasis-free survival | 0.587 | ||
| Distant metastasis | 27 (14.3) | 31 (16.4) | |
| 3 year DMFS rate (%) | 90.1 | 86.8 | |
| 5 year DMFS rate (%) | 87.7 | 85.6 | |
| Locoregional recurrence-free survival | 0.488 | ||
| Recurrence | 11 (5.8) | 13 (6.9) | |
| 3 year LRRFS rate (%) | 98.3 | 95.9 | |
| 5 year LRRFS rate (%) | 97.0 | 93.8 | |
| Progression-free survival | 0.896 | ||
| Failures | 57 (30.2) | 52 (27.5) | |
| 3 year PFS rate (%) | 85.0 | 83.9 | |
| 5 year PFS rate (%) | 79.4 | 79.3 |
Abbreviations: OS, overall survival; DMFS, distant metastasis-free survival; LRRFS, locoregional recurrence-free survival; PFS, progression-free survival.
Prognostic factors on survival outcomes of 378 LA-NPC patients by use of univariate analysis.
| Characteristics | OS | DMFS | LRRFS | PFS | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | p | |
| Age (year) | 0.025 | 0.567 | 0.877 | 0.359 | ||||
| < 50 | Reference | Reference | Reference | Reference | ||||
| ≥ 50 | 1.656 (1.066-2.572) | 0.845 (0.475-1.504) | 1.072 (0.443-2.595) | 1.205 (0.809-1.793) | ||||
| Sex | 0.005 | 0.421 | 0.711 | 0.142 | ||||
| Female | Reference | Reference | Reference | Reference | ||||
| Male | 2.209 (1.263-3.863) | 1.273 (0.707-2.291) | 1.181 (0.489-2.854) | 1.386 (0.897-2.142) | ||||
| Smoking | 0.199 | 0.898 | 0.605 | 0.498 | ||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.355 (0.852-2.155) | 0.962 (0.535-1.732) | 0.605 (0.207-1.772) | 1.155 (0.761-1.753) | ||||
| Pathology | 0.878 | 0.636 | 0.642 | 0.821 | ||||
| WHO I/II | Reference | Reference | Reference | Reference | ||||
| WHO III | 0.947 (0.474-1.893) | 0.826 (0.375-1.821) | 0.750 (0.224-2.517) | 1.078 (0.563-2.067) | ||||
| T stage | 0.049 | 0.261 | 0.762 | 0.048 | ||||
| T1-2 | Reference | Reference | Reference | Reference | ||||
| T3 | 1.070 (0.608-1.070) | 0.814 | 1.664 (0.831-3.331) | 0.151 | 1.008 (0.366-2.773) | 0.988 | 1.107 (0.678-1.809) | 0.684 |
| T4 | 1.814 (1.026-3.208) | 0.041 | 1.810 (0.855-3.832) | 0.121 | 1.383 (0.480-3.987) | 0.548 | 1.731 (1.050-2.854) | 0.031 |
| N stage | <0.001 | <0.001 | 0.076 | <0.001 | ||||
| N0-1 | Reference | Reference | Reference | Reference | ||||
| N2 | 1.555 (0.770-3.142) | 0.218 | 2.406 (0.924-6.266) | 0.072 | 0.389 (0.150-1.009) | 0.052 | 1.393 (0.778-2.495) | 0.265 |
| N3 | 5.103 (2.540-10.254) | <0.001 | 6.418 (2.467-16.99) | <0.001 | 1.099 (0.406-2.979) | 0.852 | 4.936 (2.451-7.884) | <0.001 |
| Overall stage | <0.001 | <0.001 | 0.094 | <0.001 | ||||
| III | Reference | Reference | Reference | Reference | ||||
| IVa | 3.724 (2.296-6.043) | 2.679 (1.547-4.640) | 2.007 (0.888-4.534) | 3.082 (2.052-4.628) | ||||
| pre-EBV DNA (copies/ml) | 0.043 | 0.035 | 0.902 | 0.026 | ||||
| < 5000 | Reference | Reference | Reference | Reference | ||||
| ≥ 5000 | 1.655 (1.037-2.642) | 1.817 (1.043-3.167) | 1.054 (0.456-2.434) | 1.575 (1.052-2.356) | ||||
| IC regimen | 0.637 | 0.549 | 0.979 | 0.421 | ||||
| TPF | Reference | Reference | Reference | Reference | ||||
| TP | 1.113 (0.405-3.058) | 0.836 | 1.108 (0.345-3.555) | 0.863 | 1.131 (0.150-8.505) | 0.905 | 1.366 (0.596-3.128) | 0.461 |
| PF | 0.958 (0.543-1.688) | 0.881 | 0.540 (0.231-1.261) | 0.154 | 1.230 (0.453-3.339) | 0.685 | 0.916 (0.550-1.525) | 0.736 |
| GP | 0.276 (0.038-1.998) | 0.202 | NA | 0.927 | 1.210 (0.158-9.267) | 0.854 | 0.357 (0.087-1.454) | 0.150 |
| IC cycle | 0.991 | 0.588 | 0.490 | 0.897 | ||||
| IC = 2 | Reference | Reference | Reference | Reference | ||||
| IC = 3 | 0.997 (0.648-1.536) | 1.153 (0.688-1.932) | 1.329 (0.592-2.983) | 0.975 (0.669-1.422) | ||||
| cumulative cisplatin dose (mg/m2) | 0.352 | 0.435 | 0.315 | 0.867 | ||||
| ≥ 200 | Reference | Reference | Reference | Reference | ||||
| < 200 | 1.282 (0.760-2.162) | 0.753 (0.370-1.534) | 1.609 (0.637-4.065) | 1.042 (0.647-1.678) |
Abbreviations: WHO, World Health Organization; pre-EBV DNA, pretreatment Epstein-Barr virus DNA; TPF, docetaxel plus cisplatin plus 5-fluorouracil; TP, docetaxel plus cisplatin; GP, gemcitabine plus cisplatin; PF, cisplatin plus 5-fluorouracil; OS, overall survival; DMFS, distant metastasis-free survival; LRRFS, locoregional recurrence-free survival; PFS, progression-free survival; CI, confidence interval; HR, hazard ratio.
Kaplan-Meier OS (A), DMFS (B), LRRFS (C) and PFS (D) curves for 189 pairs of patients stratified as IC = 2 cycles and IC = 3 cycles groups. Abbreviations: OS, overall survival; DMFS, distant metastasis-free survival; LRRFS, locoregional recurrence-free survival; PFS, progression-free survival.
Prognostic factors on survival outcomes of 378 LA-NPC patients by use of multivariate analysis.
| Endpoints | Variable | HR (95% CI) | p-value |
|---|---|---|---|
| OS | Age (≥ 50 vs. < 50) | 1.161 (0.739-1.824) | 0.516 |
| Sex (Male vs. Female) | 1.782 (0.999-3.177) | 0.051 | |
| T stage (3 vs. 1-2) | 1.268 (0.714-2.252) | 0.417 | |
| T stage (4 vs. 1-2) | 2.228 (1.230-4.035) | 0.008 | |
| N stage (2 vs. 0-1) | 1.840 (0.899-3.766) | 0.095 | |
| N stage (3 vs. 0-1) | 6.074 (2.952-12.497) | <0.001 | |
| IC regimen (TPF vs. others) | 1.334 (0.797-2.230) | 0.273 | |
| DMFS | Age (≥ 50 vs. < 50) | 0.735 (0.419-1.289) | 0.283 |
| Sex (Male vs. Female) | 1.099 (0.598-2.021) | 0.761 | |
| T stage (3 vs. 1-2) | 2.109 (1.045-4.259) | 0.037 | |
| T stage (4 vs. 1-2) | 2.758 (1.262-6.027) | 0.011 | |
| N stage (2 vs. 0-1) | 3.106 (1.108-8.178) | 0.022 | |
| N stage (3 vs. 0-1) | 9.191 (3.452-24.477) | <0.001 | |
| IC regimen (TPF vs. others) | 2.021 (0.989-4.132) | 0.054 | |
| LRRFS | Age (≥ 50 vs. < 50) | 0.919 (0.381-2.218) | 0.851 |
| Sex (Male vs. Female) | 1.109 (0.445-2.767) | 0.824 | |
| T stage (3 vs. 1-2) | 0.780 (0.262-2.319) | 0.655 | |
| T stage (4 vs. 1-2) | 1.051 (0.322-3.425) | 0.935 | |
| N stage (2 vs. 0-1) | 0.377 (0.135-1.058) | 0.064 | |
| N stage (3 vs. 0-1) | 1.091 (0.371-3.212) | 0.874 | |
| IC regimen (TPF vs. others) | 0.851 (0.350-2.066) | 0.721 | |
| PFS | Age (≥ 50 vs. < 50) | 1.974 (0.654-1.452) | 0.897 |
| Sex (Male vs. Female) | 1.111 (0.707-1.744) | 0.648 | |
| T stage (3 vs. 1-2) | 1.348 (0.817-2.225) | 0.242 | |
| T stage (4 vs. 1-2) | 2.356 (1.389-3.999) | 0.001 | |
| N stage (2 vs. 0-1) | 1.696 (0.935-3.075) | 0.082 | |
| N stage (3 vs. 0-1) | 5.584 (3.046-10.237) | <0.001 | |
| IC regimen (TPF vs. others) | 1.220 (0.787-1.891) | 0.374 |
Abbreviations: OS, overall survival; DMFS, distant metastasis-free survival; LRRFS, locoregional recurrence-free survival; PFS, progression-free survival; CI, confidence interval; HR, hazard ratio.
The variables included in multivariate analysis were prognostic factors identified by univariate analysis. Through PSM, there was still significant difference of IC regime in the different IC cycles group (Table 1). Thus, we also included IC regime into the multivariate analysis to test whether it was an independent prognostic factor. The outcomes of the multivariate analysis revealed that IC regime was not associated with significantly improved survival rates. The visual details of this step for the OS, DMFS, LRRFS and PFS were shown in Table 4. By means of Spearman correlation analysis, we found that T stage and N stage were correlated with clinical stage, with the correlation coefficients of 0.486 (p < 0.001) and 0.421 (p < 0.001), respectively. Thus, clinical staging didn't be included in multivariate analysis in Table 4, and we conducted another multivariate analysis combined clinical staging and other prognostic factors besides T, N stage in Supplementary Table 1. According to multivariate analyses, T stage, N stage, and clinical stage remained independent prognosticators.
Subgroup analysis.
| Characteristic | OS (%) | DMFS (%) | LRRFS (%) | PFS (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC=2 | IC=3 | P | IC=2 | IC=3 | P | IC=2 | IC=3 | P | IC=2 | IC=3 | P | |
| T stage | ||||||||||||
| T1-2 | 81.3 | 84.2 | 0.743 | 92.2 | 84.8 | 0.296 | 97.4 | 93.5 | 0.753 | 77.1 | 78.6 | 0.855 |
| T3 | 81.5 | 79.3 | 0.773 | 82.0 | 80.6 | 0.625 | 95.5 | 90.2 | 0.199 | 73.6 | 70.9 | 0.571 |
| T4 | 72.2 | 68.5 | 0.978 | 77.7 | 81.4 | 0.610 | 92.1 | 91.7 | 0.568 | 60.1 | 64.9 | 0.395 |
| N stage | ||||||||||||
| N0-1 | 95.1 | 84.9 | 0.617 | 93.1 | 95.0 | 0.702 | 97.4 | 83.3 | 0.194 | 88.3 | 77.4 | 0.596 |
| N2 | 80.8 | 85.7 | 0.488 | 83.4 | 87.7 | 0.541 | 95.8 | 97.5 | 0.263 | 73.9 | 83.0 | 0.182 |
| N3 | 52.8 | 56.8 | 0.810 | 69.0 | 61.1 | 0.268 | 80.8 | 87.7 | 0.468 | 36.4 | 44.5 | 0.735 |
| Clinical stage | ||||||||||||
| III | 88.7 | 90.2 | 0.651 | 88.6 | 90.8 | 0.691 | 97.0 | 93.8 | 0.666 | 81.8 | 85.9 | 0.423 |
| IVa | 65.5 | 65.4 | 0.875 | 76.3 | 72.8 | 0.522 | 91.3 | 88.9 | 0.732 | 53.9 | 58.2 | 0.984 |
| pre-EBV DNA (copies/ml) | ||||||||||||
| < 5000 | 77.7 | 85.0 | 0.401 | 84.7 | 90.6 | 0.447 | 93.1 | 92.8 | 0.817 | 69.7 | 81.6 | 0.153 |
| ≥ 5000 | 75.5 | 72.4 | 0.937 | 81.2 | 74.9 | 0.312 | 98.2 | 90.1 | 0.214 | 67.8 | 63.3 | 0.647 |
Abbreviations: pre-EBV DNA, pretreatment Epstein-Barr virus DNA; OS, overall survival; DMFS, distant metastasis-free survival; LRRFS, locoregional recurrence-free survival; PFS, progression-free survival.
Acute toxicity in patients during IC.
| Variable | IC = 2 (n = 189) | IC = 3 (n = 189) | p-value |
|---|---|---|---|
| Haematological | |||
| Leukocytopenia | |||
| Grade 1/2 | 65 | 102 | <0.001 |
| Grade 3/4 | 23 | 18 | 0.408 |
| Neutropenia | |||
| Grade 1/2 | 47 | 69 | 0.014 |
| Grade 3/4 | 33 | 28 | 0.485 |
| Thrombocytopenia | |||
| Grade 1/2 | 8 | 10 | 0.629 |
| Grade 3/4 | 3 | 4 | 1.000 |
| Anemia | |||
| Grade 1/2 | 30 | 48 | 0.022 |
| Grade 3/4 | 1 | 2 | 1.000 |
| Hepatoxicity | |||
| ALT increase | |||
| Grade 1/2 | 53 | 70 | 0.131 |
| Grade 3/4 | 5 | 5 | 1.000 |
| AST increase | |||
| Grade 1/2 | 42 | 57 | 0.079 |
| Grade 3/4 | 3 | 1 | 0.623 |
| Bilirubin increase | |||
| Grade 1/2 | 20 | 57 | <0.001 |
| Grade 3/4 | 0 | 0 | 1.000 |
| Gastrointestinal reactions | |||
| Grade 1/2 | 75 | 109 | <0.001 |
| Grade 3/4 | 2 | 4 | 0.685 |
Note: All data are presented as number of patients (%). Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase.
Subgroup analysis
According to the results of multivariate analyses, patients with advanced T stage, N stage, or clinical stage would associate with higher rate of treatment failure. Then we examined whether patients with different risk stratification would benefit differently for different IC cycles or not, so we conducted subgroup analysis to further examine the treatment efficiency of different IC cycles on different risk subgroups. In addition, pre-EBV DNA was showed to be a predictor by univariate analysis in this study. Because not all patients in this study had pre-EBV data, we did not include it in the multivariate analysis. However, previous studies used pre-EBV DNA to conduct risk stratification [22, 23], we also divided patients into two subgroups according to pre-EBV DNA and investigated the role of number of cycles of IC in these two subgroups. The results of subgroup analysis indicated that no significant differences were found between two or three cycles of IC for patients stratified by pre-EBV DNA, T stage, N stage, and clinical stage. Details regarding subgroup analysis of survival outcomes are provided in Table 5.
Acute toxicity
During IC period, complete hematology results were available for all patients and we evaluated the treatment-related acute toxicity between different IC cycles groups. Details of patients with Grade 1-2, and 3-4 acute toxicities are presented in Table 6. There was no significant difference in terms of the incidence of Grade 3-4 acute toxicities within the two groups. However, three cycles of IC significantly increased the prevalence of Grade 1-2 leukocytopenia (P < 0.001), neutropenia (p = 0.014), anemia (P = 0.022), hepatoxicity (bilirubin increase, P < 0.001), and gastrointestinal reactions (P < 0.001), as compared to two cycles of IC.
Discussion
In the present study, we retrospectively analyzed 378 locoregionally advanced NPC with IC and CCRT and revealed the treatment efficiency of the number of cycles of IC through the PSM method. The above findings indicated that the treatment efficacy of two cycles of IC is equivalent to three cycles of IC in LA-NPC, but decrease the incidence of treatment-related Grade 1-2 acute toxicities during the IC period. In addition, the stratified subgroups analysis results demonstrated two cycles of IC offered similar survival benefit over three cycles for patients in both the high-risk group and the low-risk group. These results revealed that two cycles of IC might be sufficient and additional more cycles of IC did not lead to survival benefit.
For non-metastatic LA-NPC, CCRT has been proved as the standard of care according to previous studies [2, 3, 24]. With the wide application of intensity-modulated radiotherapy (IMRT), the local control rate of NPC has been significantly improved and the occurrence of distant metastasis has become the main cause for treatment failure [25-27]. In recent years, adding IC to CCRT has received considerable attention. A previous randomized phase 2 study reported that the application of IC to CCRT was superior to CCRT alone for 3-year overall survival, and also led to a trend to improve progression-free survival and distant control [8]. Later several studies provided evidence that IC plus CCRT could afford a survival benefit for LA-NPC patients [6, 11, 28], and IC has since played an important role of the treatment regimen for LA-NPC. In most randomized trial and clinical practice, physicians may prefer three cycles as initial IC regimen. However, a considerable number of patients could not successfully complete the three cycles IC due to the treatment-related toxicity and cost. It is also unclear whether three is the optimal number of IC cycles for maximizing patient survival or not. Considering the toxicity and economic cost of IC, accurate judgment of optimal number of cycles of IC merits further study.
Theoretically, LA-NPC patients with advanced stage are likely to have a higher risk of occurrence of disease progression. Some of them would develop subclinical micrometastasis at initial of diagnosis, which indicates they may benefit from intensive treatment. Although it is not recommended as a high level of evidence for application in clinical practice, clinicians may choose more cycles of IC for these “high-risk” subgroups. In the current study, we identified which variables would affect survival outcomes and assessed the prognostic value of these variables by univariate and multivariate Cox analyses. We found that T stage, N stage, and clinical stage were independent prognostic factors of OS, DMFS, LRRFS, or PFS, and the number of IC cycles was not a predictor of any survival outcome. Thus, according to the prognostic factors chosen by multivariate analysis with different tumor burden and risk of treatment failure, we conducted subgroups analysis to further investigate the efficacy of cycles of IC. The results of this step showed that non-significant differences were observed in all endpoints between different IC cycles groups. These results indicated that additional more cycles after two could not add benefit for patients with higher pre-EBV DNA or advanced stage.
Our study showed that three cycles IC could not improve OS, DMFS, LRRFS, or PFS in LA-NPC patients, which were consistent with previous study by Wei et al [16]. The subgroup analysis further demonstrated no survival difference between the patient subgroups, regardless of pre-EBV DNA or tumor stage. We speculated there were two reasons for this situation. One reasonable explanation for the finding is that two cycles of IC may be sufficient for LA-NPC patients to eradicate micrometastases and decrease tumor volume. Another potential reason why the difference was not significant is the prolonged waiting time for radiotherapy (WRT) by additional IC cycles. WRT was defined as the interval between cancer diagnosis and the implementation of radical radiotherapy. Generally, oncologists think that early radical treatment is reasonable and cancer patients should receive radical treatment as soon as possible after a definite diagnosis. However, timely treatment is hampered in many patients due to lack of policy support, limited medical resources, or inefficient healthcare process [29]. Some medical strategy-related factors are also important factors in clinical practice, especially IC, which is one of the main treatment-related factors prolonging WRT. The association between prolonged WRT and poor prognosis has been found in many cancers, such as breast cancer, rectal cancer, and bladder cancer [30-33]. Also, a number of previous retrospective studies suggested prolonged WRT was correlated with worse survival outcome for NPC patients. Chen et al reported that an interval time of > 4 weeks between diagnosis and radical radiotherapy was an independent negative predictor for PFS [34]. Another later study with a larger population and longer follow-up indicated that increased WRT correlated with poor clinical outcome of NPC patients with advanced stage [29]. The above studies reminded us that IC could prolong WRT and may decrease survival rate even though it has been proven to ameliorate clinical outcomes for NPC patients. NPC patients receiving three cycles of IC usually experience longer WRT than that of patients treated with only two cycles, which may “counteract” the benefit of additional cycles of IC. Thus, we suspect that WRT might be a potential confounding variable that subtly influences the results of this study. These hypotheses could explain why this study failed to observe significant differences of treatment efficacy between the different IC cycles groups.
Studies of the optimal number of cycles of IC for LA-NPC patients treated with IC plus CCRT are scarce and their conclusions were controversial. Peng et al evaluated 247 pairs of NPC patients with advanced stage and found no significant difference in survival between patients with 2 cycles and 3 to 4 cycles of IC, while in N2-3 stage subgroup, patients treated with 2 cycles IC had better OS than those of patients with 3 to 4 cycles IC (92.4% vs. 80.8%, P = 0.029) [17]. In another retrospective analysis, Wang et al demonstrated that 2 cycles of IC could significantly improve DMFS (HR, 0.499; P=0.038) and PFS (HR, 0.585; P=0.049) compared with 3 to 4 cycles, and the results of stratified analysis indicated that LRRFS, PFS, and OS were comparable between the different IC cycles groups for N0-1 category patients [15]. However, both studies defined high cycle group as patient with 3 or 4 cycles of IC and the 3 cycles IC were not explored separately. As we all know, three cycles were usually used in randomized controlled clinical trials and clinical practice. Additionally, the population of the two studies was all staged by the 7th AJCC/UICC staging system, which would lack generalizability in real-world clinical practice with the advent of the 8th staging system. A recent study by He et al compared survival outcome from patients treated with 2, 3 and 4 IC cycles and found similar survival between 2 and 3 cycles IC groups, while 4 cycles IC was associated with worse overall survival and higher incidence of treatment-related toxicities [16]. However, the above study also used the 7th staging system and included stage II patients for whom IC is not routinely recommended according to the latest guideline [35]. Also, this study set in a non-endemic area of China with higher rate of non-keratinizing diferentiated subtype (approximate 30%) than endemic area (< 5%), and this histological type is an adverse prognostic factor for patient survival [36, 37]. Although the results were not entirely consistent, these studies corroborated the fact that additional more cycles of IC might not lead to better survival outcomes. In contrast to these three studies, the scholars in another study reported that the number of IC appeared to be an independent predictor and for N2-3 NPC, survival data of the 4 cycles of IC were better than those of 2 cycles [20]. It is noted that the study paid attention to advance N stage patients and lack of patient receiving 3 cycles of IC. These published inconsistent results above mentioned implied that the effect of the number of IC cycles on prognosis is an important problem to be noticed and requires additional research. According to most findings of above studies, we speculated that the treatment efficacy of three cycles of IC may not be superior to two cycles and our results supported this hypothesis. Compared to other studies, our study paid attention to stage III-IVa NPC in endemic area of China and performed based on the 8th AJCC/UICC staging system. Our finding may provide better guidance for treatment decisions in areas with high incidence of NPC.
During the period of IC, the most commonly observed acute adverse events included hematologic toxicities and gastrointestinal reactions. Our study showed that additional more cycles increase the incidence of treatment-related Grade 1-2 toxicity. Although Grade 1-2 acute toxicity was acceptable and easily managed, patients may be affected by it for subsequent CCRT. In a randomized controlled trial, only 30% of patients with three cycles IC completed three cycles of concurrent cisplatin successfully [6]. In clinical practice, the most common reasons that patients do not continue concurrent cisplatin are patients' refusal and treatment-related toxicities. If patient experienced too much adverse events during IC period, patients' fear of acute adverse events may decrease their tolerance to subsequent CCRT.
In this study, we used PSM to evaluate the treatment efficiency of two and three IC cycles for patients with LA-NPC, which increase the reliability of the results. However, several limitations should be stated. First, this is a retrospective study, and inherent selective bias was unavoidable. The IC regimen was not balanced among the IC = 2 and IC = 3 group because there were no standard IC regimens at the time and the determination of the treatment decision would take individual patient's situation into account. This factor might be a confounding factor when evaluated survival rates. Of note, the multivariate analyses including this factor could effectively reduce this bias. In this study, IC regime failed to be an independent prognostic factor for survival outcomes. Additionally, all regimens included in this study (TPF, TP, PF, GP) were platinum-based and recommended according to the guideline, which have been widely used in many hospitals [16, 18, 21]. Second, the data from a single institution do not provide robust evidence. Therefore, these results must be validated by other institutions.
Conclusion
In summary, our retrospective study indicated that two cycles of IC appear to provide similar survival benefit over additional more cycles for patients with LA-NPC and may be associated with lower incidence of treatment-related adverse events. This finding will help patients avoiding overtreatment and financial burden. Additionally, patients may have better compliance to CCRT if appropriate cycles of IC were administered. Based on the above evidence, we believe that two cycles of IC may be more reasonable for patients with LA-NPC than three cycles. This conclusion needs to be confirmed by multi-center prospective study with large cohort.
Supplementary Material
Supplementary methods and table.
Acknowledgements
This work was supported by the Key Research and Development Program Project of Guangxi Zhuang Autonomous Region (Grant No. GuikeAB18221007), Youth Science Foundation Project of Guangxi Medical University (Grant No. GXMUYSF202122), and the Independent Project of Key Laboratory of Early Prevention & Treatment for Regional High-Incidence-Tumor (Grant No. GKE-ZZ202014).
Ethics Committee Approval and Patient Consent
The study was approved by the Medical Ethics Committee of Guangxi Medical University Cancer Hospital. The ethics committee waived the requirement of written informed consent for participation. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Study conception and design: XiaoDong Zhu and YuTing Jiang. Data acquisition and quality control: all authors; Statistical analysis: YuTing Jiang and KaiHua Chen; Manuscript preparation: YuTing Jiang; Manuscript review: all authors.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ma H, Qiu Y, Li H, Xie F, Ruan G, Liu L. et al. Prognostic Value of Nodal Matting on MRI in Nasopharyngeal Carcinoma Patients. J Magn Reson Imaging. 2021;53:152-64
2. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T. et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310-7
3. Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B. et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20:2038-44
4. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631-7
5. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y. et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163-71
6. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY. et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509-20
7. Blake DD, Penk WE, Mori DL, Kleespies PM, Walsh SS, Keane TM. Validity and clinical scale comparisons between the MMPI and MMPI-2 with psychiatric inpatients. Psychol Rep. 1992;70:325-32
8. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK. et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27:242-9
9. Kerr DJ, Burt AD, Brewin TB, Boyle P. Divergence between mortality and incidence rates of thyroid cancer in Scotland. Lancet. 1985;2:149
10. Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y. et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: Long-term results of phase 3 randomized controlled trial. Int J Cancer. 2019;145:295-305
11. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY. et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med. 2019;381:1124-35
12. Bae WK, Hwang JE, Shim HJ, Cho SH, Lee JK, Lim SC. et al. Phase II study of docetaxel, cisplatin, and 5-FU induction chemotherapy followed by chemoradiotherapy in locoregionally advanced nasopharyngeal cancer. Cancer Chemother Pharmacol. 2010;65:589-95
13. Du C, Ying H, Zhou J, Hu C, Zhang Y. Experience with combination of docetaxel, cisplatin plus 5-fluorouracil chemotherapy, and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Int J Clin Oncol. 2013;18:464-71
14. Kong L, Hu C, Niu X, Zhang Y, Guo Y, Tham IW. et al. Neoadjuvant chemotherapy followed by concurrent chemoradiation for locoregionally advanced nasopharyngeal carcinoma: interim results from 2 prospective phase 2 clinical trials. Cancer. 2013;119:4111-8
15. Fangzheng W, Chuner J, Zhimin Y, Quanquan S, Tongxin L, Min X. et al. Association of the neoadjuvant chemotherapy cycle with survival outcomes in patients with locoregionally advanced nasopharyngeal carcinoma: a propensity-matched analysis. Oncotarget. 2017;8:94117-28
16. He Y, Zhao Z, Wang Y, Chai J, He J, Wang J. et al. Optimizing number of cycles of induction chemotherapy for patients with nasopharyngeal carcinoma: Retrospective survival analysis. Head Neck. 2020;42:2067-76
17. Peng H, Chen L, Li WF, Zhang Y, Liu LZ, Tian L. et al. Optimize the cycle of neoadjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: A propensity score matching analysis. Oral Oncol. 2016;62:78-84
18. Jiang YT, Chen KH, Yang J, Liang ZG, Qu S, Li L. et al. Establishment of a Prognostic Nomogram for Patients With Locoregionally Advanced Nasopharyngeal Carcinoma Incorporating TNM Stage, Post-Induction Chemotherapy Tumor Volume and Epstein-Barr Virus DNA Load. Front Oncol. 2021;11:683475
19. Jiang Y, Qu S, Pan X, Huang S, Zhu X. Prognostic Nomogram For Locoregionally Advanced Nasopharyngeal Carcinoma. Sci Rep. 2020;10:861
20. Wei J, Feng H, Xiao W, Wang Q, Qiu B, Liu S. et al. Cycle number of neoadjuvant chemotherapy might influence survival of patients with T1-4N2-3M0 nasopharyngeal carcinoma. Chin J Cancer Res. 2018;30:51-60
21. Wen DW, Li ZX, Chen FP, Lin L, Peng BY, Kou J. et al. Individualized cumulative cisplatin dose for locoregionally-advanced nasopharyngeal carcinoma patients receiving induction chemotherapy and concurrent chemoradiotherapy. Oral Oncol. 2020;107:104675
22. Liu SL, Sun XS, Liu LT, Sun R, Luo DH, Chen QY. et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients based on plasma Epstein-Barr virus DNA level after induction chemotherapy. Aging (Albany NY). 2020;12:4931-44
23. Xu C, Zhang S, Li WF, Chen L, Mao YP, Guo Y. et al. Selection and Validation of Induction Chemotherapy Beneficiaries Among Patients With T3N0, T3N1, T4N0 Nasopharyngeal Carcinoma Using Epstein-Barr Virus DNA: A Joint Analysis of Real-World and Clinical Trial Data. Front Oncol. 2019;9:1343
24. Chan AT, Leung SF, Ngan RK, Teo PM, Lau WH, Kwan WH. et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97:536-9
25. Sun X, Su S, Chen C, Han F, Zhao C, Xiao W. et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110:398-403
26. Tang LQ, Chen QY, Fan W, Liu H, Zhang L, Guo L. et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2013;31:2861-9
27. Zhang MX, Li J, Shen GP, Zou X, Xu JJ, Jiang R. et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51:2587-95
28. Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ. et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14-23
29. Liang H, Xiang YQ, Lv X, Xie CQ, Cao SM, Wang L. et al. Survival impact of waiting time for radical radiotherapy in nasopharyngeal carcinoma: A large institution-based cohort study from an endemic area. Eur J Cancer. 2017;73:48-60
30. Chen Z, King W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16
31. Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Longer wait times increase overall mortality in patients with bladder cancer. J Urol. 2009;182:1318-24
32. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353:1119-26
33. Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY. et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012;23:2731-7
34. Chen YP, Mao YP, Zhang WN, Chen L, Tang LL, Li WF. et al. Prognostic value of wait time in nasopharyngeal carcinoma treated with intensity modulated radiotherapy: a propensity matched analysis. Oncotarget. 2016;7:14973-82
35. Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH. et al. Chemotherapy in Combination With Radiotherapy for Definitive-Intent Treatment of Stage II-IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. J Clin Oncol. 2021;39:840-59
36. Zang J, Li C, Xu M, Xu W, Kang X, Wang J. et al. Induction chemotherapy followed by concurrent chemoradiotherapy is benefit for advanced stage nasopharyngeal carcinoma with different nonkeratinizing carcinoma subtypes. Sci Rep. 2018;8:13318
37. Zang J, Li C, Zhao LN, Wang JH, Xu M, Luo SQ. et al. Prognostic Model of Death and Distant Metastasis for Nasopharyngeal Carcinoma Patients Receiving 3DCRT/IMRT in Nonendemic Area of China. Medicine (Baltimore). 2016;95:e3794
Author contact
![]() Corresponding author: Xiao-Dong Zhu, Email: zhuxdonggxmucom.
Corresponding author: Xiao-Dong Zhu, Email: zhuxdonggxmucom.

 Global reach, higher impact
Global reach, higher impact