Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(1):243-252. doi:10.7150/jca.65297 This issue Cite
Research Paper
Shikonin induced Apoptosis Mediated by Endoplasmic Reticulum Stress in Colorectal Cancer Cells
1. Experiment Center for Science and Technology, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, P.R. China.
2. Oncology and Immunology BU, Research Service Division, WuXi Apptec, Shanghai, China.
*These authors contributed equally to this work.
Received 2021-7-23; Accepted 2021-11-22; Published 2022-1-1
Abstract
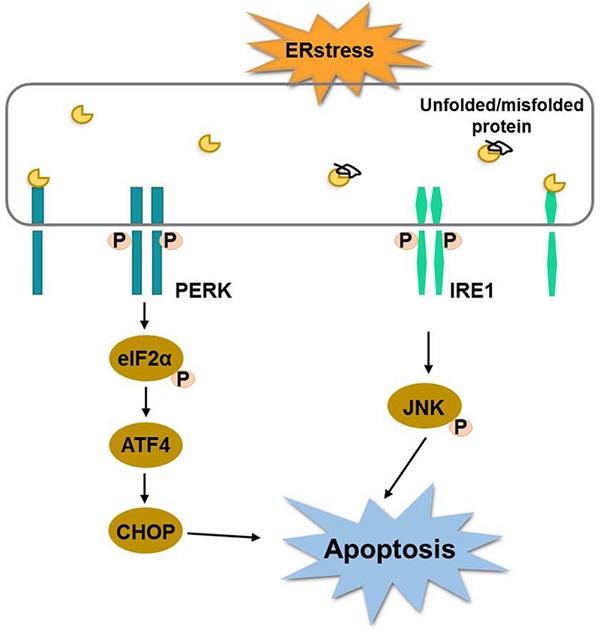
Shikonin is a naphthoquinone pigment isolated from the root of Lithospermum erythrorhizon, which has displayed potent anti-tumor properties. However, the effects of shikonin in colorectal cancer cells have not been yet fully investigated. In this study, we demonstrated that shikonin significantly inhibited the activity of colorectal cancer cells in a time- and dose-dependent manner. The flow cytometry and western blot results indicated that shikonin induced cell apoptosis by down-regulating BCL-2 and activating caspase-3/9 and the cleavage of PARP. The expression of BiP and the PERK/elF2α/ATF4/CHOP and IRE1α /JNK signaling pathways were upregulated after shikonin treatment. The pre-treatment with N-acetyl cysteine significantly reduced the cytotoxicity of shikonin. Taken together, shikonin could inhibit proliferation of the colorectal cancer cell through the activation of ROS mediated-ER stress. The in vivo results showed that shikonin effectively inhibited tumor growth in the HCT-116 and HCT-15 xenograft models. In conclusion, shikonin inhibited the proliferation of colorectal cancer cells in vitro and in vivo and warrants future investigation.
Keywords: Shikonin, Colorectal cancer, Endoplasmic reticulum stress, Apoptosis
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer related deaths worldwide [1]. According to the latest data published by the National Cancer Center of China, annually there are more than 420,000 individuals diagnosed with colorectal cancer, accounting for 9.88% of the newly diagnosed cancers in China [2].
Treatments which provide a limited increase of overall survival for colorectal cancer are surgical resection, radiotherapy and chemotherapy [3]. Immunotherapy and targeted therapy with monoclonal antibodies can also improve the outcomes in some patients, but in many others the benefits are only modest or lacking [4, 5]. Thus, there is an urgent need for new bioactive molecules with anti-cancer properties. In the traditional Chinese medicine, different natural products have been demonstrated to possess antitumor activities [6].
Nowadays, several natural products have been successfully used in cancer therapy, such as, paclitaxel (Taxol) that discovered in the bark of a Pacific yew tree, which has been commonly used in the treatment of breast, ovarian and other cancers [7]. Shikonin is a naturally occurring naphthoquinone isolated from the root of the plant Lithospermum erythrorhizon, which has been widely used in the treatment of various disease [8]. In the recent years, studies have shown that shikonin and its derivatives have antitumor effects in wide range of cancer types including breast [9], lung [10], ovarian [11], liver [12], prostate [13], gastric [14] and pancreatic cancer [15]. However, the effects of shikonin in colorectal cancer cells have not been yet fully investigated yet.
Endoplasmic reticulum (ER) is a relatively complex organelle which regulates protein synthesis, folding, assembly and trafficking by utilizing its dynamic structural change [16]. Therefore, it is necessary to ensure the homeostasis of endoplasmic reticulum environment. Alteration of ER stability will prime a series of signaling events known as endoplasmic reticulum stress (ERS). Although the main function of unfolded protein response is to restore the organelle's homeostasis, continuous ER stress and unfolded protein response can also cause cell death. One study published recently showed that shikonin induces apoptosis in human melanoma A375 cells through activating reactive oxygen species (ROS)-mediated endoplasmic reticulum stress and mitochondrial apoptotic pathway [17]. Meanwhile, there are growing studies reveal that ROS and ER stress play an essential role in the development of colorectal cancer. For example, one study has shown that ER stress-related ATF6 upregulates CIP2A, which results in poor prognosis of colon cancer [18]. ER stress induces apoptosis through inducing ROS, and that elevated ROS also play an important role in down-regulating cell cycle progression and cell survival. Therefore, targeting ER stress is a potential new option in CRC therapy. We hypothesized that shikonin have antitumor activity against human colorectal cancer, and tested this hypothesis in both in vitro and in vivo prospective.
Materials and Methods
Materials and reagents
The colorectal cancer cell lines HCT-116 (ATCC® CCL-247™) and HCT-15 (ATCC® CCL-225™) were purchased from the American Type Culture Collection (ATCC, USA). Shikonin was purchased from Meilun Biotechnology Co., Ltd (Shanghai, China). Trypsin was purchased from Gibco (New York, USA). Dimethyl sulfoxide (DMSO), tween 80, poly (ethylene glycol) 400 and N-acetyl cysteine (NAC) were obtained from Sigma (St. Louis, MO, USA).
Cell culture and treatment
HCT-116 and HCT-15 cells were maintained in McCoy's 5A (Invitrogen, Carlsbad, USA) or RPMI-1640 medium containing 10% heat inactivated fetal bovine serum (Gibco, New York, USA) at 37 ºC in a 5% CO2 atmosphere. Cells were treated with different doses of shikonin at different time points during several experiments.
Cell viability assay
Cell viability was examined by the CellTiter-Glo luminescent cell viability assay kit (Promega, Madison, WI, USA). Cells were seeded in 96-well plates at densities of 5000 cells (HCT-116) and 6000 cells (HCT-15) per well and treated with shikonin at various time points. After treatment, luminescence was recorded utilizing a Perkin Elmer 2104 EnVision plate reader (PerkinElmer, Hopkinton, MA).
Cell colony formation
After treatment, cells were seeded in 6-well plates at a density of 1000 cells per well and cultivated for 10 days. Then, the cells were fixed with 4% paraformaldehyde (Sigma, St. Louis, MO, USA) for 30 min and stained with 1% crystal violet (Beyotime, Shanghai, China) for 1 h at room temperature. After that, the 6-well plates were washed with PBS. Images were taken using a microscope (Leica, Wetzlar, Germany) and the total number of cell colonies was counted.
Apoptosis assay
Cells were seeded in 6-well plates at a density of 3 × 105 cells per well and treated with shikonin. After 24 h treatment, the cells were harvested and stained with Annexin V-FITC and propidium iodide (PI) according to the manufacturer's instructions (BD Biosciences, San Diego, CA, USA). The cell apoptosis rate was detected by flow cytometry (FACSCanto II, BD Biosciences, USA).
qRT-PCR
Total RNAs were extracted using the RNeasy mini kit (Qiagen, Hilden, Germany). The cDNA was synthesized using the cDNA reverse transcription kit (ABI, Carlsbad, USA). The qRT-PCR was performed using the Real-Time PCR System and the SYBR® Green PCR Master Mix (ABI, Carlsbad, USA). QuantStudio7 (ABI, Carlsbad, USA) was used to acquire real-time quantitative PCR data. RNA expression was quantified using the 2-ΔΔCt method. The sequences of the primer are shown in Table 1.
Western blotting analysis
The total protein was extracted by using RIPA lysis buffer containing protease and phosphatase inhibitors (Sigma, St. Louis, MO, USA). The cell extracts were centrifuged at 15,000 rpm for 20 min at 4 °C. The protein concentrations were measured by using the pierce™ BCA protein assay kit (Thermo Scientific, Lithuania). A total of 20 μg of protein per sample was separated on SDS-PAGE gels and transferred onto PVDF membranes. After blocking with 5% nonfat dry milk, the PVDF membranes were incubated overnight with the primary antibody at 4 °C, then incubated for 2 h with the HRP-conjugated secondary antibody at room temperature. Later, the HRP substrate from West Femto Maximum Sensitivity kit (Thermo Scientific, Lithuania) was added to the PVDF membranes. Luminescence was detected with ImageQuant LAS 4000 (GE, USA).
The primers used for quantitative PCR
| Gene | Primer sequence (5'---3') | Base (bp) |
|---|---|---|
| BiP forward | CACAGTGGTGCCTACCAAGA | 20 |
| BiP reverse | TGTCTTTTGTCAGGGGTCTTT | 21 |
| ATF4 forward | CCCTTCACCTTCTTACAACCTC | 22 |
| ATF4 reverse | TGCCCAGCTCTAAACTAAAGGA | 22 |
| CHOP forward | AGCCAAAATCAGAGCTGGAA | 20 |
| CHOP reverse | TGGATCAGTCTGGAAAAGCA | 20 |
| Bcl-2 forward | GGTCATGTGTGTGGAGAGAGCG | 22 |
| Bcl-2 reverse | CCGTACAGTTCCACAAAGGC | 20 |
| GAPDH forward | GGAGTCAACGGATTTGGTCG | 21 |
| GAPDH reverse | ACGGTGCCATGGAATTTGC | 19 |
Xenograft mouse model
Female BALB/c nude mice were purchased from Shanghai Sino-British BK Laboratory Animal Co., LTD (Shanghai, China). Mice were injected with HCT-116 or HCT-15 tumor cells (5 × 106) at the right flank. When the tumor volume reached up to approximately 100 mm3, mice were randomly assigned into two groups. Shikonin was injected intraperitoneally three times per week, for two weeks. After therapy, tumor sizes were measured twice weekly with calipers. The volume was expressed in mm3 using the formula: Tumor Volume = 0.5 a × b2, where a and b are the long and short diameters of the tumor, respectively. In another study, six mice were randomly grouped into two groups, after three doses, the tumor tissues were collected to conduct western blot. Mice were monitored daily for general health, activity, and well-being. During the study, the care and use of animals were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of WuXi AppTec.
Statistical analysis
All experiments were performed in biologically independent triplicates. Results are presented as mean ± standard deviation (SD). Comparisons between different treatment groups were tested using one-way analysis of variance (ANOVA). A value of p < 0.05 was considered to be statistically significant. Double asterisks (**) indicate p < 0.01, triple asterisks (***) indicate p < 0.001.
Results
Shikonin inhibited the proliferation of colorectal cancer cells
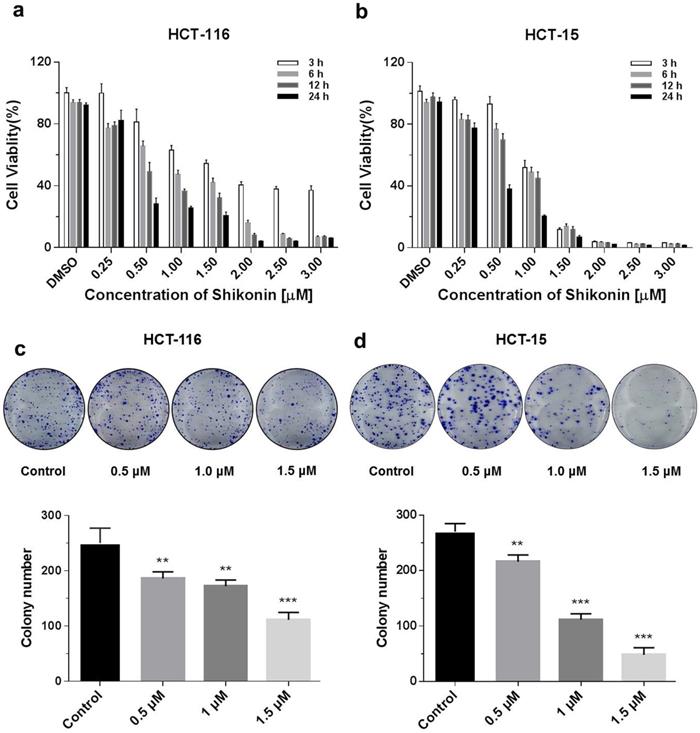
The cytotoxicity of shikonin on HCT-116 and HCT-15 colorectal cell lines was investigated using the Promega CellTiter-Glo luminescent cell viability assay kit. Colorectal cells were treated with different doses (0.25, 0.5, 1, 1.5, 2, 2.5 and 3 μM) of shikonin at various time points (3, 6, 12 and 24 h). Shikonin significantly inhibited the proliferation of colorectal cancer cells in a dose- and time-dependent manner (Figure 1a). The detailed statistic has been included in supplement 1 and supplement 2. HCT-15 cells were more sensitive to shikonin treatment compare with HCT-116. Moreover, cell colonies decreased at 10 days after treatment of shikonin (0.5 μM and higher doses), compared with the control group. Finally, a significant inhibition of colony formation was observed in the treatment of shikonin under the dosage of 1.5 μM (Figure 1b).
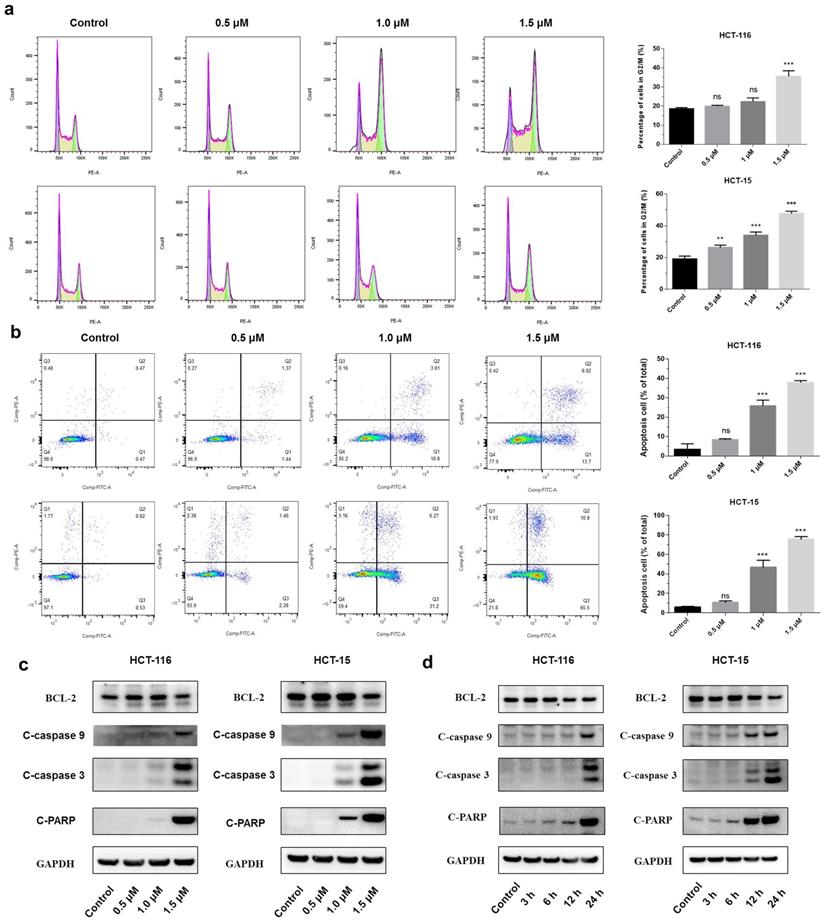
Shikonin induced apoptosis of colorectal cancer cells
A significant increase in the percentage of the G2/M phase was observed in both HCT-116 and HCT-15 cells after the treatment with shikonin (Figure 2a). The flow cytometry showed that shikonin induced apoptosis in a dose-dependent manner, with the percentages of cell apoptosis being 8.25%, 25.8%, 37.8%, and 10.62%, 46.80%, and 75.53% in HCT-116 and HCT-15 cells, respectively (Figure 2b). Western blot results showed that shikonin down regulated the expression level of anti-apoptotic Bcl-2, induced the cleavage of PARP and activated the caspase-3/9 (Figures 2c-d).
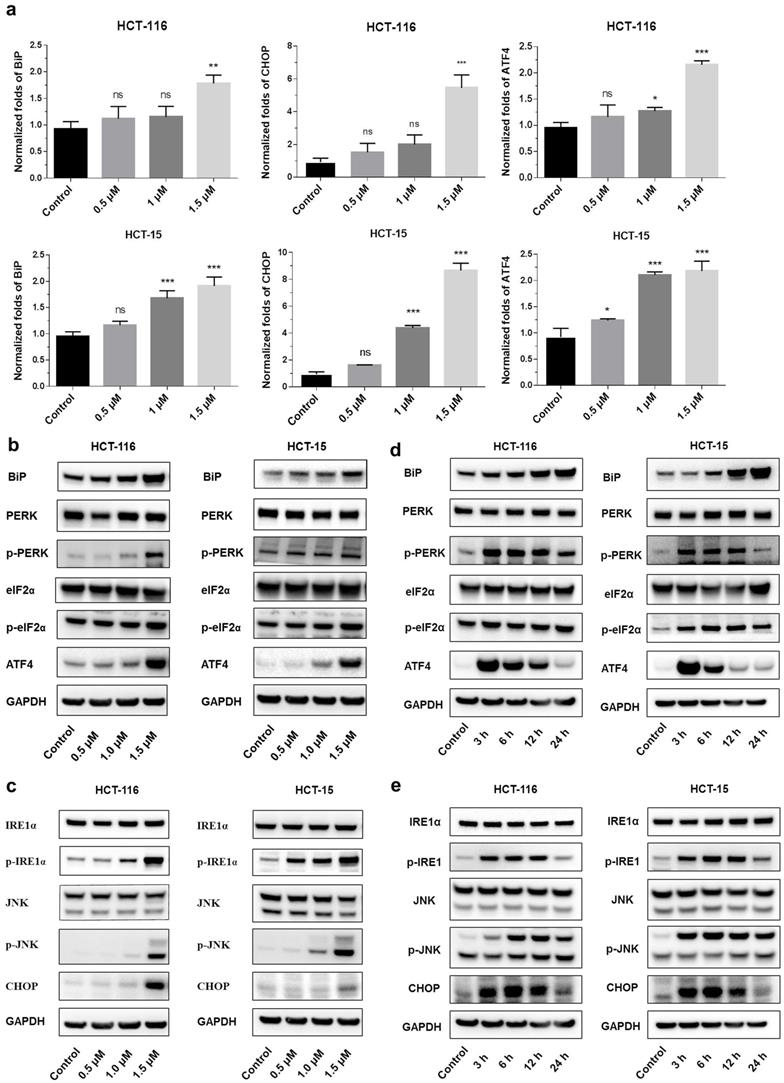
Shikonin induced endoplasmic reticulum stress
Endoplasmic reticulum stress (ER stress) occurs when proteins are not properly folded or conformed. Multiple studies have described the relation between ER stress and cancer [19, 20]. Consistent with this concept, we extracted RNA from shikonin treated HCT-116 and HCT15, examined the expression level of the principal genes involved in endoplasmic reticulum (ER) stress by conducting RT_PCR. Results showed that the expression of binding immunoglobulin protein (BiP), ATF4 and CHOP were significantly increased after shikonin treatment in HCT116 and HCT15 cells (Figures 3a-c). The phosphorylation pattern of protein kinase R-like ER kinase (P-ERK) pathway was also assessed. Western blot found that protein level of p-PERK, p-eIF2α and ATF4 increased in HCT116 and HCT15 cells after shikonin treatment (Figure 3b-c). Regarding the IRE1α pathway, shikonin treatment showed similar phosphorylation patterns in colorectal cancer cells. Shikonin significantly increased the protein level of p-IRE1α, p-JNK and CHOP in HCT116 and HCT15 cells (Figure 3d-e).
Shikonin inhibited the proliferation of colorectal cancer cells. (a) Shikonin inhibited colorectal cancer cell viability. HCT-116 and HCT-15 cells were treated with different doses (0.25, 0.5, 1, 1.5, 2, 2.5 and 3 µM) of shikonin at various time points (3, 6, 12 and 24 h). (b) Colony formation and number of colonies per well. DMSO was used as a negative control. Data are presented as mean ± SD of three independent experiments. Significance is indicated by * p < 0.05, ** p < 0.01 and *** p < 0.001 compared to the control group.
Shikonin induced reactive oxygen species-mediated ER stress
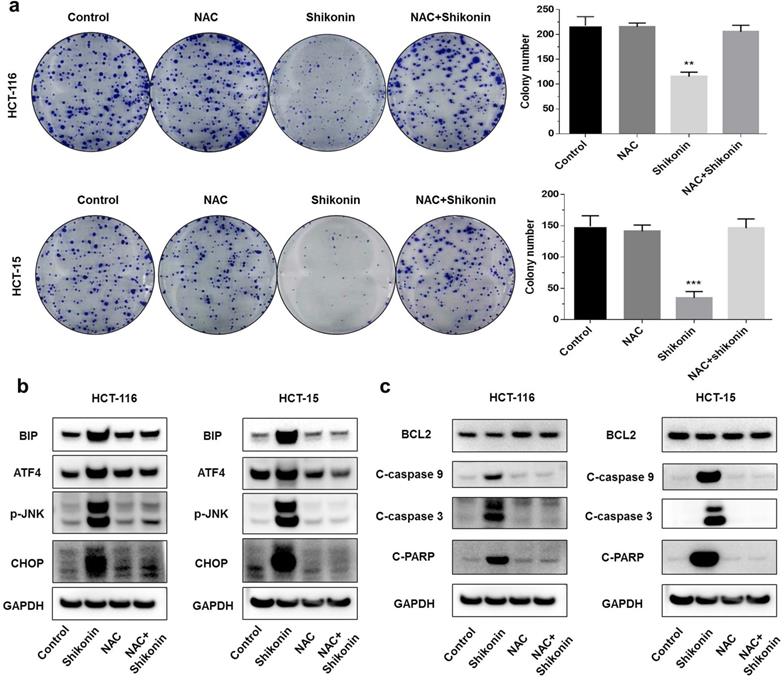
Shikonin is an inducer of reactive oxygen species [21, 22]. A previous study found that shikonin induced apoptosis through the production of reactive oxygen species. To investigate the pro-apoptotic mechanisms of shikonin, we pre-treated the colorectal cancer cells with N-acetyl cysteine (NAC) for 2 hours before treating with shikonin. The colony formation assay showed that the pre-treatment with NAC reduced the cytotoxic effects of shikonin significantly (Figure 4a). Pre-treatment with NAC also significantly suppressed the shikonin-induced expression of BiP, p-JNK, ATF4 and CHOP (Figure 4b). The protein level of cleaved- caspase-3/9 and cleaved-PARP were completely neutralized in the presence of NAC (Figure 4c).
Shikonin suppressed the growth of colorectal cancer cells in vivo
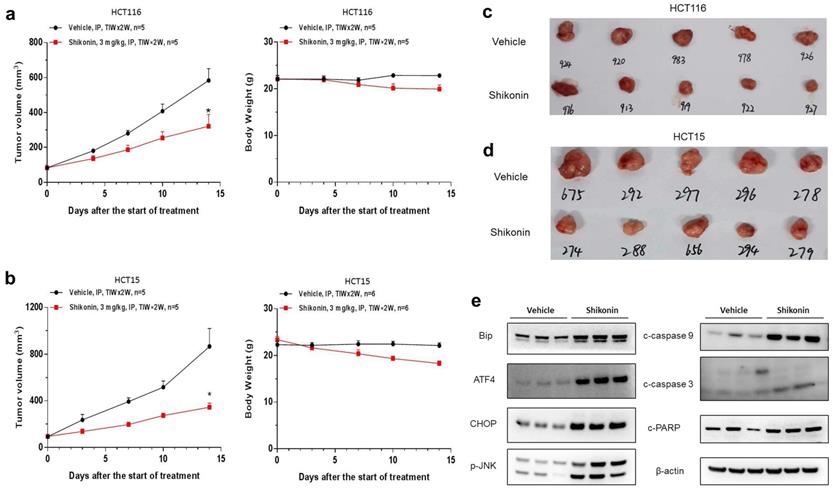
To investigate should shikonin have anticancer activity in vivo, HCT-116 and HCT-15 cells were implanted subcutaneously in BALB/c nude mice. Shikonin effectively inhibited tumor growth in vivo, with the tumor growth inhibition (TGI) rates of HCT-116 and HCT-15 cells being 52.3% and 67.8%, respectively (Figure 5a-d). Please note that this is the primary study, which means the dosing schedule and roots could be optimized to archive better efficacy. Furthermore, western blot was conducted to evaluate the protein levels in HCT-15 tumor tissues collected from anther in vivo study. We also found that the expression of BiP, ATF4, CHOP and p-JNK were significantly increased after shokonin treatment. Moreover, cleaved caspase-3/9 and cleaved PARP were also upregulated in the tumor tissues (Figure 5e). Taken together, these results showed that shikonin has a powerful antitumor effect in vivo.
Shikonin treatment induced apoptosis of colorectal cancer cells. (a-b) Cell cycle and cellular apoptosis were determined by flow cytometric analysis. HCT-116 and HCT-15 cells were treated with shikonin for 24 h. (c) Western blot was performed to evaluate the apoptosis-related proteins after shikonin treatment. Cells were treated with different doses of shikonin (0, 0.5, 1, and 1.5 µM) for 24 h. (d) Western blot was performed to evaluate the apoptosis-related proteins after shikonin treatment. Cells were treated with shikonin (1.5 µM) at different time points (3, 6, 12, and 24 h). Data are presented as mean ± SD of three independent experiments. Significance is indicated by * p < 0.05, ** p < 0.01 and *** p < 0.001 compared to the control group.
Shikonin induced apoptosis of colorectal cancer cells. (a) Gene expression levels of BiP, ATF4 and CHOP were analyzed by qRT-PCR. (b-c) Protein expression of PERK and IRE1α pathways after shikonin treatment. Cells were treated with different doses of shikonin (0, 0.5, 1, and 1.5 µM) for 24 h. (d-e) Protein expression of PERK and IRE1α pathways after shikonin treatment. Cells were treated with shikonin (1.5 µM) at different time points (0, 3, 6, 12, and 24 h). Data are presented as mean ± SD of three independent experiments. Significance is indicated by * p < 0.05, ** p < 0.01 and *** p < 0.001 compared to the control group.
Shikonin induced reactive oxygen species-mediated ER stress. (a) Colony formation and number of colonies per well. Cells were pre-treated with N-acetyl cysteine (NAC) for 2 h and then treated with shikonin (1.5 µM) for 10 days. (b-c) Western blot evaluated the apoptosis-related proteins and ER stress-related proteins when cells were pre-treated with NAC for 2 h and then treated with shikonin (1.5 µM) for 24 h. DMSO was used as a negative control. Data are presented as mean ± SD of three independent experiments. Significance is indicated by * p < 0.05, ** p < 0.01 and *** p < 0.001 compared to the control group.
Discussion
Shikonin is a major naphthoquinone compound found in the roots of Lithospermum erythrorhizon that exhibits powerful anticancer activities. Shikonin also exerts other activities, including antimicrobial, anti-inflammatory, and antioxidant [23, 24]. Most of the studies on shikonin were focused on cellular responses, while the effects of shikonin in vivo are largely unknown [25, 26]. In the current study, we found that shikonin induced apoptosis in colorectal cancer cells through activating ER stress and upregulate reactive oxygen species, antioxidants treatment could neutralize the induction in vitro.
Previous studies found that shikonin induce cell cycle arrest in G0/G1 phase and G2/M phase, thereby inhibiting cancer cell proliferation [27, 28]. In accordance, we demonstrated that shikonin induced G2/M phase arrest, down-regulated protein level of Bcl-2, along with increased protein level of cleaved-PARP and cleaved-caspase-3/9 suggesting that apoptosis was elevated in both HCT-116 and HCT-15 cells. Study has reported that Shikonin-induced apoptosis through the mitochondrial pathway, and generation of reactive oxygen species, the activation of c-Jun-N-terminal kinase (JNK), p38, caspase-3/9 and the cleavage of PARP [29].
Cancer cells grow continuously, increase reactive oxygen species and induce hypoxia, thus activating ER stress. ER stress in turn activates the unfolded protein response (UPR), that is both apoptotic and adaptive in tumor cells. The purpose of the UPR is to balance the ER folding environment under stress. If the stress is prolonged and the UPR fails to restore ER homeostasis, tumor cells will undergo apoptosis. It has been reported that shikonin induced ER stress-mediated apoptosis in melanoma cells via the production of reactive oxygen species and the up-regulation of p-eIF2α, CHOP and caspase-3 [17]. In colon cancer cells, the main components of shikonin-induced apoptosis were the generation of reactive oxygen species, the down-regulation of Bcl-2 and the activation of the caspase cascade [30]. In the present study, we found that shikonin induced the cell apoptosis of colorectal cancer cells by activating the ER stress through the PERK/elF2α/ATF4/CHOP and IRE1α/JNK signaling pathways, up-regulating the anti-apoptotic protein Bcl-2 and increasing the expression of caspase-3/9. There is a functional crosstalk between the production of reactive oxygen species, the mitochondrial apoptosis pathway and the ER-stress apoptosis pathway, which leads to cell death in colorectal cancer cells.
In our previous in vivo studies, oral administration of shikonin did not show antitumor activities in colorectal cancer xenograft models (supplement 3). Here, we found that shikonin effectively inhibited tumor growth through intraperitoneal injection (3 mg/kg, 3 times per week). It is possible that the anti-tumor activities of shikonin are more evident when injected intraperitoneally, however, PK analyze is required to compare differences of in vivo exposures and metabolites between oral and intraperitoneal injection. The new treatment schedule and new option of dosing roots should have great potential to improve efficacy of shikonin in xenograft mouse models.
ER stress and mitochondrial apoptotic pathway contribute to tumorigenesis in wide range of cancer types [31]. Shikonin can activate the apoptosis-related signal regulation network and reduce the expression of Bcl-2 by interfering the PI3K/AKT signaling pathway in afatinib-resistant non-small cell lung cancer cell lines H1650/R and H1975/R in-vitro [32]. However, these studies didn't conduct studies in xenograft models. Our study found that intraperitoneal injection gave better efficacy comparing with oral administration, therefore, intraperitoneal injection of shikonin should have potential to give promising efficacy against afatinib-resistant non-small cell lung cancer. Taken together, shikonin should have great potential in treatments of variety cancer types, and more studies need to be conducted in investigate the mechanism of action and druggability.
Shikonin suppressed the growth of colorectal cancer cells in vivo. HCT-116 and HCT-15 cells were implanted subcutaneously in BALB/c nude mice. The tumor-bearing mice were administered with shikonin (3 mg/kg) or vehicle via intraperitoneal injection for two weeks (n = 5 per group). (a-b) The tumor growth curve and the body weight change of HCT-116 and HCT-15 cells treated with shikonin. (c-d) The representative images of tumors from the implanted mice with HCT-116 and HCT-15 cells. (e) Western blot was performed to evaluate the protein level after shikonin treatment in HCT-15 xenograft model. Data are shown as mean ± SD. Significance is indicated by * p < 0.05 compared to the control group.
Supplementary Material
Supplementary figure and tables.
Acknowledgements
We would like to thank TopEdit (www.topeditsci.com) for English language editing of this manuscript.
Ethics approval and consent to participate
Experiments were performed according to the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of WuXi AppTec.
Funding
The present study was supported by the National Natural Science Foundation of China (No. 81503102), the “Xinlin Young Talent Program”.
Author Contributions
Yifu Y, Xuzhen Tang and Xiaoyu W designed the experiments. Hui Q, HuanHuan L, Meng H and Shulan Q performed the experiments and analyzed the data. Hui Q and Xing Z wrote the manuscript. All authors read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (London, England). 2019;394:1467-80
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians. 2016;66:115-32
3. Johdi NA, Sukor NF. Colorectal Cancer Immunotherapy: Options and Strategies. Frontiers in immunology. 2020;11:1624
4. Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. 2019;125:4139-47
5. Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH. et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nature reviews Gastroenterology & hepatology. 2019;16:361-75
6. Fulda S. Modulation of apoptosis by natural products for cancer therapy. Planta medica. 2010;76:1075-9
7. Wachtel-Galor S, Benzie IFF. Herbal Medicine: An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. Boca Raton (FL): CRC Press/Taylor & Francis Copyright © 2011 by Taylor and Francis Group, LLC. 2011
8. Guo C, He J, Song X, Tan L, Wang M, Jiang P. et al. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacological research. 2019;149:104463
9. Shahsavari Z, Karami-Tehrani F, Salami S. Targeting Cell Necroptosis and Apoptosis Induced by Shikonin via Receptor Interacting Protein Kinases in Estrogen Receptor Positive Breast Cancer Cell Line, MCF-7. Anti-cancer agents in medicinal chemistry. 2018;18:245-54
10. Zang F, Rao Y, Zhu X, Wu Z, Jiang H. Shikonin suppresses NEAT1 and Akt signaling in treating paclitaxel-resistant non-small cell of lung cancer. Molecular medicine (Cambridge, Mass). 2020;26:28
11. Hao Z, Qian J, Yang J. Shikonin induces apoptosis and inhibits migration of ovarian carcinoma cells by inhibiting the phosphorylation of Src and FAK. Oncology letters. 2015;9:629-33
12. Liu T, Li S, Wu L, Yu Q, Li J, Feng J. et al. Experimental Study of Hepatocellular Carcinoma Treatment by Shikonin Through Regulating PKM2. Journal of hepatocellular carcinoma. 2020;7:19-31
13. Wang L, Stadlbauer B, Lyu C, Buchner A, Pohla H. Shikonin enhances the antitumor effect of cabazitaxel in prostate cancer stem cells and reverses cabazitaxel resistance by inhibiting ABCG2 and ALDH3A1. American journal of cancer research. 2020;10:3784-800
14. Lee MJ, Kao SH, Hunag JE, Sheu GT, Yeh CW, Hseu YC. et al. Shikonin time-dependently induced necrosis or apoptosis in gastric cancer cells via generation of reactive oxygen species. Chemico-biological interactions. 2014;211:44-53
15. Ruan Z, Liang M, Shang L, Lai M, Deng X, Su X. Shikonin-mediated PD-L1 degradation suppresses immune evasion in pancreatic cancer by inhibiting NF-κB/STAT3 and NF-κB/CSN5 signaling pathways. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al]. 2021;21:630-41
16. Chen X, Cubillos-Ruiz JR. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nature reviews Cancer. 2021;21:71-88
17. Liu Y, Kang X, Niu G, He S, Zhang T, Bai Y. et al. Shikonin induces apoptosis and prosurvival autophagy in human melanoma A375 cells via ROS-mediated ER stress and p38 pathways. Artificial cells, nanomedicine, and biotechnology. 2019;47:626-35
18. Liu CY, Hsu CC, Huang TT, Lee CH, Chen JL, Yang SH. et al. ER stress-related ATF6 upregulates CIP2A and contributes to poor prognosis of colon cancer. Molecular oncology. 2018;12:1706-17
19. Sisinni L, Pietrafesa M, Lepore S, Maddalena F, Condelli V, Esposito F. et al. Endoplasmic Reticulum Stress and Unfolded Protein Response in Breast Cancer: The Balance between Apoptosis and Autophagy and Its Role in Drug Resistance. International journal of molecular sciences. 2019 20
20. Fu X, Cui J, Meng X, Jiang P, Zheng Q, Zhao W. et al. Endoplasmic reticulum stress, cell death and tumor: Association between endoplasmic reticulum stress and the apoptosis pathway in tumors (Review). Oncology reports. 2021;45:801-8
21. Chen Y, Zheng L, Liu J, Zhou Z, Cao X, Lv X. et al. Shikonin inhibits prostate cancer cells metastasis by reducing matrix metalloproteinase-2/-9 expression via AKT/mTOR and ROS/ERK1/2 pathways. International immunopharmacology. 2014;21:447-55
22. Zheng H, Huang Q, Huang S, Yang X, Zhu T, Wang W. et al. Senescence Inducer Shikonin ROS-Dependently Suppressed Lung Cancer Progression. Frontiers in pharmacology. 2018;9:519
23. Andújar I, Ríos JL, Giner RM, Recio MC. Pharmacological properties of shikonin - a review of literature since 2002. Planta medica. 2013;79:1685-97
24. Wang A, Liu J, Yang Y, Chen Z, Gao C, Wang Z. et al. Shikonin promotes ubiquitination and degradation of cIAP1/2-mediated apoptosis and necrosis in triple negative breast cancer cells. Chinese medicine. 2021;16:16
25. Tang JC, Zhao J, Long F, Chen JY, Mu B, Jiang Z. et al. Efficacy of Shikonin against Esophageal Cancer Cells and its possible mechanisms in vitro and in vivo. Journal of Cancer. 2018;9:32-40
26. Wang F, Yao X, Zhang Y, Tang J. Synthesis, biological function and evaluation of Shikonin in cancer therapy. Fitoterapia. 2019;134:329-39
27. Markowitsch SD, Juetter KM, Schupp P, Hauschulte K, Vakhrusheva O, Slade KS. et al. Shikonin Reduces Growth of Docetaxel-Resistant Prostate Cancer Cells Mainly through Necroptosis. Cancers. 2021 13
28. Zhai T, Hei Z, Ma Q, Liang H, Xu Y, Zhang Y. et al. Shikonin induces apoptosis and G0/G1 phase arrest of gallbladder cancer cells via the JNK signaling pathway. Oncology reports. 2017;38:3473-80
29. Alam MM, Kariya R, Boonnate P, Kawaguchi A, Okada S. Induction of apoptosis by Shikonin through ROS-mediated intrinsic and extrinsic apoptotic pathways in primary effusion lymphoma. Translational oncology. 2021;14:101006
30. Liang W, Cui J, Zhang K, Xi H, Cai A, Li J. et al. Shikonin induces ROS-based mitochondria-mediated apoptosis in colon cancer. Oncotarget. 2017;8:109094-106
31. Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants & redox signaling. 2014;21:396-413
32. Li B, Yuan Z, Jiang J, Rao Y. Anti-tumor activity of Shikonin against afatinib resistant non-small cell lung cancer via negative regulation of PI3K/Akt signaling pathway. Bioscience reports. 2018 38
Author contact
![]() Corresponding authors: Yifu Yang, Experiment Center for Science and Technology, Shanghai University of Traditional Chinese Medicine, 1200 Cailun Road, Shanghai, 201203, P.R. China. E‑mail: yangyifushcnc.ac.cn; Xiaoyu Wang, Experiment Center for Science and Technology, Shanghai University of Traditional Chinese Medicine, 1200 Cailun Road, Shanghai, 201203, P.R. China. E‑mail: wangdxiaoyucom.
Corresponding authors: Yifu Yang, Experiment Center for Science and Technology, Shanghai University of Traditional Chinese Medicine, 1200 Cailun Road, Shanghai, 201203, P.R. China. E‑mail: yangyifushcnc.ac.cn; Xiaoyu Wang, Experiment Center for Science and Technology, Shanghai University of Traditional Chinese Medicine, 1200 Cailun Road, Shanghai, 201203, P.R. China. E‑mail: wangdxiaoyucom.

 Global reach, higher impact
Global reach, higher impact