Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(10):2866-2876. doi:10.7150/jca.54408 This issue Cite
Research Paper
A Novel Immunotype-based Risk Stratification Model Predicts Postoperative Prognosis and Adjuvant TACE Benefit in Chinese Patients with Hepatocellular Carcinoma
1. Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
2. Department of General Surgery, Qilu Hospital, Shandong University, Jinan 250012, China.
3. Department of General Surgery, Huashan Hospital & Cancer Metastasis Institute, Fudan University, Shanghai 200040, China.
4. Institute of Advanced Surgical Technology and Engineering, First Affiliated Hospital of Medical College, Xi'an Jiaotong University, Xi'an 710061, China.
* These authors contributed equally to this work.
Received 2020-10-12; Accepted 2021-2-28; Published 2021-3-15
Abstract
Background and Aims: The tumor microenvironment can be divided into inflamed, immune-excluded and immune-desert phenotypes according to CD8+ T cell categories with differential programmed cell death protein 1 (PD-L1) expression. The study aims to construct a novel immunotype-based risk stratification model to predict postsurgical survival and adjuvant trans-arterial chemoembolization (TACE) response in patients with hepatocellular carcinoma (HCC).
Methods: A total of 220 eligible HCC patients participated in this study. CD8+ T cell infiltration and PD-L1 expression mode were estimated by immunohistochemical staining. A risk stratification model was developed and virtualized by a nomogram that integrated these independent prognostic factors. The postoperative prognosis and adjuvant TACE benefits were evaluated with a novel immunotype-based risk stratification model.
Results: A total of 220 patients were finally identified. Immune-desert, immune-excluded, and inflamed immunotypes represented 45%, 24%, and 31% of HCC, respectively. Univariate and multivariate analyses identified immunotype and PD-L1 expression mode as independent prognostic factors for overall survival time (OS) and recurrence-free survival time (RFS). The nomogram was constructed by integrating immunotype, PD-L1 expression, Barcelona Clinic Liver Cancer (BCLC) stage and tumor grade. The C-index was 0.794 in the training cohort and 0.813 in the validation cohort. A risk stratification system was constructed based on the nomogram classifying HCC patients into 3 risk groups. The average OS times in the low-risk, intermediate-risk and high-risk groups in all cohorts were 77.1 months (95% CI 71.4-82.9), 53.7 months (95% CI 48.2-59.2), and 25.6 months (95% CI 21.4-29.7), respectively. Further analysis showed that OS was significantly improved by adjuvant TACE in the low- and intermediate-risk groups (P=0.041 and P=0.010, respectively) but not in the high-risk group (P=0.398).
Conclusion: A novel immunotype-based risk stratification model was built to predict postoperative prognosis and adjuvant TACE benefit in HCC patients. These tools can assist in building a more customized method of HCC treatment.
Keywords: Hepatocellular carcinoma, adjuvant TACE, tumor microenvironment, immunotype, prognosis.
Introduction
As the fifth most prevalent cancer and the third most lethal cancer, HCC causes 700,000 deaths each year [1-4]. Moreover, HCC is more prevalent in East Asia, Southeast Asia and sub-Saharan Africa [4]. Despite the development of surgical techniques, HCC still shows a poor prognosis, mainly attributable to the high rate of metastasis and relapse after curative treatments [5, 6]. Therefore, the prevention of relapse is one of the toughest challenges to improve surgical efficacy. Currently, TACE is a major adjuvant management to prevent relapse of HCC and prolong the survival time of patients after hepatectomy [7]. Although some clinical studies have reported that HCC patients with large tumors, portal vein tumor thrombus or microvascular invasion benefit from TACE, the role of postoperative adjuvant TACE has not yet been fully established [8, 9]. In particular, controversial results reported by several previous studies suggest the need to set a more specific subtype classification and markers to select suitable patients for TACE in addition to the traditional clinical risk factors for recurrence [10, 11].
Recent studies have identified the adaptive or exhausted immune-specific subtypes of HCC on the basis of molecular features [12]. However, limited evidence was provided in evaluating prognosis or chemotherapy-related benefits. Multitudinous prognostic signatures have been developed to stratify patients with HCC [13, 14]; however, they are mainly concentrated on malignant cells and do not represent stromal cells and the immune microenvironment. Previous studies have shown that the immune response plays a key role in chemotherapy-induced cytotoxicity, which may indicate the patients' response to chemotherapy [15-19]. In muscle-invasive bladder cancer, the stromal immunotype has been demonstrated to predict postsurgical survival and adjuvant platinum-based chemotherapy after surgery[15]. Anticancer immunity can be segregated into three main phenotypes: immune-desert, immune-excluded and inflamed phenotypes [20-22]. More importantly, collective clinical studies suggest that checkpoint immunotherapy is the most effective in inflamed tumors, as characterized by a high density of IFN-γ-producing CD8+ T cells and the expression of PD-L1 in tumors [23-26]. However, the prognostic value and precise function of these three immunotypes in HCC are not quite clear and need to be further investigated.
Therefore, in the present study, we classified HCC patients into three distinctive subtypes of immune desert, immune excluded, or inflamed immunotype and into two PD-L1 expressed modes, and we verified that immunotype and PD-L1 expression were independent prognostic factors. Integrating immunotype, PD-L1 expression and other clinicopathologic features, we constructed a novel risk stratification model, aiming to predict the impact of immunotype on postoperative survival and the benefits of adjuvant TACE therapy. These results may unravel the important role of immune status in HCC and provide a predictive system that could possibly evaluate outcomes for patients who received adjuvant TACE.
Materials and Methods
Patients and procedures
This retrospective study was based on clinical data collected from patients diagnosed with HCC for the first time who underwent curative resections at the Department of General Surgery, Huashan Hospital, Fudan University, from January 2012 to December 2015.
The inclusion criteria for patients enrolled in the study were as follows: (1) tumor tissue was histopathologically confirmed as HCC; (2) age >18 and ≤75; and (3) Child-Pugh was classified as A or B by evaluation. Patients were excluded when they met the following criteria: (1) received any preoperative radiotherapy and/or chemotherapy; (2) had recurrent liver cancer or were diagnosed with other cancers; (3) had refractory ascites or severe portal hypertension; and (4) had organ dysfunction. All specimens were pathologically reassessed by two pathologists independently. Human tissues were used with patients' informed consent and the approval of the Clinical Research Ethics Committee of the authors' institution.
A total of 220 HCC patients who underwent curative resections were included in the study. Patients undergoing surgical resection were recommended to undergo adjuvant TACE one or two months after surgery if they encountered any of the following circumstances: having a single tumor with microvascular invasion (MVI), having major vascular invasion, having a single tumor larger than 5 cm or having two or three tumors [27].
TACE was conducted with the Seldinger technique. First, the arterial angiographic catheter was inserted into an appropriate hepatic artery through the femoral artery, and artery angiography was then carried out to show the real artery anatomy. A mixed emulsion of lipiodol, doxorubicin hydrochloride, pirarubicin, or pharmorubicin was slowly infused into the remnant liver by the catheter.
Specimens and tissue microarray (TMA)
Clinical and follow-up information was analyzed for all patients. OS was calculated from the date of operation to death or the last follow-up. RFS was calculated from the date of resection to the date of tumor recurrence, death or the last follow-up.
Formalin-fixed and paraffin-embedded tumor tissues were employed to build TMAs (in collaboration with Shanghai Biochip Company Ltd, Shanghai, China) as previously described [28]. Briefly, to make the TMA, two 4 μm diameter core biopsies from the center and lateral of each donor block were taken. Then, they were transferred to the recipient paraffin block at predefined array positions. In total, 220 pairs of TMA blocks were constructed in this study.
Immunohistochemistry and evaluation of immunostaining
Immunohistochemical (IHC) staining was carried out as described previously [29]. In short, TMAs were deparaffinized and rehydrated, and then the antigen of the tissue was retrieved. Then, the TMAs were incubated with CD8 (BD Pharmingen, 550372, diluted at 1:50) and PD-L1 (Abcam, ab205921, 1:30) primary antibodies at 4°C for 12 hours. After that, the secondary antibody (Dako Denmark A/S, Glostrup, Denmark) was applied and incubated at 37°C for 30 minutes. Diaminobenzidine (DAB) and hematoxylin were used to perform the staining and counterstaining. Two independent pathologists evaluated all specimens.
Three major T cell categories (inflamed, immune excluded, and immune desert) were defined on the basis of the CD8+ T cell infiltration pattern at the tumor core and stroma. “Immune desert” was characterized as no significant CD8+ T cell infiltration into the tumor core and stroma. CD8+ T cell infiltration limited to the invasive margin only was referred to as “immune-excluded”. “Inflamed” immunotype represented diffuse intratumoral CD8+ T cell infiltrate. PD-L1+ tumor tissues were defined as PD-L1+ tumor cells over 5%.
Microvascular invasion (MVI) is a nesting mass of cancer cells observed by microscopy in the lumen of endothelium-lined vasculature, mainly in the branches of portal veins (including intraperitoneal vessels). All the tumor tissues from the HCC patients were pathologically examined by pathologists, and MVI was provided on the pathological report.
Nomogram construction and validation
A nomogram was constructed based on the data of the multivariable model using R 3.4.3. The 3-year and 5-year OS rates were used to develop a nomogram. C-indexes were calculated to calculate the predictive accuracy. One thousand bootstrap resamples were performed to determine the C-indexes and create calibration plots. Moreover, the patients were divided into three prognostic groups by the risk stratification model depending on each patient's total nomogram score.
Statistical analysis
All data were expressed as the means ± SEM. Statistical analyses were executed with the aid of SPSS 22.0 (IBM, Armonk, USA) and GraphPad Prism 7 statistical software. Comparisons of categorical and continuous variables were performed with the χ2 test or Fisher's exact test. Survival curves were calculated by Kaplan-Meier analysis and compared with the log-rank test. Univariate analysis and Cox regression multivariate analysis were performed to evaluate the independent predictive variables for survival. Differences were defined as significant with two-sided P<0.05.
Baseline clinicopathological characteristics of HCC patients.
| Variables | Training cohort (N=105) | Validation cohort (N=115) |
|---|---|---|
| Sex | ||
| Male | 94 (89.5%) | 99 (86.1%) |
| Female | 11 (10.5%) | 16 (13.9%) |
| Age | ||
| <50 | 33 (31.4%) | 47 (40.9%) |
| ≥50 | 72 (68.6%) | 68 (59.1%) |
| HBV infection | ||
| No | 8 (7.6%) | 11 (9.6%) |
| Yes | 97 (92.4%) | 104 (90.4%) |
| Cirrhosis | ||
| No | 24 (22.9%) | 26 (22.6%) |
| Yes | 81 (77.1%) | 89 (77.4%) |
| ALT (U/L) | ||
| <40 | 48 (45.7%) | 51 (44.3%) |
| ≥40 | 57 (54.3%) | 64 (55.7%) |
| AFP (μg/L) | ||
| <20 | 35 (33.3%) | 41 (35.7%) |
| ≥20 | 70 (66.7%) | 74 (64.3%) |
| Grade | ||
| G 1-2 | 59 (56.2%) | 84 (73.0%) |
| G 3-4 | 46 (43.8%) | 31 (27.0%) |
| Vascular invasion | ||
| No | 61 (58.1%) | 69 (60.0%) |
| MVI | 34 (32.4%) | 34 (29.6%) |
| Major vascular invasion | 10 (9.5%) | 12 (10.4%) |
| Immunotype | ||
| Inflamed | 25 (23.8%) | 34 (29.6%) |
| Immune-excluded | 33 (31.4%) | 36 (31.3%) |
| Immune-desert | 47 (44.8%) | 45 (39.1%) |
| PD-L1 expression | ||
| High | 25 (23.8%) | 40 (34.8%) |
| Low | 80 (76.2%) | 75 (65.2%) |
| TNM stage | ||
| I | 43 (41.0%) | 58 (50.4%) |
| II | 35 (33.3%) | 26 (22.6%) |
| III | 20 (19.0%) | 20 (17.4%) |
| IV | 7 (6.7%) | 11 (9.6%) |
| BCLC stage | ||
| 0-A | 26 (24.8%) | 30 (26.1%) |
| B | 32 (30.5%) | 32 (27.8%) |
| C | 47 (44.8%) | 53 (46.1%) |
| TACE | ||
| Yes | 53 (50.5%) | 58 (50.4%) |
| No | 52 (49.5%) | 57 (49.6%) |
AFP, alpha fetoprotein; ALT, alanine aminotransferase; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; MVI, microvascular invasion; PD-L1, programmed cell death ligand 1;; TACE, trans-arterial chemoembolization.
Patient selection flowchart.
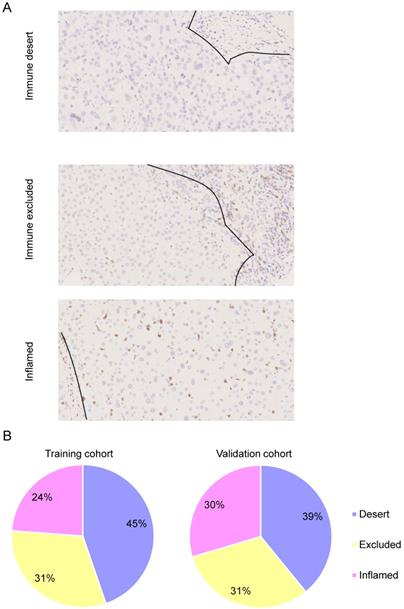
Immunotype classification in HCC. Representative photos of immunohistochemistry staining of CD8+ T cells. HCC is characterized as “immune desert” when CD8+ T cell infiltration is absent, “immune excluded” when CD8+ T cell infiltration is at the invasive margin only, and “inflamed immunotype” when CD8+ T cells infiltrate among tumor cells (A). Scale bar: 50 µm. Relative rates of different immunotypes in the training cohort and validation cohort (B).
Results
Baseline clinicopathological characters
The selection of patients was conducted in accordance with the procedure in Figure 1. In total, two hundred and twenty patients were included: 105 patients and 115 patients were included in the training and verification cohorts. First, the baseline clinicopathological features of each subgroup were analyzed (Table 1). In the training cohort, 89.5% (94/105) were male patients, 68.6% (72/105) of patients were aged ≥50, 92.4% (97/105) were affected with HBV, and 77.1% (81/105) suffered from liver cirrhosis. Patients with AFP ≥20 μg/L accounted for 66.7% (70/105), and those with ALT ≥40 U/L accounted for 54.3% (57/105). In addition, 56.2% (59/105) and 43.8% (46/105) of the patients had Grade 1-2 and Grade 3-4 disease, respectively. In addition, 32.4% (34/105) and 9.5% (10/105) of the patients had HCC with MVI or major vascular invasion, respectively. Moreover, the proportions of patients bearing TNM (AJCC Cancer Staging Manual, Eighth Edition Version) I, II, III and IV tumors were 41.0% (43/105), 33.3% (35/105), 19% (20/105) and 6.7% (7/105), respectively, and the proportions of patients bearing BCLC stage 0-A, B and C tumors were 24.8% (26/105), 30.5% (32/105) and 44.8% (47/105), respectively. Furthermore, 50.5% (53/105) of the patients received TACE therapy postoperatively. Among these 220 patients, 170 patients were recommended adjuvant TACE, and 111 patients received adjuvant TACE after surgical resection.
Immunotype classification in HCC
To assess the categories of CD8+ T cell infiltration within HCC and the distribution of intratumoral CD8+ T cells in the tumor core and stroma, we performed IHC staining of CD8 in TMAs. Each specimen was categorized on the basis of the distribution of CD8+ T cells in relation to the tumor core and stroma. Representative immunohistochemistry images of three major CD8+ T cell categories are shown in Figure 2A. Among these, 45% had no significant CD8+ T cell infiltrate, which was referred to as the “immune desert” (Figure 2B). Thirty-one percent had CD8+ T cell infiltration limited to the invasive margin only, referred to as “immune-excluded” (Figure 2B). Only 24% had diffuse intratumoral CD8+ T cell infiltrate, named the “inflamed” immunotype (Figure 2B). The relationship between immunotype and clinicopathological parameters is shown in Table 2. Immunotype was found to be significantly correlated with HCC grade (P=0.024) but not with other clinicopathological features.
Relationship between clinicopathological features and immunotypes in the training cohort.
| Variables | Immunotype | P | ||
|---|---|---|---|---|
| Inflamed (N=25) | Immune-excluded (N=33) | Immune-desert (N=47) | ||
| Sex | 0.839 | |||
| Male | 22 (88.0%) | 29 (87.9%) | 43 (91.5%) | |
| Female | 3 (12.0%) | 4 (12.1%) | 4 (8.5%) | |
| Age | 0.852 | |||
| <50 | 9 (36.0%) | 10 (30.3%) | 14 (29.8%) | |
| ≥50 | 16 (64.0%) | 23 (69.7%) | 33 (70.2%) | |
| HBV infection | 0.733 | |||
| Yes | 24 (96.0%) | 30 (90.9%) | 43 (91.5%) | |
| No | 1 (4.0%) | 3 (9.1%) | 4 (8.5%) | |
| Cirrhosis | 0.413 | |||
| No | 4 (16.0%) | 10 (30.3%) | 10 (21.3%) | |
| Yes | 21 (84.0%) | 23 (69.7%) | 37 (78.7%) | |
| ALT (U/L) | 0.813 | |||
| <40 | 10 (40.0%) | 16 (48.5%) | 22 (46.8%) | |
| ≥40 | 15 (60.0%) | 17 (51.5%) | 25 (53.2%) | |
| AFP (μg/L) | 0.712 | |||
| <20 | 10 (40.0%) | 10 (30.3%) | 15 (31.9%) | |
| ≥20 | 15 (60.0%) | 23 (69.7%) | 32 (68.1%) | |
| Grade | 0.024 | |||
| G 1-2 | 12 (48.0%) | 25 (75.8%) | 22 (46.8%) | |
| G 3-4 | 13 (52.0%) | 8 (24.2%) | 25 (52.2%) | |
| Vascular invasion | 0.325 | |||
| No | 19 (76.0%) | 18 (54.5%) | 24 (51.1%) | |
| MVI | 5 (20.0%) | 11 (33.3%) | 18 (38.3%) | |
| Major vascular invasion | 1 (4.0%) | 4 (12.1%) | 5 (10.6%) | |
| TNM stage | 0.820 | |||
| I | 11 (44.0%) | 15 (45.5%) | 17 (45.5%) | |
| II | 11 (44.0%) | 8 (24.2%) | 16 (44.0%) | |
| III | 3 (12%) | 7 (21.2%) | 10 (8.0%) | |
| IV | 0 (0%) | 3 (9.1%) | 4 (4.0%) | |
| BCLC stage | 0.061 | |||
| 0-A | 6 (24.0%) | 10 (30.3%) | 10 (21.3%) | |
| B | 13 (52.0%) | 7 (21.2%) | 12 (25.5%) | |
| C | 6 (24.0%) | 16 (48.5%) | 25 (53.2%) | |
AFP, alpha fetoprotein; ALT, alanine aminotransferase; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; MVI, microvascular invasion.
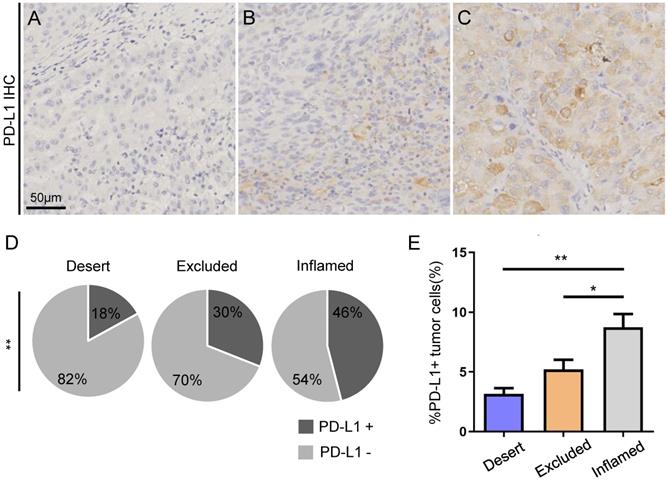
Moreover, PD-L1 staining by IHC was performed in TMAs. PD-L1+ tumor tissues were defined as PD-L1+ tumor cells over 5%. Representative immunohistochemistry images of the major PD-L1 expression modes are shown in Figure 3A-C. The ratio of PD-L1+ tumor cells was found to be significantly higher in HCC patients with inflamed immunotypes than in those with immune-desert and immune-excluded immunotypes (Figure 3D). Furthermore, patients with inflamed immunotypes expressed significantly higher PD-L1 than those with immune-desert and immune-excluded immunotypes (Figure 3E).
Impact of immunotype on OS and RFS
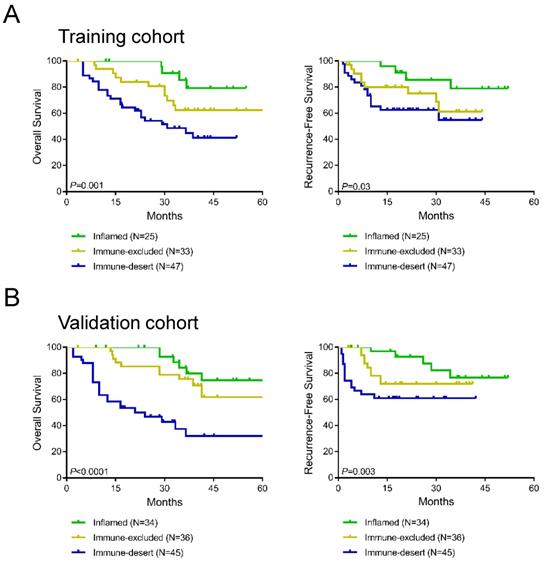
To further evaluate the prognostic value of immune-desert, immune-excluded, and inflamed immunotypes, Kaplan-Meier analysis was performed to compare OS and RFS between the three immunotype subgroups. In the training cohort, a significant difference was found in OS among all three immunotypes (P=0.001, Figure 4A), and RFS differed significantly among all three immunotypes (P=0.03, Figure 4A). Patients with inflamed immunotypes showed the best prognosis and lowest relapse rate, while patients with immune-desert immunotypes showed the poorest prognosis and highest relapse rate. Additionally, the validation cohort showed consistent results with the training cohort (P<0.0001 for OS and P=0.003 for RFS; Figure 4B).
Univariate and Multivariate Regression Analyses
The Cox hazards model was carried out in the training cohort to exhibit the predictive variables for survival. Univariate analysis showed that clinical factors such as tumor grade (P=0.001), BCLC stage (P<0.001), immunotype (P=0.001) and PD-L1 expression (P=0.002) were associated with overall survival time (Table 3). Multivariate Cox regression analysis further showed that tumor grade (G 3-4: HR 2.160, 95% CI 1.085-4.292; G 1-2 as reference), BCLC stage (B stage: HR 1.845, 95% CI 1.104-4.237; C stage: HR 3.846, 95% CI 1.304-11.363; 0-A stage as reference), immunotype (immune-excluded immunotype: HR 2.365, 95% CI 1.734-7.616; immune-desert immunotype: HR 4.655, 95% CI 1.600-13.540; inflamed immunotype as reference) and PD-L1 expression (PD-L1 high expression: HR 2.146, 95% CI 1.067-4.310; PD-L1 low expression as reference) were independent predictive variables for OS (Table 3).
In addition, univariate analysis and multivariate Cox regression analysis were executed to identify the independent predictive variable for RFS (Supplementary Table 1). Tumor grade (G 3-4: HR 4.566, 95% CI 1.832-11.364; G 1-2 as reference), BCLC stage (B stage: HR 5.051, 95% CI 1.621-15.625; C stage: HR 7.692, 95% CI 1.761-33.333; 0-A stage as reference), immunotype (immune-excluded immunotype: HR 3.313, 95% CI 1.133-11.757; immune-desert immunotype: HR 4.330, 95% CI 1.324-14.159; inflamed immunotype as reference) and PD-L1 expression (high expression: HR 6.667, 95% CI 2.660-16.667; low expression as reference) were found to be independent predictive variables for RFS.
Collectively, these results further proved that immunotype and PD-L1 expression were independent prognostic factors for liver cancer. Furthermore, all the independent predictive prognostic factors were included for nomogram construction.
Higher expression of PD-L1 by tumor cells in HCC with inflamed immunotype. Representative photos of immunohistochemistry staining of PD-L1 (A-C). Example of tumors with no expression of PD-L1 (%PD-L1+ tumor cells <1%) (A), weak expression (%PD-L1+ tumor cells ≥1% and <5%) (B), and strong expression (%PD-L1+ tumor cells ≥ 5%) (C). Scale bar: 50 µm. Relative rates of PD-L1+ and PD-L1- expression in the patients with different immunotypes in all cohorts (D). PD-L1 expression by tumor cells in HCC with different immunotypes (E).
Kaplan-Meier analysis of the impact of immunotypes on prognosis in the training cohort (A) and validation cohort (B).
Univariate and multivariate analyses for overall survival in the training cohort.
| Variables | Univariable analysis P | Multivariable analysis | |
|---|---|---|---|
| Hazard ratio (95% CI) | P | ||
| Immunotype | 0.001 | 0.002 | |
| Inflamed | Reference | ||
| Immune-excluded | 2.365 (1.734-7.616) | 0.015 | |
| Immune-desert | 4.655 (1.600-13.540) | 0.008 | |
| PD-L1 expression | 0.002 | 0.029 | |
| Low | Reference | ||
| High | 2.146 (1.067-4.310) | ||
| Sex | 0.392 | ||
| Male | |||
| Female | |||
| Age | 0.540 | ||
| <50 | |||
| ≥50 | |||
| HBV infection | 0.147 | ||
| Yes | |||
| No | |||
| Cirrhosis | 0.939 | ||
| Yes | |||
| No | |||
| ALT (U/L) | 0.631 | ||
| <40 | |||
| ≥40 | |||
| AFP (μg/L) | 0.141 | ||
| <20 | |||
| ≥20 | |||
| Grade | 0.001 | 0.028 | |
| G 1-2 | Reference | ||
| G 3-4 | 2.160 (1.085-4.292) | ||
| BCLC stage | <0.001 | 0.007 | |
| 0-A | Reference | ||
| B | 1.845 (1.104-4.237) | 0.032 | |
| C | 3.846 (1.304-11.363) | 0.015 | |
HBV, hepatitis B virus; ALT, alanine aminotransferase; AFP, alpha fetoprotein; BCLC, Barcelona Clinic Liver Cancer.
Nomogram scores of clinical variables in each subgroup.
| Variables | Points |
|---|---|
| Immunotype | |
| Inflamed | 0 |
| Immune-excluded | 56 |
| Immune-desert | 100 |
| PD-L1 | |
| Low | 0 |
| High | 50 |
| Grade | |
| G 1-2 | 0 |
| G 3-4 | 50 |
| BCLC stage | |
| 0-A | 0 |
| B | 47 |
| C | 87 |
Nomogram construction and validation
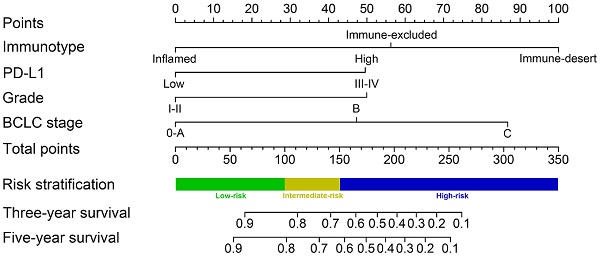
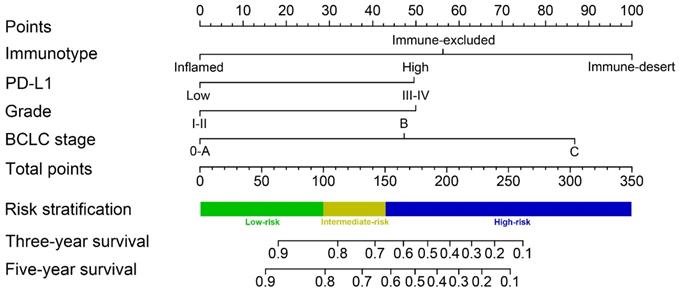
A predictive nomogram was constructed by integrating 3 independent risk factors for survival prognosis (Figure 5). Each clinical variable was assigned a score: immunotype (inflamed immunotype scored 0, immune-excluded immunotype scored 56 and immune-desert immunotype scored 100), PD-L1 (PD-L1 low expression scored 0 and PD-L1 high expression scored 50), tumor grade (G 1-2 scored 0 and G 3-4 scored 50) and BCLC stage (0-A stage scored 0, B stage scored 47 and C stage scored 87) (Table 4). Furthermore, the total scores were calculated by adding all scores of the included variables. The probability of 3-year and 5-year survival rates were estimated based on the nomogram model.
The C-indexes of the nomogram in the training cohort (0.794, 95% CI 0.732-0.855) and validation cohort (0.813, 95% CI 0.751-0.875) suggested the excellent discrimination ability of the model. In addition, the calibration curves also demonstrated consistency between the predicted and actual 3- and 5-year survival rates in the training cohort (Supplementary Figure 1A). The calibration curves of the validation cohort showed good consistency as well (Supplementary Figure 1B).
Risk stratification model development
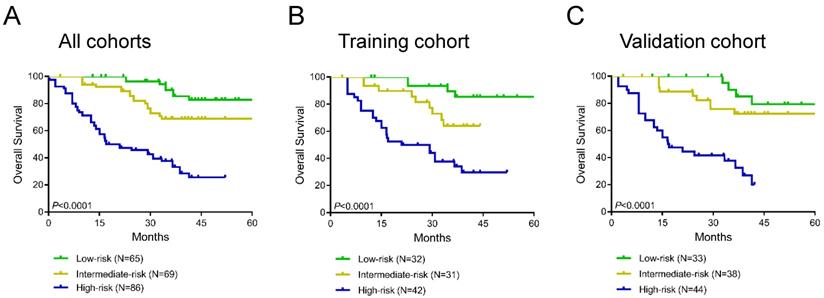
Additionally, we constructed a novel risk stratification model by determining total scores from the nomogram, which separated the patients into three groups with different prognoses. We divided patients into the low-risk group (65/220, total score <100), the intermediate-risk group (69/220, total score 100-150) and the high-risk group (86/220, total score ≥150) on the basis of this model. The Kaplan-Meier survival analysis exhibited the excellent accuracy of this model in predicting prognosis among the 3 risk groups in the training cohort, validation cohort, and all cohorts (Figure 6A-C). In all cohorts, the average OS times in the low-risk group, intermediate-risk group and high-risk group were 77.1 months (95% CI 71.4-82.9), 53.7 months (95% CI 48.2-59.2), and 25.6 months (95% CI 21.4-29.7), respectively.
Survival benefit of adjuvant TACE in groups of stratified risk
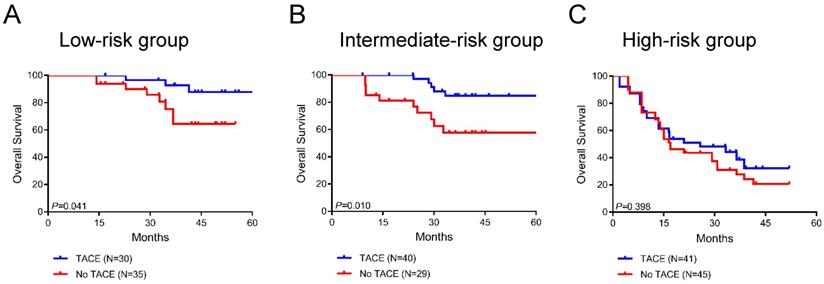
Furthermore, we analyzed the survival benefit from adjuvant TACE in three stratified risk groups. Interestingly, in the low-risk and intermediate-risk groups, postoperative adjuvant TACE significantly prolonged overall survival compared to untreated patients (P=0.041 and P=0.010, respectively; Figure 7A-B). In the low-risk group, the average overall survival times in the TACE and non-TACE groups were 76.6 months (95% CI 70.0-83.2) and 45.9 months (95% CI 40.7-51.2), respectively. In the intermediate-risk group, the average overall survival times in the TACE and non-TACE groups were 61.7 months (95% CI 56.7-66.6) and 58.5 months (95% CI 45.2-71.8), respectively. However, the overall survival of patients in the high-risk group did not show a significant difference between the TACE group and the non-TACE group (P=0.398, Figure 7C). In addition, adjuvant TACE significantly enhanced OS in the inflamed and immune-excluded groups (P=0.023 and P=0.003, respectively, Supplementary Figure 2A-B) but not in the immune-desert group (P=0.771, Supplementary Figure 2C). Taken together, these results indicated that the novel immunotype-based risk stratification model could successfully predict adjuvant TACE benefit in HCC patients after surgery.
Immunotype-based nomogram for predicting 3- and 5-year overall survival in HCC patients after surgery.
Kaplan curves of low-, intermediate- and high-risk groups in all cohorts (A), the training cohort (B) and the validation cohort (C).
Survival benefit of postoperative TACE in the low-risk (A), intermediate-risk (B) and high-risk (C) groups.
Discussion
In the present study, CD8+ T cell categories and PD-L1 expression mode were ascertained as two independent predictive factors for prognosis. To precisely predict prognosis in HCC patients, we developed a nomogram incorporating immunotype, PD-L1 expression, BCLC stage and tumor grade. The accurate discrimination of the nomogram was explicated with C-index and calibration curves. This novel stratification model was developed on the basis of total scores calculated by the nomogram and was used to analyze the postoperative overall survival and to evaluate the survival benefit of adjuvant TACE in stratified risk groups.
HCC is a complex, heterogeneous disease with a widely distinct prognosis [30, 31]. Currently, several prognostic models for patients with HCC rely on tumor size, tumor number, vascular invasion, differentiation, tumor-node-metastasis (TNM) stage and BCLC stage, which are derived from tumor-cell centered biological phenotypes [1, 32]. In fact, accumulative studies have found that various infiltrated immune cells are important prognostic factors for HCC [33-38]. Obviously, traditional prognostic models cannot reflect all the biological features of cancer because they neglect the influence of the immunocyte or immune microenvironment. CD8+ T cells are the main effector immune cells infiltrating the immune microenvironment. PD-L1 is one of the most important factors inducing T cell exhaustion and immune escape in HCC tumor cells [39]. Accumulating evidence indicates that CD8 and PD-L1 can reflect the immune status of the tumor environment, and oncologists tend to use these two indicators to screen candidate patients for PD-L1/PD-1 blockade therapies [26, 40]. Concerning the clinical application, we chose CD8 and PD-L1 to reflect the immune status of the tumor microenvironment. The present study characterized CD8+ T cell infiltration categories and PD-L1 expression in HCC, which specifically addressed the spatial heterogeneity and function of effective T cells and their prognostic value.
The novel risk stratification model was based on the immunotype, which also included the factors of PD-L1 expression mode, BCLC stage and tumor grade. In comparison with the traditional prognostic model, this novel risk stratification model integrated both immune features and tumor cell-centered biological features, which were reflected in the BCLC stage and tumor grade. Furthermore, risk stratification can visually recognize low-risk, intermediate-risk or high-risk patients by calculating the assigned score of each factor. Additionally, accurate grouping can predict the prognosis more precisely, which is close to the actual survival.
Currently in clinical practice, HCC patients with intermediate and high risks of recurrence (patients who have a single tumor with MVI or major vascular invasion, a single tumor larger than 5 cm, or two or three tumors) in the remnant liver are candidates for enrollment in postoperative adjuvant TACE, but the debate is still ongoing [41, 42]. Thus, the identification of predictive biomarkers to be sensitive to postoperative adjuvant TACE is critical, which would prevent excessive toxicities. Accumulated evidence has proven that the immune microenvironment also determines the efficacy of chemotherapy in several tumors [43-48]. The persistent immune response targeting tumor cells is essential for successful cytotoxic chemotherapy [49]. A previous study indicated that postoperative TACE showed limited prognostic benefit for HCC patients [50]. However, our novel immunotype-based risk stratification model showed that postoperative TACE discriminatively benefited patients in different risk groups. Patients in the low-risk and intermediate-risk groups benefited from postoperative TACE, while those in the high-risk group did not benefit from TACE. When these results have confirmed the therapeutic effect of adjuvant TACE, especially for low-risk and intermediate-risk patients, more effective therapeutic strategies are needed to benefit high-risk patients. This model emphasizes the role of the immune response (including the PD-L1 expression mode and CD8+ T cell infiltration categories) in evaluating the benefit of TACE. Thus, the mechanical interaction between tumor-infiltrated immunocytes and chemotherapy drugs needs to be thoroughly explored. This study demonstrated the value of our novel immunotype-based risk stratification model for predicting the prognosis of survival and benefit from postoperative adjuvant TACE.
However, several limitations must be acknowledged in the present study. This is a single-center, retrospective study, and the sizes of our cohorts are relatively small. The indication of adjuvant TACE is virtually not standard. Although we have recommended postoperative TACE for patients with intermediate and high risks of recurrence, only some patients accepted adjuvant TACE, while the other patients rejected it. We can only assume that various factors, such as socioeconomic status, compliance, and fear, influenced their decision. Therefore, a multicenter prospective study is needed to potently validate this conclusion in the future. In addition, because we selected only CD8+ T cells, other crucial immune phenotypes might be neglected. Other types of infiltrated immune cells also play important roles in the immune response, such as tumor-associated macrophages [51-53], tumor-associated neutrophils [52, 54] and myeloid-derived suppressor cell infiltration [54, 55]. Further studies should evaluate the role of the whole immunocyte landscape and infiltration heterogenicity.
In conclusion, our data identify that the immune desert, immune exclusion, and inflamed immunotypes and PD-L1 expression are correlated with HCC outcomes and can be used as independent prognostic factors. In addition, we built a novel risk stratification model for predicting postoperative prognosis and adjuvant TACE benefit for HCC patients by integrating this immunotype based on CD8 T cell infiltration, PD-L1 expression and two clinicopathological features. These findings provide a novel predictive tool to predict the prognosis of HCC patients and may improve current predictive systems in terms of counseling patients. Furthermore, immune status can redefine the different risk subgroups of patients with HCC who will benefit from postoperative adjuvant TACE, which could help to develop a more customized method of treatment.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This is a short text to acknowledge the contributions of the two pathologists from Huashan Hospital, Fudan University, Qiong Li and Jia-Li Li, who aided the efforts of the authors. They selflessly helped us to review the pathology slides.
Data availability statement
The datasets of this study are available on request to the corresponding author.
Ethnics statement
Clinical samples were obtained after acquiring consent of patients and the protocol was approved by the Ethics Boards of Huashan Hospital, Fudan University (Shanghai, China). This research was carried out in accordance with the Declaration of Helsinki.
Funding
This work was supported by the following: National Natural Science Foundation of China (82002773, 81902390 and 81902952), China Postdoctoral Science Foundation (2019M651529), Shanghai Sailing Program (17YF1401600), Ruijin Youth NSFC Cultivation Fund (2019QNPY01046).
Author Contributions
YZ and ZW designed research; T-EL, ZZ and DX performed research; T-EL, DX and JD analysed data; YW and ZZ supervised research; T-EL and ZZ drafted the manuscript; YZ, T-EL and JD revised the paper; all authors approved the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-53
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87-108
3. Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-55
4. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C. et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA oncology. 2017;3:1683-91
5. El-Serag HB. Hepatocellular carcinoma. The New England journal of medicine. 2011;365:1118-27
6. Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA. et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Annals of surgery. 2006;243:229-35
7. Zheng Z, Liang W, Wang D, Schroder PM, Ju W, Wu L. et al. Adjuvant chemotherapy for patients with primary hepatocellular carcinoma: a meta-analysis. International journal of cancer. 2015;136:E751-9
8. Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM. et al. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Annals of surgical oncology. 2016;23:1344-51
9. Lau WY, Lai EC, Leung TW, Yu SC. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial-update on 5-year and 10-year survival. Annals of surgery. 2008;247:43-8
10. Samuel M, Chow PK, Chan Shih-Yen E, Machin D, Soo KC. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. The Cochrane database of systematic reviews. 2009: CD001199.
11. Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. The Lancet Oncology. 2002;3:593-603
12. Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M. et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology. 2017;153:812-26
13. Boyault S, Rickman DS, de Reynies A, Balabaud C, Rebouissou S, Jeannot E. et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology (Baltimore, Md). 2007;45:42-52
14. Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY. et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer research. 2009;69:7385-92
15. Fu H, Zhu Y, Wang Y, Liu Z, Zhang J, Xie H. et al. Identification and Validation of Stromal Immunotype Predict Survival and Benefit from Adjuvant Chemotherapy in Patients with Muscle-Invasive Bladder Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2018;24:3069-78
16. Zhang H, Liu H, Shen Z, Lin C, Wang X, Qin J. et al. Tumor-infiltrating Neutrophils is Prognostic and Predictive for Postoperative Adjuvant Chemotherapy Benefit in Patients With Gastric Cancer. Annals of surgery. 2018;267:311-8
17. Hao D, Liu J, Chen M, Li J, Wang L, Li X. et al. Immunogenomic Analyses of Advanced Serous Ovarian Cancer Reveal Immune Score is a Strong Prognostic Factor and an Indicator of Chemosensitivity. Clinical cancer research: an official journal of the American Association for Cancer Research. 2018;24:3560-71
18. Lin Z, Liu L, Xia Y, Chen X, Xiong Y, Qu Y. et al. Tumor infiltrating CD19(+) B lymphocytes predict prognostic and therapeutic benefits in metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitors. Oncoimmunology. 2018;7:e1477461
19. Wang J, Lin C, Li H, Li R, Wu Y, Liu H. et al. Tumor-infiltrating gammadeltaT cells predict prognosis and adjuvant chemotherapeutic benefit in patients with gastric cancer. Oncoimmunology. 2017;6:e1353858
20. Hegde PS, Karanikas V, Evers S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:1865-74
21. Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Annals of oncology: official journal of the European Society for Medical Oncology. 2016;27:1492-504
22. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-7
23. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321-30
24. Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Seminars in oncology. 2015;42:663-71
25. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-71
26. Hegde PS, Chen DS. Top 10 Challenges in Cancer Immunotherapy. Immunity. 2020;52:17-35
27. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L. et al. Adjuvant Transarterial Chemoembolization for HBV-Related Hepatocellular Carcinoma After Resection: A Randomized Controlled Study. Clin Cancer Res. 2018;24:2074-81
28. Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL. et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut. 2019;68:1653-66
29. Zhu Y, Wang XY, Zhang Y, Xu D, Dong J, Zhang Z. et al. Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8(+) T-cell immune responses. Cancer management and research. 2018;10:4113-23
30. Zhai W, Lim TK, Zhang T, Phang ST, Tiang Z, Guan P. et al. The spatial organization of intra-tumour heterogeneity and evolutionary trajectories of metastases in hepatocellular carcinoma. Nature communications. 2017;8:4565
31. Schulze K, Nault JC, Villanueva A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. Journal of hepatology. 2016;65:1031-42
32. Yan X, Qiu Y. Impact of current staging systems on treatment strategy for HBV-related hepatocellular carcinoma. Cancer letters. 2016;379:220-4
33. Gabrielson A, Wu Y, Wang H, Jiang J, Kallakury B, Gatalica Z. et al. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in HCC. Cancer immunology research. 2016;4:419-30
34. Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA. et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer cell. 2006;10:99-111
35. Li X, Yao W, Yuan Y, Chen P, Li B, Li J. et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157-67
36. Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S. et al. TGF-beta-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer cell. 2012;22:291-303
37. Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS. et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. Journal of hepatology. 2011;54:497-505
38. Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A. et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. Journal of hepatology. 2010;52:370-9
39. Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74-80
40. Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell. 2020;37:443-55
41. Feng L, Jin-Hua Z, Yong-Yi Z, Jing-Feng L. Clinical efficacy of transcatheter arterial chemoembolization combined with surgery in the treatment of hepatocellular carcinoma. Indian J Cancer. 2015;52(Suppl 2):e99-e101
42. Chen X-h, Zhang B-h, Yin X, Qiu S-j, Fan J, Ren Z-g. et al. Effect of postoperative adjuvant transarterial chemoembolization upon early recurrence after radical resection of hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi. 2010;90:826-9
43. Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A. et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451-8
44. Verma C, Eremin JM, Robins A, Bennett AJ, Cowley GP, El-Sheemy MA. et al. Abnormal T regulatory cells (Tregs: FOXP3+, CTLA-4+), myeloid-derived suppressor cells (MDSCs: monocytic, granulocytic) and polarised T helper cell profiles (Th1, Th2, Th17) in women with large and locally advanced breast cancers undergoing neoadjuvant chemotherapy (NAC) and surgery: failure of abolition of abnormal treg profile with treatment and correlation of treg levels with pathological response to NAC. J Transl Med. 2013;11:16 -
45. Bernal-Estévez DA, García O, Sánchez R, Parra-López CA. Monitoring the responsiveness of T and antigen presenting cell compartments in breast cancer patients is useful to predict clinical tumor response to neoadjuvant chemotherapy. BMC Cancer. 2018;18:77 -
46. Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M. et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105-13
47. Aoto K, Mimura K, Okayama H, Saito M, Chida S, Noda M. et al. Immunogenic tumor cell death induced by chemotherapy in patients with breast cancer and esophageal squamous cell carcinoma. Oncol Rep. 2018;39:151-9
48. Tao H, Shien K, Soh J, Matsuda E, Toyooka S, Okabe K. et al. Density of tumor-infiltrating FOXP3+ T cells as a response marker for induction chemoradiotherapy and a potential prognostic factor in patients treated with trimodality therapy for locally advanced non-small cell lung cancer. Ann Thorac Cardiovasc Surg. 2014;20:980-6
49. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74-88
50. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology (Baltimore, Md). 2010;52:762-73
51. Wan S, Kuo N, Kryczek I, Zou W, Welling TH. Myeloid cells in hepatocellular carcinoma. Hepatology (Baltimore, Md). 2015;62:1304-12
52. Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402-10
53. Tacke F. Targeting hepatic macrophages to treat liver diseases. Journal of hepatology. 2017;66:1300-12
54. Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Seminars in immunology. 2016;28:187-96
55. Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nature reviews Cancer. 2013;13:739-52
Author contact
![]() Corresponding authors: Zheng Wang, zhengwangedu.cn, Tel: & Fax: +86-21-64370045; Ying Zhu, xj_zy2009com, Tel: & Fax: +86-21-5288-7174.
Corresponding authors: Zheng Wang, zhengwangedu.cn, Tel: & Fax: +86-21-64370045; Ying Zhu, xj_zy2009com, Tel: & Fax: +86-21-5288-7174.

 Global reach, higher impact
Global reach, higher impact