3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(6):1804-1814. doi:10.7150/jca.50509 This issue Cite
Review
CAR-T cells for Colorectal Cancer: Target-selection and strategies for improved activity and safety
Department of Gastrointestinal Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, P.R. China.
Abstract
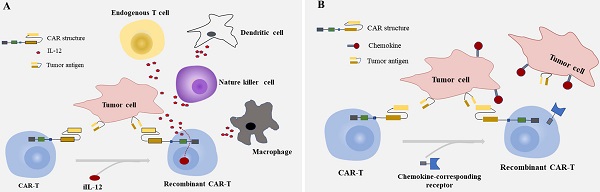
Chimeric antigen receptor-T (CAR-T) cell immunotherapy is a novel method that is genetically engineered to recruit T cells against malignant disease. Administration of CAR-T cells has led to progress in hematological malignancies, and it has been proposed for solid tumors like colorectal cancer (CRC) for years. However, this method was not living up to expectations for the intrinsic challenges posed to CAR-T cells by solid tumors, which mainly due to the lacking of tumor-restricted antigens and adverse effects following treatment. New approaches are proposed to overcome the multiple challenges to alleviate the difficult situation of CAR-T cells in CRC, including engineering T cells with immune-activating molecules, regional administration of T cell, bispecific T cell engager, and combinatorial target-antigen recognition. In this review, we sum up the current stage of knowledge about target-selection, adverse events like on/off-tumor toxicity, the preclinical and clinical studies of CAR-T therapy, and the characteristics of strategies applied in CRC.
Keywords: colorectal cancer, chimeric antigen receptor-T (CAR-T) cell, tumor associated antigens, toxicity, strategies

 Global reach, higher impact
Global reach, higher impact