3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(5):1483-1492. doi:10.7150/jca.52395 This issue Cite
Research Paper
miR-361-3p Regulates Liver Tumor-initiating Cells Expansion and Chemo-resistance
1. Department of Hepatic Surgery, Third Affiliated Hospital of Second Military Medical University, Shanghai, 200438, China.
2. Department of General surgery, First Affiliated Hospital of Second Military Medical University, Shanghai, 200433, China.
#These authors contributed equally to this work.
Abstract
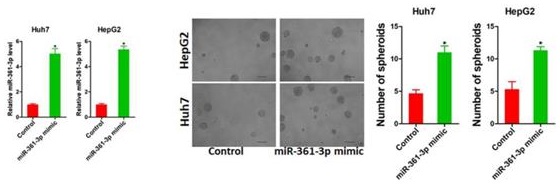
Increasing evidence shows that liver tumor-initiating cells (T-ICs) closely associated with the progression, metastasis, recurrence and chemo-resistance of hepatocellular carcinoma (HCC). However, the underlying mechanism for the propagation of liver T-ICs remains unclear. Here we show that miR-361-3p is upregulated in liver T-ICs. Knockdown of miR-361-3p impairs the self-renewal and tumorigenicity liver T-ICs. Conversely, forced miR-361-3p expression enhances the self-renewal and tumorigenicity liver T-ICs. Mechanistically, miR-361-3p directly targets SOX1 via binding its 3'-UTR in liver T-ICs. Moreover, miR-361-3p knockdown hepatoma cells are more sensitive to cisplatin or sorafenib treatment. Clinical cohort analysis demonstrates that miR-361-3p low HCC patients are benefited from TACE (transcatheter arterial chemoembolization) or sorafenib treatment. In conclusion, our findings revealed the crucial role of the miR-361-3p in liver T-IC expansion and TACE or sorafenib response, rendering miR-361-3p an optimal target for the prevention and intervention in HCC.
Keywords: Tumor-initiating cells, hepatocellular carcinoma, miR-361-3p, SOX1, cisplatin, sorafenib

 Global reach, higher impact
Global reach, higher impact