3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(24):7157-7165. doi:10.7150/jca.46721 This issue Cite
Research Paper
Complement sC5b-9 and CH50 increase the risk of cancer-related mortality in patients with non-small cell lung cancer
1. Department of Endocrinology, Suzhou Xiangcheng People's Hospital, Suzhou, China
2. Department of Medicine, Respiratory, Emergency and Intensive Care Medicine, The Affiliated Dushu Lake Hospital of Soochow University, Suzhou, China
3. Department of General Surgery, The First Affiliated Hospital of Soochow University, Suzhou, China
4. Department of Urology, The Ninth People's Hospital of Suzhou, Suzhou, China
5. Department of Cardiovascular, The First Affiliated Hospital of Soochow University, Suzhou, China
# These authors contributed equally to this work
Received 2020-4-6; Accepted 2020-10-8; Published 2020-10-18
Abstract
Objectives: Immunologic dysfunction occurred in most of patients with non-small cell lung cancer (NSCLC), which worsened the overall survival (OS) of patients. Complement activation plays a significant role in abnormal activation of immune system. However, the prognostic value of complement components such as CH50 and sC5b-9 in NSCLC patients remains unclear. This study evaluated the risk factors of NSCLC and created a prediction model.
Methods: A real-world study was conducted including data from 928 patients with NSCLC between April 1, 2005 and June 1, 2015. CH50 and sC5b-9 were recorded during the admission. Cox proportional hazard model was applied for survival analyses and for assessing risk factors of cancer-related mortality and to create a nomogram for prediction. The accuracy of the model was evaluated by C-index and calibration curve.
Results: In this study, the mortality in group with high CH50 level (≥ 480.56 umol/L) was 92.0%. Based on univariate analysis, we put factors (P <0.05) into a multivariate regression model, patients with high CH50 level (P <0.001, HR=1.59) and sC5b-9 >1422.18 μmol/L (P <0.001, HR=2.28) remained statistically factors for worsened OS and regarded as independent risk factors. These independently associated risk factors were applied to establish an OS estimation nomogram. Nomogram revealed good accuracy in estimating the risk, with a bootstrap-corrected C index of 0.741.
Conclusion: sC5b-9 and CH50 increased the risk of cancer-related mortality in patients with NSCLC. Nomogram based on multivariate analysis demonstrated good accuracy in estimating the risk of overall mortality.
Keywords: non-small cell lung cancer, sC5b-9, CH50, overall survival, prediction model
Introduction
Lung cancer is a fatal malignant tumor, and its incidence rate and mortality rate have been greatly improved internationally [1]. Previous reports have demonstrated that about 1.82 million cases were identified as lung cancer, with 1.59 million deaths in 2012 [2]. Non-small cell lung cancer (NSCLC) is the most common lung cancer, accounting for 85% of all lung cancer types [3]. NSCLC mainly includes adenocarcinoma (AC) and squamous cell carcinoma (SCC) [4]. At present, the treatment of NSCLC mainly includes surgery, chemotherapy and radiotherapy [5]. However, due to the late diagnosis and high rate of recurrence and metastasis, the 5-year survival rate is only 16% [6]. Therefore, timely diagnosis is important to improve the survival rate of lung cancer patients.
Complement is a key factor in the innate immune immunity and in the maintenance of host homeostasis [7]. It consists of more than 50 plasma proteins produced mainly by the liver as well as receptors expressed on the membranes of different kinds of cells [8]. The complement system can be activated via classical, lectin, or alternative pathways, all of which unite at the level of central complement component C3 activation, leading to the formation of the membrane attack complex (MAC) and to cell lysis [9]. Recent discoveries have made it clear that complement proteins exist in the tumor microenvironment and that malignant cells have the ability to produce in situ a large spectrum of the components [10, 11].
A solid body of evidence has accumulated to reveal that the complement system plays a role in cancer cell growth by promoting proliferation, angiogenesis, and antitumor immunity [12]. Evidence supporting complement activation associated with C5b-9 deposition during the antitumoral response exists in various human malignancies [13]. Niculescu et al. provided the first support for the presence of C5b-9 deposition in breast cancer [14]. However, the correlation of serum complement level and prognosis of patients with NSCLC remains unclear.
In this study, we aimed to detect the variation in expression levels of serum complement sC5b-9 and CH50 in patients with NSCLC and explored the potential prognostic factors for NSCLC. We also showed a nomogram that could provide individualized, evidence-based, highly accurate risk estimates. Nomograms were easy to performed and could facilitate management-related decision making.
Materials and methods
Study design and patient characteristics
We did a real-world and retrospective study, including data from 928 patients with NSCLC between April 2005 and June 2015, in the First Affiliated Hospital of Soochow University. Those who withdrew from treatment, lacked information on complement components data or had no follow-up information were excluded. A flow chart of the screening process was shown in Figure 1. Patients' gender, age, BMI, serum CEA level, albumin level, C-reactive protein (CRP) level, lymphocytes count, neutrophils count, hemoglobin level, platelet count, PNI score, neutrophil lymphocyte ratio (NLR), stage of NSCLC, pathologic type, surgery, therapy of radiation, target therapy, application of platinum, application of vascular endothelial growth factor (VEGF) inhibitor, application of Tyrosine Kinase Inhibitor (TKI), Karnofsky Performance Status (KPS) score, smoking, heart failure, diabetes, acute coronary syndrome (ACS), and hyperlipemia were recorded. Diagnosis of NSCLC was confirmed by histopathological examination. The definition and details of all the variables above were provided in Supplemental Materials Part I. The median length of follow-up was 23.6 months. Inform, and consent was obtained from all patients or their immediate family members. All protocols were in line with the guidelines of the ethic committee of the First Affiliated Hospital of Soochow University, and following the Declaration of Helsinki.
Assays for plasma sC5b-9 and CH50 levels
Peripheral blood samples of NSCLC patients were collected when diagnosed. Then, all of the samples were treated with EDTA as an anticoagulant, and centrifuged at 800 × g for 10 min at room temperature to collect the plasma. Next, all the plasma samples were stored at -80℃ for further research. Plasma levels of sC5b-9 and CH50 were detected by ELISA kits (Xitang, Shanghai, China) according to the manufacturer's instruction.
Statistical analysis
Sample size assessment was performed using NCSS-PASS software version 11.0 (https://www.ncss.com/software/pass/). Power was set as 0.99, and alpha was 0.5. The mortalities of both CH50 high-level group and CH50 low-level group in our previous data (2008-2009) (0.600 and 0.950) were entered into the PASS. The Actual Hazard Ratio was set as 1.50. Then the sample size was calculated via PASS, and the minimum sample size was 330 (control = 165, experiment = 165). Our sample size was 928 (464 for each group), which was suitable. The report of the sample size assessment was displayed in Supplemental Material Part II. The missing data (<5.0%) were estimated by random forest algorithm using the mice package in RStudio (R version 3.6.1). Categorical variates were presented as percentages and compared via the κ² test. Continuous variates with skewed and normal distributions were presented as median with interquartile ranges and mean ± standard deviation. The Mann-Whitney U test and the unpaired t-test were used for comparison between Groups. Cumulative mortality was displayed by the Kaplan-Meier curve and analyzed by the log-rank test. Univariate and multivariate survival analyses for OS were assessed using the Cox regression model. The forest plots were applied to visualize the significance of covariates to the prognosis. The restricted cubic spline analyses were conducted with Harrell's Regression Modelling Strategies (rms) package.
To establish a prognostic risk model, the least absolute shrinkage and selection operator (LASSO) method, which is suitable for the regression of high-dimensional data, was performed to identify risk factors associated with prognosis. The contribution of each covariate was quantified and visualized in a prognostic nomogram with internal validation through 1000-times bootstrapping. The consistency of the resulting model was evaluated by the calibration assay. Decision curve analyses were used to assess net clinical benefits of the model compared with conventional prognostic scores. The scatter plots were used for visualization of the consistency of each model. A 1000-time bootstrapping was employed as indicated. Kaplan-Meier curves and log-rank test were applied to analyze the correlation of CH50, sC5b-9 class with survival endpoints. Statistical analysis was conducted using the RStudio (R version 3.6.1) with the following packages: 'ggplot2', 'rms', 'PredictABLE', 'risk regression', and 'survminer'.
A flow chart of the screening process.
Results
Baseline characteristics
During the study period, a total of 928 NSCLC patients who were diagnosed between April 2005 and June 2015 were included. The median age was 64 years old (58-69), and it contains 630 (68.0%) males. Median serum CEA and CRP level was 3.35 ng/ml and 6.46 μmol/L respectively. The pathologic type of these patients was as follow: 631 (68.0%) with adenocarcinoma, 6 (1.0%) with mixed lung cancer, 42 (5.0%) with large cell lung cancer, 242 (26.0%) with squamous carcinoma, and 7 (1.0%) with other types. For the stage of NSCLC, 136 (15.0%) patients were diagnosed with stage I, 78 (8.0%) patients were stage II, 207 (22.0%) were stage III, while 507 (55.0%) patients got stage IV. 356 (38.0%) patients accepted the surgery therapy, and 307 (33.0%) patients got the therapy of radiation. Platinum was applied to 895 (96.0%) patients and TKI was used for 266 (28.0%) patients. We also estimated the KPS score of these patients, and 584 (62.9%) patients got 80 or higher points. Basic diseases were also assessed in these patients. Diabetes was found in 91 (9.0%) patients, while hyperlipemia was 80 (9.0%). Cardiovascular diseases such as heart failure and ACS were found in 10 (1.0%) and 18 (2.0%) patients, respectively. 351 (38.0%) patients suffered from hypertension. In addition, 457 (49.0%) had a habit of smoking among all of the patients. The baseline characteristics of these patients were listed in Table 1.
Among all the 928 patients, the overall mortality was 78.5%. The mortality in high CH50 level group was 92.0%, while the low group was 65.0%. Moreover, in the high CH50 level group, patients with stage III or IV were 382 (82.0%), while the low group was 332 (71.0%) (Table 1).
Study Participant Characteristics at Enrollment
| Variation | Total (n=928) | Cohort, median (IQR) | |||
|---|---|---|---|---|---|
| CH50<480,(n=464) | CH50>480,(n=464) | p.value | |||
| Age, (year) | 64(58,69) | 63(58,69) | 64(58,70) | 0.19 | |
| BMI, (kg/m2) | 22.86(20.44,24.86) | 22.76(20.44,24.97) | 22.98(20.52,24.78) | 0.803 | |
| Serum CEA level, (ng/ml) | 3.35(1.64,13.33) | 3.18(1.49,11.73) | 3.88(1.86,17.08) | 0.009** | |
| Serum CRP level, (umol/L) | 6.46(1.65,12.59) | 5.95(1.49,12.78) | 7.28(1.88,12.47) | 0.357 | |
| Albumin level, (g/L) | 40(36.6,43.62) | 40.1(36.6,43.52) | 40(36.5,43.73) | 0.915 | |
| Neutrophils count, (10^9/L) | 4.67(3.46,6.27) | 4.58(3.41,6.06) | 4.84(3.59,6.45) | 0.107 | |
| Lymphocytes count, (10^9/L) | 1.61(1.25,1.97) | 1.59(1.26,1.99) | 1.64(1.24,1.94) | 0.794 | |
| Hemoglobin level, (g/L) | 132(122,142) | 132(122,142.25) | 132(122,142) | 0.893 | |
| Platelet count, (10^9/L) | 223.5(179,279) | 219(174.75,275.5) | 228(182,279.25) | 0.131 | |
| PNI score | 48.5(44.2,53.05) | 48.7(44.2,52.85) | 48.38(44.14,53.26) | 0.781 | |
| NLR | 2.9(2.07,4.31) | 2.87(1.99,4.24) | 2.95(2.14,4.38) | 0.131 | |
| sC5b-9 (μmol/L) | 1422.18(1182.69,1620.32) | 1355.82(1084.96,1586.72) | 1466.33(1283.25,1650.99) | <0.001*** | |
| CH50 (μmol/L) | 480.56±110.07 | 391.11±62.73 | 570.01±65.46 | <0.001*** | |
| C3a | 570.61(488.22,650.37) | 545.03(466.06,636.14) | 595.89(522.21,666.22) | <0.001*** | |
| C5a | 664.47±115.42 | 641.85±119.72 | 687.09±106.37 | <0.001*** | |
| KPS score, IQR | 90(80,90) | 90(80,100) | 90(80,90) | 0.006** | |
| Gender, (n%) | 0.725 | ||||
| Female | 298(32) | 152(33) | 146(31) | ||
| Male | 630(68) | 312(67) | 318(69) | ||
| Pathologic type, (n%) | 0.974 | ||||
| Adenocarcinoma | 631(68) | 314(68) | 317(68) | ||
| Mixed lung cancer | 6(1) | 3(1) | 3(1) | ||
| Large cell lung cancer | 42(5) | 23(5) | 19(4) | ||
| Squamous carcinoma | 242(26) | 121(26) | 121(26) | ||
| Others | 7(1) | 3(1) | 4(1) | ||
| Metastasis, n(%) | 0.012* | ||||
| No | 419(45) | 229(49) | 190(41) | ||
| Yes | 509(55) | 235(51) | 274(59) | ||
| Stage of NSCLC | 0.002** | ||||
| Stage I | 136(15) | 84(18) | 52(11) | ||
| Stage II | 78(8) | 48(10) | 30(6) | ||
| Stage III | 207(22) | 98(21) | 109(23) | ||
| Stage IV | 507(55) | 234(50) | 273(59) | ||
| Surgery, (n%) | <0.001*** | ||||
| No | 572(62) | 249(54) | 323(70) | ||
| Yes | 356(38) | 215(46) | 141(30) | ||
| Therapy of radiation, (n%) | 0.026* | ||||
| No | 621(67) | 327(70) | 294(63) | ||
| Yes | 307(33) | 137(30) | 170(37) | ||
| Application of platinum, (n%) | 1 | ||||
| No | 33(4) | 16(3) | 17(4) | ||
| Yes | 895(96) | 448(97) | 447(96) | ||
| Chemotherapy | 0.863 | ||||
| AP | 436(47) | 221(48) | 215(46) | ||
| DP | 169(18) | 78(17) | 91(20) | ||
| EP | 50(5) | 25(5) | 25(5) | ||
| GP | 40(4) | 21(5) | 19(4) | ||
| Others | 233(25) | 119(26) | 114(25) | ||
| Target therapy, (n%) | 0.064 | ||||
| No | 600(65) | 314(68) | 286(62) | ||
| Yes | 328(35) | 150(32) | 178(38) | ||
| Application of TKI, (n%) | 0.046* | ||||
| No | 662(71) | 347(75) | 315(68) | ||
| TKI I | 206(22) | 92(20) | 114(25) | ||
| TKI II | 3(0) | 0(0) | 3(1) | ||
| TKI III | 57(6) | 25(5) | 32(7) | ||
| Application of VEGF inhibitor, n(%) | 1 | ||||
| No | 791(85) | 395(85) | 396(85) | ||
| Yes | 137(15) | 69(15) | 68(15) | ||
| Smoking, n(%) | 0.694 | ||||
| No | 471(51) | 239(52) | 232(50) | ||
| Yes | 457(49) | 225(48) | 232(50) | ||
| Hypertension, n(%) | 0.042* | ||||
| No | 577(62) | 304(66) | 273(59) | ||
| Yes | 351(38) | 160(34) | 191(41) | ||
| Diabetes, n(%) | 0.508 | ||||
| No | 837(90) | 415(89) | 422(91) | ||
| Yes | 91(10) | 49(11) | 42(9) | ||
| Hyperlipemia, n(%) | 0.726 | ||||
| No | 848(91) | 422(91) | 426(92) | ||
| Yes | 80(9) | 42(9) | 38(8) | ||
| Heart failure, n(%) | 0.34 | ||||
| No | 918(99) | 461(99) | 457(98) | ||
| Yes | 10(1) | 3(1) | 7(2) | ||
| ACS, n(%) | 0.812 | ||||
| No | 910(98) | 456(98) | 454(98) | ||
| Yes | 18(2) | 8(2) | 10(2) | ||
Abbreviation: IQR, interquartile range; CRP, C-reactive protein; PNI, neutrophil lymphocyte ratio; NLR, neutrophil lymphocyte ratio; NSCLC, non-small-cell lung cancer; TKI, Tyrosine Kinase Inhibitor; VEGF, vascular endothelial growth factor; KPS, Karnofsky Performance Status; ACS, acute coronary syndrome. ***p<0.001, **p<0.01, *p<0.05.
Plasma sC5b-9 and CH50, and clinical risk factors predict the development of NSCLC
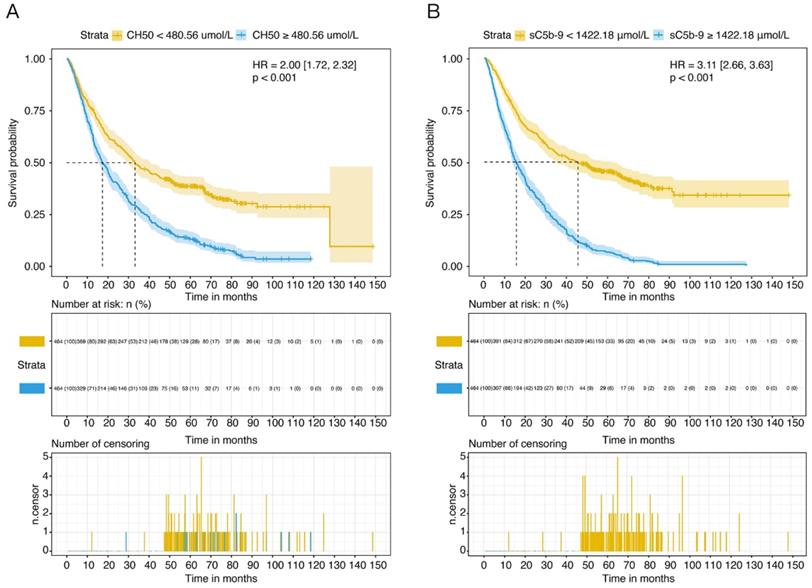
According to the univariate analysis, high CH50 levels (≥ 480.56 μmol/L) was a strong predictor of cancer-related mortality (HR 1.59, 95% CI 1.36-1.85, P <0.001) (Table 2). Kaplan-Meier curve displayed that patients with CH50 high levels class had increased cumulative incidence of death compared to those with the CH50 low levels class (log-rank P <0.001) (Figure 2A). Meanwhile, patients who had high level of sC5b-9 (≥ 1422.18 μmol/L) also showed a high morality compared to those patients with low levels group in the survival curve (HR 3.11, 95% CI 2.66-3.63, P <0.001) (Figure 2B).
Cox Regression Analysis of Hazard Ratio on NSCLC patients
| Variation | Non-adjustment | ||
|---|---|---|---|
| Hazard Ratio (95% CI) | p.value | ||
| Gender, male vs. female | 1.53 [1.30, 1.79] | <0.001*** | |
| Age (year), ≥60 vs. <60 | 1.20 [1.03, 1.40] | 0.022* | |
| BMI, ≥22.86 kg/m2 vs. <22.86kg/m2 | 0.80 [0.69, 0.92] | 0.002** | |
| Serum CEA level, >3.35 ng/ml vs. ≤3.35 ng/ml | 1.63 [1.41, 1.89] | <0.001*** | |
| Serum CRP level, >6.46 μmol/L vs. ≤6.46 μmol/L | 1.75 [1.51, 2.03] | <0.001*** | |
| Albumin level, ≤40 g/L vs. >40 g/L | 1.70 [1.46, 1.96] | <0.001*** | |
| Neutrophils count, >4.67x10^9/L vs. ≤ 4.67x10^9/L | 1.59 [1.38, 1.84] | <0.001*** | |
| Lymphocytes count, >1.61x10^9/L vs. ≤ 1.61x10^9/L | 0.89 [0.77, 1.03] | 0.105 | |
| Hemoglobin level, >132 g/L vs. ≤ 132 g/L | 0.94 [0.81, 1.08] | 0.385 | |
| Platelet count, >223.5x10^9/L vs. ≤ 223.5x10^9/L | 1.24 [1.08, 1.44] | 0.003** | |
| PNI score, >48.5 vs. ≤48.5 | 0.61 [0.53, 0.71] | <0.001*** | |
| NLR, >2.9 vs. ≤2.9 | 1.78 [1.54, 2.06] | <0.001*** | |
| Pathologic type, Adenocarcinoma vs. others | 0.95 [0.81, 1.11] | 0.526 | |
| Metastasis, Yes vs. No | 1.56 [1.35, 1.81] | <0.001*** | |
| Stage of NSCLC, IV, III vs. II and I | 2.00 [1.65, 2.43] | <0.001*** | |
| Surgery, Yes vs. No | 0.30 [0.25, 0.35] | <0.001*** | |
| Therapy of radiation, Yes vs. No | 1.21 [1.04, 1.41] | 0.013* | |
| Application of platinum, Yes vs. No | 0.55 [0.38, 0.80] | 0.002** | |
| Target therapy, Yes vs. No | 1.22 [1.05, 1.42] | 0.009** | |
| Application of TKI, Yes vs. No | 1.20 [1.03, 1.40] | 0.022* | |
| Application of VEGF inhibitor, Yes vs. No | 1.08 [0.88, 1.33] | 0.442 | |
| Chemotherapy, AP vs. others | 0.85 [0.74, 0.99] | 0.035* | |
| Smoking, Yes vs. No | 1.61 [1.39, 1.87] | <0.001*** | |
| Hypertension, Yes vs. No | 1.05 [0.90, 1.22] | 0.524 | |
| Diabetes, Yes vs. No | 1.07 [0.84, 1.36] | 0.603 | |
| Hyperlipemia, Yes vs. No | 0.59 [0.45, 0.79] | <0.001*** | |
| Heart failure, Yes vs. No | 2.71 [1.40, 5.23] | 0.003** | |
| ACS, Yes vs. No | 1.30 [0.78, 2.17] | 0.311 | |
| KPS score, >80 vs. ≤ 80 | 0.43 [0.37, 0.50] | <0.001*** | |
| CH50, >480.56 μmol/L vs. ≤480.56 μmol/L | 2.00 [1.72, 2.32] | <0.001*** | |
| sC5b-9, >1422.18 μmol/L vs ≤1422.18 μmol/L | 3.11 [2.66, 3.63] | <0.001*** | |
Abbreviation: HR, hazard risk; BMI, Body Mass Index; CRP, C-reactive protein; PNI, neutrophil lymphocyte ratio; NLR, neutrophil lymphocyte ratio; NSCLC, non-small-cell lung cancer; TKI, Tyrosine Kinase Inhibitor; VEGF, vascular endothelial growth factor; KPS, Karnofsky Performance Status; ACS, acute coronary syndrome. ***p<0.001, **p<0.01, *p<0.05.
In addition, gender, age, BMI, serum CEA and CRP level, albumin level, neutrophils and platelet counts, PNI score, NLR, metastasis, stage of NSCLC, surgery, therapy of radiation, application of platinum or TKI, target therapy, chemotherapy, smoking, hyperlipemia, heart failure, and KPS score were also associated with overall mortality (Table 2). When adjusted by age and gender, CH50 (HR 2.01, 95% CI 1.73-2.33, P <0.001) and sC5b-9 (HR 3.02, 95% CI 2.58-3.53, P <0.001) high level also displayed a high cumulative incidence of death compared to those with the low level classes.
Independent prognostic factors for OS of NSCLC patients
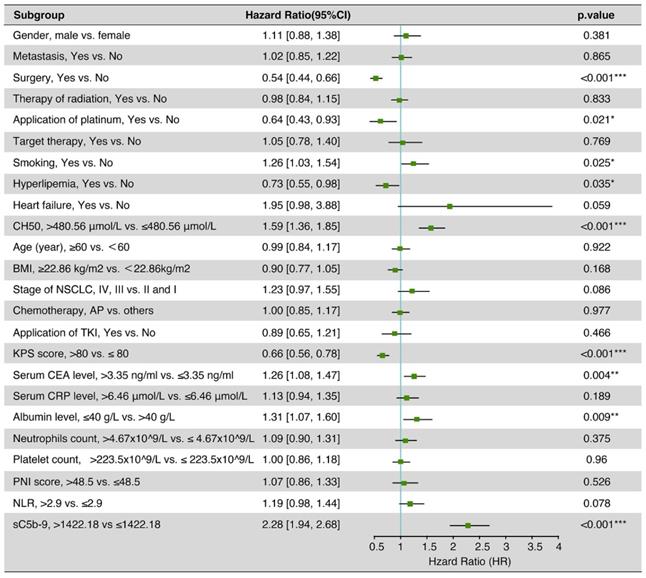
After multivariate adjustment, the sC5b-9 (HR 2.28, 95% CI 1.94-2.68, P < 0.001) and CH50 (HR 1.59, 95% CI 1.36-1.85, P <0.001) were also correlated with a high increase in the risk of death (Figure 3). Meanwhile, smoking, hyperlipemia, albumin level, serum CEA level, KPS score, surgery, and application of platinum were also the independent risk factors for OS of NSCLC patients.
Development and validation of an OS-predicting nomogram
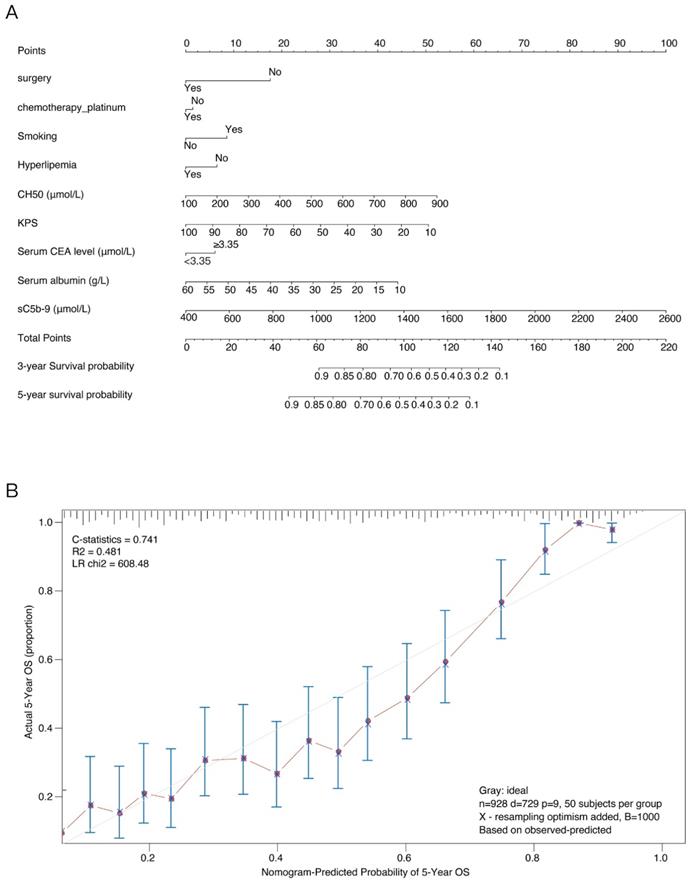
These independently associated risk factors that obtained from the multivariate analysis were used to form an OS estimation nomogram (Figure 4A). The prognostic model was internally validated through the bootstrap validation method. With an unadjusted C index of 0.700 and a bootstrap-corrected C index of 0.741, the nomogram displayed good accuracy in estimating the risk of OS. In the validation cohort, the nomogram showed a C index of 0.700 for the estimation of OS. A suitable calibration curve for risk estimation was also displayed (R2=0.481, LR chi2=608.48) (Figure 4B).
Discussion
In this study, we detected the level of complement components, including sC5b-9 and CH50, in a large cohort of NSCLC patients at a single institution, between April 2005 and June 2015. We found that patients in high sC5b-9 or CH50 expression group had worsen OS compared with those in low sC5b-9 or CH50 expression group, respectively. The multivariate analysis indicated that sC5b-9 and CH50 were independent risk factors for OS. In addition, to propose, and retrospectively evaluate in an independent cohort, these independent prognostic factors were applied to form a nomogram for OS estimation. The nomogram suggested good accuracy in estimating the risk of OS.
Carcinogenesis involves various biological processes, including many key genes or proteins [15]. The characteristics of cancer formation represent properties that a cell needs to achieve some abilities in order to become and maintain as a cancer cell [16]. These hallmarks guide the immune response related to initiation and progression of cancer [17]. Complement system represents a vital component of inflammatory response, which functions as a bridge linking innate and adaptive immune response [18]. The contribution of complement system in the pathophysiology has been recently considered involvement in carcinogenesis [19, 20]. A study investigated by Osther K et al. showed that the complement system in cancer patients may be stimulated through the complement lectin pathway [21]. In addition, complement convertases C5 and C3 were found to be involved in the development of lung cancer because these 2 mediators could have an effect on classical, lectin, or alternative complement pathways [22, 23].
Overall survival (OS) of NSCLC patients in different levels of complement components. (A) OS of NSCLC patients with high or low level of CH50. (B) OS of NSCLC patients with high or low level of sC5b-9.
Previous studies have suggested that many complement components can serve as a valuable biomarker for cancer [24, 25]. Recently, several complement components have been identified as a biomarker for prognostic prediction in NSCLC. Tuberk et al. found that complement components (C3 and C4) levels were abnormally expressed in lung cancer patients with multiple cell types compared to that in control group, and aberrant C3 or C4 could function as a prognostic biomarker for patients with lung cancer [26]. As a member of complement components, sC5b-9 has been proved to participate in other diseases. Many studies indicated that complement system activation could give rise to some diseases through the formation of sC5b-9 [27, 28]. However, no studies have been conducted to explore the potential role of sC5b-9 for biomarker in cancer, especially in NSCLS. To the best of our knowledge, this study was the first attempt ever made to comprehensively elucidate the prognostic role for biomarker based on sC5b-9 or CH50 level in patients with NSCLC. In the current study, we detected the expression levels of sC5b-9 and CH50 in NSCLC patients. Moreover, the univariate analysis suggested that high CH50 level was a strong predictor for cancer-related mortality. Kaplan-Meier curve showed that patients with high sC5b-9 or CH50 level had increased cumulative incidence of death compared to those with low sC5b-9 or CH50 level, respectively. Furthermore, gender, age, BMI, serum CEA and CRP level, albumin level, neutrophils and platelet counts, PNI score, NLR, metastasis, stage of NSCLC, surgery, therapy of radiation, application of platinum or TKI, target therapy, chemotherapy, smoking, hyperlipemia, heart failure, and KPS score were correlated with overall mortality. The multivariate analysis displayed that sC5b-9, CH50, smoking, hyperlipemia, albumin level, serum CEA level, KPS score, surgery, and application of platinum were independent risk factors.
It is reported that nomograms are used for visualization of statistical models, calculation of predicted values, and graphical assessment of variable significance [29, 30]. The nomograms have been widely applied for prediction of cancer risk and treatment outcomes [31, 32]. Recently, some studies have successfully established prognostic nomograms that integrated miRNAs and clinical-related variables in different cancers [33-35]. However, few studies have built prognostic models using combination of sC5b-9 and CH50 and clinical risk factors in NSCLC patients. In this analysis, based on the combination of sC5b-9 and CH50 and independent clinicopathological variables, we created a prognostic nomogram model that could provide an individual estimation of OS in NSCLC patients. The nomogram displayed excellent accuracy in estimating the risk of OS. The suitable calibration curve for risk estimation showed good agreements between observation and prediction. Thus, this is the first prognostic nomogram for NSCLC patients that considers clinicopathological variables in addition to sC5b-9 and CH50. Based on this model, the potential higher risk patients with low survival rate could be selected for a specific treatment.
There are some limitations existing in this study. Firstly, experiments that explore the biological implications of sC5b-9 and CH50 are needed. Therefore, the investigation of molecular mechanism of sC5b-9 and CH50 should be considered in further study in NSCLC. Secondly, the prognostic nomogram needs to be further verified by a large-scale and prospective multicenter research before it can be used in clinical practice.
Collectively, we found that sC5b-9 and CH50 were independent risk factors for prognosis prediction in patients with NSCLC. In addition, prognostic nomogram based on multivariate analysis had excellent accuracy in estimating the risk of OS.
Multivariate cox regression analysis of 5-year overall survival on data in the patients with NSCLC.
Nomogram for overall survival (OS) risk estimation of NSCLC patients and its predictive performance. (A) Nomogram to estimate the OS risk of patients with NSCLC in different variations. To build the nomogram, find the position of each variable on the corresponding axis, draw a line to the points axis for the number of points, add the points from all of the variables, and draw a line from the total points axis to determine the OS probabilities at the lower line of the nomogram. (B) Validity of the predictive performance of the nomogram in estimating the OS risk of NSCLC patients.
Abbreviations
IQR: interquartile range; CRP: C-reactive protein; PNI: neutrophil lymphocyte ratio; NLR: neutrophil lymphocyte ratio; NSCLC: non-small-cell lung cancer; TKI: Tyrosine Kinase Inhibitor; VEGF: vascular endothelial growth factor; KPS: Karnofsky Performance Status; ACS: acute coronary syndrome; HR: hazard risk; BMI: Body Mass Index.
Supplementary Material
Supplementary details of variants and report of sample size assessment.
Acknowledgements
Author Contributions
JL conducted the analysis and drafted the manuscript. ZC, LM and ZX carried out the analysis. JL and XW designed the study and finalized the manuscript. All authors read and approved the final manuscript.
Ethics committee approval and patient consent
Use of patient samples and clinical data in this study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Soochow University.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wang J, Lu X, Ding X, Cao DB. Left lung cancer in a patient with congenital unilateral absence of the left pulmonary artery: a case report and literature review. World J Surg Oncol. 2020;18:32
2. Ferlay J, Soerjomataram I, Dikshit R. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359-386
3. Zou JG, Ma LF, Li X. et al. Circulating microRNA array (miR-182, 200b and 205) for the early diagnosis and poor prognosis predictor of non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2019;23:1108-1115
4. Sun R, Wang R, Chang S. et al. Long non-coding RNA in drug resistance of non-small cell lung cancer: a mini review. Front Pharmacol. 2019;10:1457
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30
6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30
7. Pio R, Corrales L, Lambris JD. The role of complement in tumor growth. Adv Exp Med Biol. 2014;772:229-262
8. Borceux P, Morelle J, Goffin E. Complement system activation and peritoneal membrane alterations: culprit or innocent bystander. Perit Dial Int. 2020;40:115-123
9. Afshar-Kharghan V. The role of the complement system in cancer. J Clin Invest. 2017;127:780-789
10. Kochanek DM, Ghouse SM, Karbowniczek MM, Markiewski MM. Complementing cancer metastasis. Front Immunol. 2018;9:1629
11. Zirakzadeh AA, Sherif A, Rosenblatt R. et al. Tumour-associated B cells in urothelial urinary bladder cancer. Scand J Immunol. 2020;91:e12830
12. Kemper C, Kohl J. Back to the future-non-canonical functions of complement. Semin Immunol. 2018;37:1-3
13. Kolev M, Le Friec G, Kemper C. Complement-tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811-820
14. Niculescu F, Rus HG, Retegan M, Vlaicu R. Persistent complement activation on tumor cells in breast cancer. Am J Pathol. 1992;140:1039-1043
15. Pisani G, Baron B. Nuclear paraspeckles function in mediating gene regulatory and apoptotic pathways. Noncoding RNA Res. 2019;4:128-134
16. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674
17. Lemos de Matos A, Franco LS, McFadden G. Oncolytic viruses and the immune system: the dynamic duo. Mol Ther Methods Clin Dev. 2020;17:349-358
18. Arthur CM, Chonat S, Fasano R. et al. Examining the role of complement in predicting, preventing, and treating hemolytic transfusion reactions. Transfus Med Rev. 2019;33:217-224
19. Noh EM, Kim JM, Lee HY. et al. Immuno-enhancement effects of platycodon grandiflorum extracts in splenocytes and a cyclophosphamide-induced immunosuppressed rat model. BMC Complement Altern Med. 2019;19:322
20. Cserhalmi M, Papp A, Brandus B, Uzonyi B, Jozsi M. Regulation of regulators: role of the complement factor H-related proteins. Semin Immunol. 2019;45:101341
21. Osther K, Fornvik K, Liljedahl E, Salford LG, Redebrandt HN. Upregulation of C1-inhibitor in pancreatic cancer. Oncotarget. 2019;10:5703-5712
22. Ramos AA, Castro-Carvalho B, Prata-Sena M. et al. Can marine-derived fungus Neosartorya siamensis KUFA 0017 extract and its secondary metabolites enhance antitumor activity of doxorubicin? An in vitro survey unveils interactions against lung cancer cells. Environ Toxicol. 2020;35:507-517
23. Boonruang S, Prakobsri K, Pouyfung P, Prasopthum A, Rongnoparut P, Sarapusit S. Structure-activity relationship and in vitro inhibition of human cytochrome CYP2A6 and CYP2A13 by flavonoids. Xenobiotica. 2020;50:630-639
24. Yokoyama S, Hamada T, Higashi M. et al. Predicted prognosis of pancreatic cancer patients by machine learning. Clin Cancer Res. 2020;26:2411-2421
25. Muntel J, Gandhi T, Verbeke L. et al. Surpassing 10 000 identified and quantified proteins in a single run by optimizing current LC-MS instrumentation and data analysis strategy. Mol Omics. 2019;15:348-360
26. Oner F, Savas I, Numanoglu N. Immunoglobulins and complement components in patients with lung cancer. Tuberk Toraks. 2004;52:19-23
27. Palikhe A, Sinisalo J, Seppanen M. et al. Serum complement C3/C4 ratio, a novel marker for recurrent cardiovascular events. Am J Cardiol. 2007;99:890-895
28. Iltumur K, Karabulut A, Toprak G, Toprak N. Complement activation in acute coronary syndromes. APMIS. 2005;113:167-174
29. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364-1370
30. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:173-180
31. Yang Y, Qu A, Zhao R. et al. Genome-wide identification of a novel miRNA-based signature to predict recurrence in patients with gastric cancer. Mol Oncol. 2018;12:2072-2084
32. Kawai K, Ishihara S, Yamaguchi H. et al. Nomogram prediction of metachronous colorectal neoplasms in patients with colorectal cancer. Ann Surg. 2015;261:926-932
33. Lv Y, Duanmu J, Fu X, Li T, Jiang Q. Identifying a new microRNA signature as a prognostic biomarker in colon cancer. PLoS One. 2020;15:e0228575
34. Zhang L, Chen J, Wang L. et al. Linc-PINT acted as a tumor suppressor by sponging miR-543 and miR-576-5p in esophageal cancer. J Cell Biochem. 2019;120:19345-19357
35. Yang Y, Qu A, Wu Q. et al. Prognostic value of a hypoxia-related microRNA signature in patients with colorectal cancer. Aging (Albany NY). 2020;12:35-52
Author contact
![]() Corresponding authors: dr_xiangmeiwucom (Xiangmei Wu) or dr_zhihuaxuedu.cn (Zhihua Xu)
Corresponding authors: dr_xiangmeiwucom (Xiangmei Wu) or dr_zhihuaxuedu.cn (Zhihua Xu)

 Global reach, higher impact
Global reach, higher impact