Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(22):6737-6747. doi:10.7150/jca.48132 This issue Cite
Research Paper
Reciprocal regulation of HIF-1α and Uroplakin 1A promotes glycolysis and proliferation in Hepatocellular Carcinoma
1. Department of Radiation Oncology, Nanfang Hospital, Southern Medical University, Guangzhou, GuangDong Province, 510515, China.
2. Hepatology Unit and Department of Infectious Diseases, Nanfang Hospital, Southern Medical University, Guangzhou, GuangDong Province, 510515, China.
#These authors contribute equally to this work.
Received 2020-5-13; Accepted 2020-9-9; Published 2020-9-25
Abstract
Uroplakin 1A (UPK1A) has recently been found dysregulation in many cancers. However, the functions of UPK1A and its underlying mechanisms in hepatocellular carcinoma (HCC) remain poorly understand. In the present study, we found that UPK1A was highly expressed in HCC tumor tissues compared with adjacent non-tumor tissues. Datasets from the Cancer Genome Atlas project (TCGA) and Gene expression Omnibus confirmed that UPK1A was highly expressed in HCC. High expression of UPK1A predicted poor overall survival (OS) in patients with HCC. Univariate and multivariate analysis showed that UPK1A was a significant and independent prognostic predictor for OS of patients with HCC. Functionally, silencing UPK1A suppressed HCC cell glycolysis and proliferation. Mechanistically, hypoxia-inducible factor 1α (HIF-1α) directly bound to the hypoxia response elements (HRE) of UPK1A promoter region, which led to the up-regulation of UPK1A under hypoxia. Furthermore, downregulation of UPK1A reduced key enzyme of glycolysis via regulating HIF-1α. Taken together, these data indicates the existence of a positive feedback loop between HIF-1α and UPK1A that modulates glycolysis and proliferation under hypoxia in HCC cells.
Keywords: UPK1A, HIF-1α, glycolysis, proliferation, Hepatocellular carcinoma
Introduction
Reprograming of cellular metabolism to satisfy the demands of rapid proliferation, survival, invasion and metastasis is a common feature of cancer [1]. This is mainly marked by the Warburg effect, which is characterized by hyper glucose metabolism [2]. Genes encoding glycolytic enzymes are reported to be responsible for the Warburg effect [3]. Transcription factors like hypoxia-inducible factor 1α (HIF-1α) play direct role in regulation of the Warburg effect [4]. HIF-1α directly regulates a majority of glycolytic genes via binding to the hypoxia-response elements (HRE) of glycolytic gene promoter [5]. HIF-1α can also indirectly regulates the Warburg effect by binding the HRE of non-glucose metabolizing enzyme gene [2, 3]. However, only a small part of non-glucose metabolizing enzyme gene which can modulate the Warburg effect directly targeted by HIF-1α has been founded so far.
Uroplakin 1A (UPK1A), a highly specialized asymmetric unit membrane (AUM) characteristic of mammalian urothelial surface cells is closely related to urothelial cell differentiation [6]. It has been reported that UPK1A is highly specific to normal urothelium tissues. Recently, UPK1A was found to be observed in normal genitourinary tract, prostate and uterus [7]. Additionally, the expression of uroplakins markedly decreased and even totally disappeared in metastasis cancer during carcinogenesis [8, 9]. However, the potential role of UPK1A in cancer development and progression remains quite contradictory. UPK1A which is significantly down-regulated in esophageal cancer acts as a tumor suppressor gene via inhibiting nuclear translocation of β-catenin and its transcriptional activity [6]. UPK1A was also found to be down-regulated in colorectal cancer and gastric cancer [10]. On the contrary, UPK1A was highly expressed in urinary bladder transitional cell carcinoma, and downregulation of UPK1A could suppress proliferation and enhance apoptosis of bladder transitional cell carcinoma cells [11, 12]. However, the expression and functions of UPK1A in HCC remains poorly understood.
Hepatocellular carcinoma (HCC) ranks the sixth most common cancer, and accounts for the fourth leading cause of cancer related mortality worldwide [13]. Adaption of glucose metabolism to satisfy the demands of rapid proliferation is commonly observed in HCC [14]. The augmented glucose uptake and metabolism is generally association with poor prognosis of HCC [15]. HIF-1α, a main transcription factor which regulates the Warburg effect, is highly expressed in HCC [16]. Although HIF-1α has been reported to regulate glucose metabolism in HCC, the mechanism involves HIF-1α- mediated UPK1A upregulation in controlling glucose metabolism in HCC has never been discussed to our knowledge.
In the present, we report a progressively increased expression of UPK1A in HCC. Furthermore, our results provide a new mechanistic insight into the existence of a positive feedback loop between HIF-1α and UPK1A that modulates glycolysis and proliferation under hypoxia in HCC cells.
Materials and Methods
Cell culture
Human HCC cell line SK-Hep-1 and MHCC-97H, hepatocyte cell line MIHA, and non-small cell lung cancer cell line A549 were purchased from Cell Bank of Type Culture Collection (CBTCC, Chinese Academy of Science, Shanghai, China). Cells were cultured in Dubecco's modified Eagle's medium (DMEM, Gibco, Geand Island, NY, USA) containing 10% fetal bovine serum (Gibco) and incubated in humidified incubator. 1% O2 was generated by flushing a 94% N2/5% CO2 mixture into the incubator.
Patients and Specimens
The currently study was reviewed and approved by the ethics committee of Nanfang Hospital, Southern Medical University. All patients were given informed consent prior to the inclusion of the clinical samples. Fresh HCC tissues and non-tumor samples collected from 17 HCC patients who underwent hepatectomies in Nanfang Hospital, were placed in liquid nitrogen after liver cancer resection and stored in -80°C before total RNA extraction. Formalin-fixed, paraffin-embedded HCC samples collected from 10 HCC patients were obtained from the same hospital. Patients with no evidence of extrahepatic metastasis and Child-Pugh class A of liver function at the initial stage were enrolled into the current study. All patients did not receive any treatment prior to surgery. The detailed clinical parameters of enrolled patients were listed in Table S1.
Expression datasets
We analyzed the Cancer Genome Atlas project (TCGA dataset, https://tcga-data.nci.nih.gov/tcga/), Gene expression Omnibus (GEO, accession number GSE22058 and GSE36376), and ICGC Data Portal (ICGC-LIRI-JP, https://dcc.icgc.org/projects) datasets to explore the expression of UPK1A in HCC. The detailed clinical parameters of enrolled patients in TCGA and ICGC-LIRI-JP datasets were listed in Table S2-3. We also analyzed the correlation between expression of UPK1A and overall survival of HCC patients using data from ICGC-LIRI-JP. Kaplan Meier Plotter (http://kmplot.com/analysis/index.php?p=service) was used to analyze the correlation between the expression of UPK1A and overall survival of HCC patients. cBioPortal online software (https://www.cbioportal.org/) was used to explore the alteration frequency of UPK1A gene.
RNA Extraction, Synthesis of cDNA and Quantitative Real-Time PCR (qRT-PCR)
These methods were described previously [17]. The primers used in the present study were listed in Table S4.
Immunohistochemistry (IHC)
IHC staining was performed to examine the expression of UPK1A in formalin, paraffin embedded HCC samples using Dako Envision System (Dako, Carpinteria, CA, USA) following the manufacturer's protocol. After baking at 65°C for 2 hours, sections were deparaffinized and rehydrated. Sections were submerged in EDTA (pH 8.0) for antigen retrieval. After incubation with 0.3% H2O2 for 15 min to block the endogenous peroxidase, the sections were incubated with antibody for UPK1A overnight at 4°C. Sections were incubated with peroxidase labeled polymer conjugated to a secondary antibody at room temperature for 50 minutes after washing. Finally, diaminobenzidine (DAB) was used for color reactions. The staining intensity was scored as follows: 0 (negative), 1 (weak), 2 (medium), 3 (strong). The score of staining extent was as follows: 0 (10%), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%). The total score for UPK1A was calculated based on the intensity and extent scores, ranging from 0 to 7. A total score ≥4 was defined as UPK1A high-expression.
Gene Set Enrichment Analysis (GSEA)
This method was described previously [18].
Small interfering RNAs (siRNA) transfection
Specific siRNAs targeting UPK1A and HIF-1α were synthesized by Genepharma Company (Jiangsu, China). The siRNAs were introduced into HCC cells using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's introduction. All target sequences were listed in Table S5.
Western blot
RIPA buffer (Beyotime Biotechnology, Shanghai, China) containing Phosphatase inhibitor cocktail A (Beyotime Biotechnology) were used to extract total protein from HCC cells. The protein lysates were then separated on a 10-12% SDS-polyacrylamide gel, and transferred onto polyvinylidene fluoride (Millipore, Bedford, MA, USA) membranes. The membranes were incubated with primary antibody overnight at 4°C after blocking with 5% skimmed milk for 50 min at room temperature. The membranes were incubated with secondary antibody conjugated to horseradish peroxidase after washing with 5% Tris-Buffered Saline Tween-20 (TBST). ECL kit (Millipore, Bedford, MA, USA) was used to detect the membrane signals. The primary antibodies were listed in Table S6.
Immunofluorescence analysis
HCC cells, cultured on coverslips, were washed twice with phosphate-buffered saline (PBS), and fixed with 4% paraformaldehyde at room temperature for 15 min. After a 10-min incubation at room temperature in 0.25% Triton X-100, the cells were blocked with 5% bovine serum albumin for 1 h at room temperature, followed by incubation overnight at 4°C with primary antibodies against UPK1A (1:100; Abcam) in PBS followed by rhodamine-conjugated goat anti-rabbit IgG (ZSCB-BIO, Beijing, China) for 1 h. The coverslips were mounted onto slides with mounting medium containing 0.2 μg/ml DAPI.
Chromatin immunoprecipitation (ChIP) assay
ChIP experiment was performed following the protocol of ChIP assay Kit (Pierce, Rockford, IL, USA). ChIP reactions were performed with 5 μg antibody against HIF-1α or with IgG used as a negative control. Purified DNA was suspended for the following PCR assays using primers of UPK1A and LDHA promoter regions. PCR product was run on 2% agarose gels and visualized with ethidium bromide.
Glucose consumption and lactate production
HCC cells were cultured for 48 h after treatment and the culture medium was then harvested for measurement of lactate or glucose concentration. Lactate and glucose levels were quantified using the Lactate Assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and Glucose Assay kit (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer' s recommendation, respectively. Values were normalized to total protein levels determined using the Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
5-ethynyl-20-deoxyuridine (EdU) incorporation assay
Proliferating HCC cells were detected using the Cell-Light EdU Apollo 567 in vitro Imaging kit (RiboBio, Guangzhou, China) according to the manufacturer's protocol. The number of EdU-positive cells was counted under a confocal microscope in five random fields. Three independent experiments were performed.
Statistical Analysis
The statistical analyses were carried out using SPSS statistical software package, version 22.0 (Abbott Laboratories, North Chicago, IL, USA). Data was shown as mean ± standard error of the mean (SEM) from three independent experiments. Survival related to UPK1A expression was evaluated using Kaplan-Meier survival analysis and log-rank test. A two-tailed t test was used to compare the variables of two groups. Significant differences were reflected with *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Dysregulation of UPK1A in various cancers
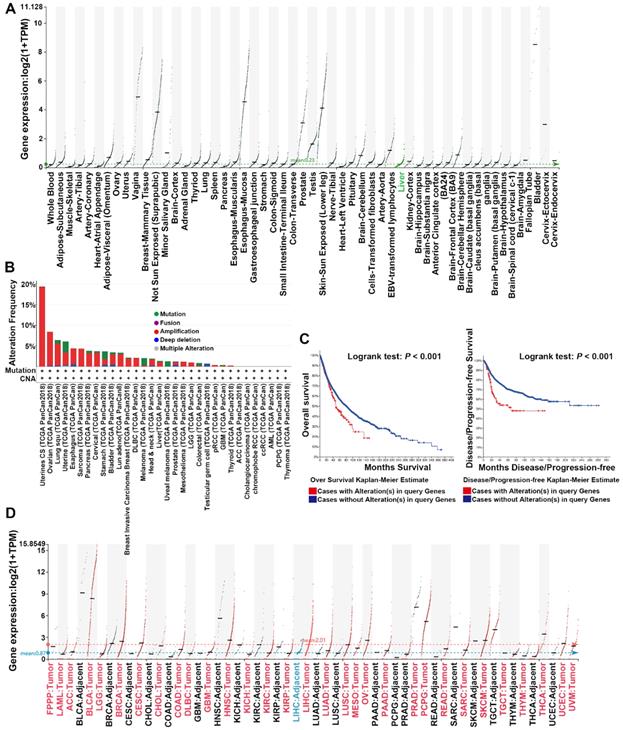
UPK1A was highly expressed in genitourinary tract, uterus, testis and prostate, but barely expressed in other organs and tissues according to the Genotype-Tissue Expression (GTEx) benign tissue RNA-seq dataset (Figure 1A). However, genomic mutations especially amplification of UPK1A gene was usually observed in various cancers, including uterine carcinosarcoma, Ovarian serous cystadenocarcinoma, lung cancer, pancreatic adenocarcinoma and HCC (Figure 1B). Moreover, patients with alterations in UPK1A gene showed poorer OS and Disease-free survival than those without alterations in UPK1A gene (Figure 1C). Consistently, cancers with high UPK1A gene amplification frequency exhibited high expression of UPK1A, including Lung squamous cell carcinoma, Lung adenocarcinoma, Uterine Corpus Endometrial Carcinoma, pancreatic adenocarcinoma and HCC (Figure 1D).
Dysregulation of UPK1A in various cancers. (A) Expression of UPK1A among the GTEx normal tissue RNA-seq dataset, spanning a myriad of different normal tissue types. (B) Alteration frequency of UPK1A gene was analyzed using cBioPortal online software. (C) Overall Survival and disease-free rate of patients with alterations in UPK1A gene was analyzed by cBioPortal online software. (D) Expression of UPK1A in various cancers from TCGA datasets.
UPK1A is highly expressed in HCC
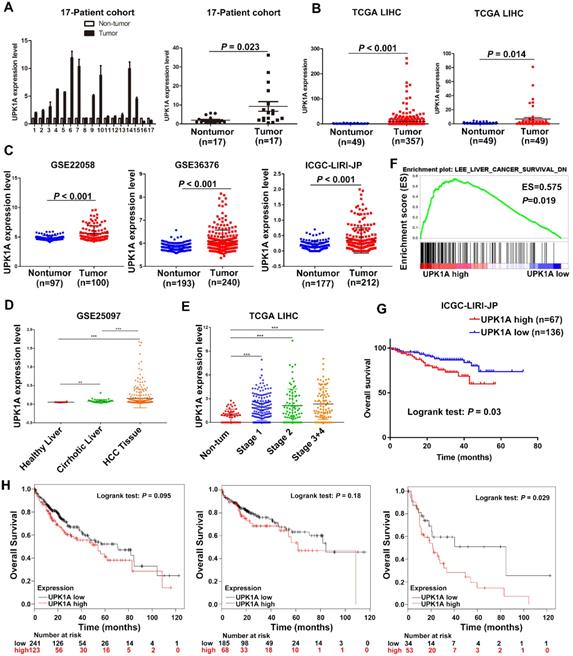
To explore the expression of UPK1A in HCC, we examined UPK1A expression in 17 paired fresh HCC tissues and adjacent non-tumor tissues (17-Patient cohort) using qRT-PCR. As shown in Figure 2A, UPK1A was highly expressed in HCC tissues compared with adjacent non-tumor tissues. To further validate the expression level of UPK1A in HCC, we analyzed RNA-seq data of 358 HCC patients from TCGA. Similarly, the results showed that the expression level of UPK1A was significantly increased in HCC (Figure 2B, left panel, P < 0.001). A similar result was found when UPK1A expression was re-assessed in 50 paired HCC tissues and adjacent non-tumor samples (Figure 2B, right panel, P = 0.014). Consistently, data from GSE22058, GSE36376 and ICGC-LIPI-JP further confirmed that UPK1A was significantly up-regulated in HCC (Figure 2C, P < 0.001). Notably, data from GSE25097 and TCGA suggested that UPK1A was closely related to the development and progression of HCC (Figure 2D-E).
Next, Hematoxylin and eosin staining was performed to further distinguish HCC samples from non-tumor samples. As shown in Figure S1, UPK1A was barely expressed in adjacent non-tumor liver sample. On the contrary, UPK1A was strongly expressed in HCC tissue. Collectively, these data indicate UPK1A is highly expressed in HCC.
High expression of UPK1A predicted poor clinical outcome of patients with HCC. (A) Expression of UPK1A was evaluated in 17-Patient cohort by qRT-PCR. (B) Left panel, analysis of UPK1A expression in HCC patients from TCGA datasets (n=358). Right panel, Re-analysis of UPK1A expression level in 49 paired HCC samples and adjacent non-tumor samples from TCGA datasets (n=49). (C) Expression of UPK1A in HCC patients from GSE22058, GSE36376, and ICGC-LIRI-JP. (D) UPK1A expression level in healthy liver, cirrhotic liver, and HCC tissues. (E) UPK1A expression level in non-tumor tissue and different stage of HCC. (F) GSEA analysis suggested high UPK1A expression was association with gene signatures of poor liver cancer survival. (G) Kaplan-Meier survival analysis of overall survival of HCC patients from ICGC-LIRI-JP database. (H) Kaplan-Meier survival analysis of overall survival of HCC patients from TCGA datasets using Kaplan Meier Plotter online program.
The associations of UPK1A expression with clinicopathological characteristics in ICGC-LIRI-JP (n=203) cohort
| Feature | Low expression of UPK1A (n=136) | High expression of UPK1A (n=67) |
|---|---|---|
| Age (years) | ||
| ≥50 | 127 | 61 |
| <50 | 9 | 6 |
| Gender | ||
| Male | 106 | 47 |
| Female | 30 | 20 |
| Virus | ||
| HBV | 30 | 23 |
| HCV | 82 | 35 |
| HBV and HCV | 2 | 2 |
| Negative | 22 | 7 |
| TNM stage | ||
| I+II | 91 | 38 |
| III+IV | 45 | 29 |
| Portal vein invasion | ||
| Positive | 29 | 20 |
| Negative | 107 | 47 |
| Vein invasion | ||
| Positive | 48 | 17 |
| Negative | 88 | 50 |
| Bile duct invasion | ||
| Positive | 47 | 7 |
| Negative | 89 | 60 |
| Fibrosis | ||
| Yes | 130 | 66 |
| No | 6 | 1 |
| Alcohol | ||
| Yes | 90 | 43 |
| No | 40 | 24 |
| NA | 6 | 0 |
| Smoking | ||
| Yes | 89 | 43 |
| No | 42 | 23 |
| NA | 5 | 1 |
Abbreviations: NA, not available.
Univariate and multivariate analyses of OS in ICGC-LIRI-JP (n=203) cohort by Cox regression analysis
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | CI (95%) | P value | HR | CI (95%) | P value | |
| Age (years) | 0.922 | 0.325-2.616 | 0.878 | |||
| Gender | 0.536 | 0.265-1.083 | 0.082 | |||
| Virus (HBV or/and HCV) | 1.654 | 0.506-5.408 | 0.406 | |||
| TNM stage | 2.830 | 1.446-5.540 | 0.002* | |||
| Portal vein invasion | 3.135 | 1.595-6.160 | 0.001* | |||
| Vein invasion | 2.394 | 1.172-4.891 | 0.017* | |||
| Fibrosis | 1.158 | 0.276-4.853 | 0.841 | |||
| Alcohol | 0.476 | 0.242-0.936 | 0.031* | 0.311 | 0.150-0.646 | 0.002* |
| Smoking | 0.758 | 0.391-1.471 | 0.413 | |||
| UPK1A | 2.121 | 1.092-4.119 | 0.026* | 2.479 | 1.243-4.943 | 0.010* |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; CI, confidence interval; HR, hazard radio; *The values had statistically significant differences.
High expression of UPK1A predicted poor clinical outcome of patients with HCC
A comparative microarray analysis of gene expression was carried out in the UPK1A-high and UPK1A-low expression groups by GSEA. The results showed that a high expression level of UPK1A was association with gene signatures of poor liver cancer survival (Figure 2F), which suggested that high expression of UPK1A correlated with poor prognosis in patients with HCC. Kaplan-Meier and log-rank test analyses were conducted to investigate an association between UPK1A expression level and OS for patients with HCC. The results from the ICGC-LIRI-JP datasets showed that patients with UPK1A overexpression exhibited a significantly shorter OS time as compared with those with low UPK1A expression level (Figure 1G, P = 0.03). Consistently, data from TCGA dataset revealed that patients with high expression of UPK1A had poorer overall survival time than those with low UPK1A expression, though the difference did not reach statistical significance. Since UPK1A closely correlated with HCC progression, we reanalyzed the data from subgroups. Interestingly, patients with high expression of UPK1A presented poorer overall survival time than those with low UPK1A expression in advanced HCC (Figure 1H). Additionally, multivariate analysis from ICGC-LIRI-JP datasets revealed that UPK1A overexpression (95% CI: 1.243-4.943, P = 0.01) was an independent prognostics factor for OS in HCC (Table 1 and Table 2).
UPK1A is a direct transcriptional target of HIF-1α in HCC cells
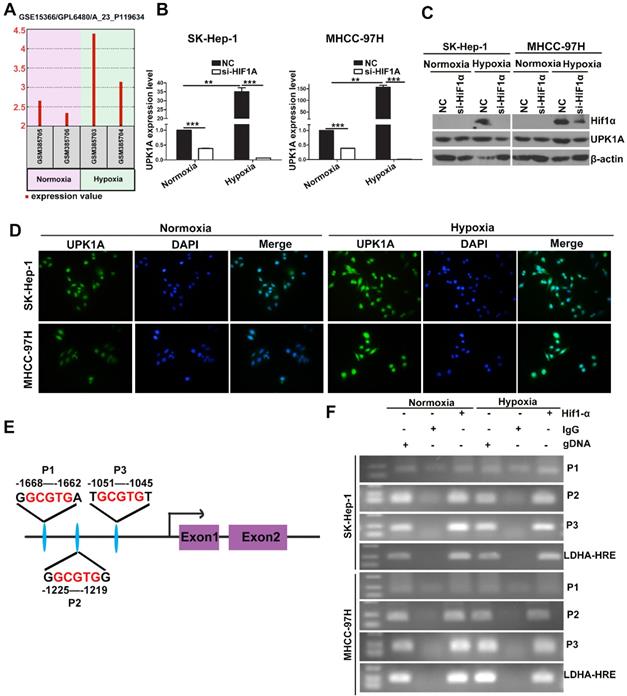
It has been reported that severe hypoxia condition does exist in solid tumor including HCC [19]. During the development of HCC, there is an important contribution of hypoxia on prognosis via abnormal regulation of gene expression [20]. To explore whether UPK1A was aberrantly expressed under hypoxic condition, we analyzed the differences between gene expression profiles (GSE15366) from Human hepatoblastoma cells HepG2 cultured under either normoxic (20% O2) or hypoxic (2% O2) conditions. Interestingly, UPK1A was highly induced under hypoxic condition (Figure 3A). HIF-1α is a key mediator of hypoxic response element. We knocked down the expression of HIF-1α in HCC cells using small interference RNAs and detected UPK1A expression level to evaluate if UPK1A was regulated by HIF-1α. As shown in Figure 3B-D, UPK1A was highly expressed under hypoxic condition. Notably, silencing HIF-1α significantly reduced the UPK1A expression either under normoxic or hypoxic conditions. We also knocked down the expression of HIF-1α in hepatocyte cell line MIHA and non-small cell lung cancer cell line A549 (Figure S2A). The expression of UPK1A remained unchanged after HIF-1α downregulation in MIHA and A549 cells (Figure S2B), indicating that hypoxic-induced upregulation of UPK1A might be tissue- and disease-specific. HIF-1α can transcriptionally regulate a vast variety of genes by directly binding to HREs within their promoters. To explore whether HIF-1α regulates UPK1A expression at the transcriptional level, we searched for the potential HREs in the promoter regions of UPK1A. Three potential HREs was found within the promoter regions of UPK1A (Figure 3E). The subsequent ChIP assays verified the binding of HIF-1α and the chromatin fragments corresponding to the three HREs in the promoter regions of UPK1A (Figure 3F). The results above demonstrate that HIF-1α transcriptionally regulates UPK1A under both normoxic and hypoxic conditions.
UPK1A is a direct transcriptional target of HIF-1α. (A) UPK1A expression levels under normoxic or Hypoxic conditions were analyzed from GSE15366. (B) mRNA expression level of UPK1A was analyzed after down-regulation of HIF-1α in SK-Hep-1 and MHCC-97H by qRT-PCR. (C) UPK1A expression level was analyzed after down-regulation of HIF-1α in SK-Hep-1 and MHCC-97H by western blot. (D) UPK1A expression level under normoxic and hypoxic conditions was analyzed in SK-Hep-1 and MHCC-97H using immunofluorescence. (E) Schematic illustration of consensus HIF-1α responsive element in UPK1A promoter region. (F) The binding of HIF-1α and the chromatin fragments corresponding to the three HREs in the promoter regions of UPK1A was verified using ChIP assay.
UPK1A is essential for hypoxia-enhanced glycolysis and proliferation. (A) Glucose consumption(A) and lactate production (B) was analyzed after down-regulation of UPK1A in SK-Hep-1 and MHCC-97H cell. (C) Effect of UPK1A down-regulation on SK-Hep-1 cell proliferation was evaluated by EdU assay.
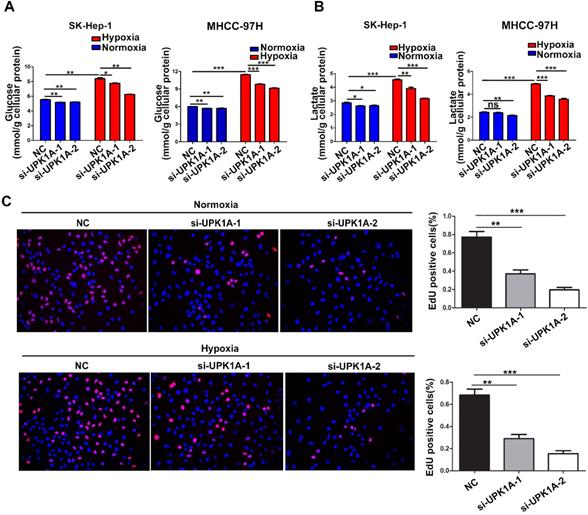
UPK1A is essential for hypoxia-enhanced glycolysis and proliferation in HCC cells
To investigate the functional role of hypoxia-induced UPK1A, we used 2 different UPK1A-targeting siRNAs to knock down UPK1A expression. The knockdown efficiency of UPK1A was examined using qRT-PCR. The results showed that the two siRNAs successfully silenced UPK1A expression (Figure 5B). It has been well known that hypoxic condition causes increased glycolysis in HCC cells [21]. Consistent with this, hypoxia treatment led to a significantly increase in glucose consumption and lactate production (Figure 4A-B). Notably, all these hypoxia-induced effects were strongly reversed by UPK1A knockdown (Figure 4A-B). However, downregulation of UPK1A had no or little impact on the glucose consumption in MIHA and A549 cells (Figure S2C). Given that glycolysis contributes to HCC progression, we examined the biological function of UPK1A in HCC. Silencing UPK1A obviously inhibited HCC cell proliferation both in normoxic and hypoxic conditions (Figure 4C).
Downregulation of UPK1A inhibits HCC cell glycolysis via regulating HIF-1α signaling
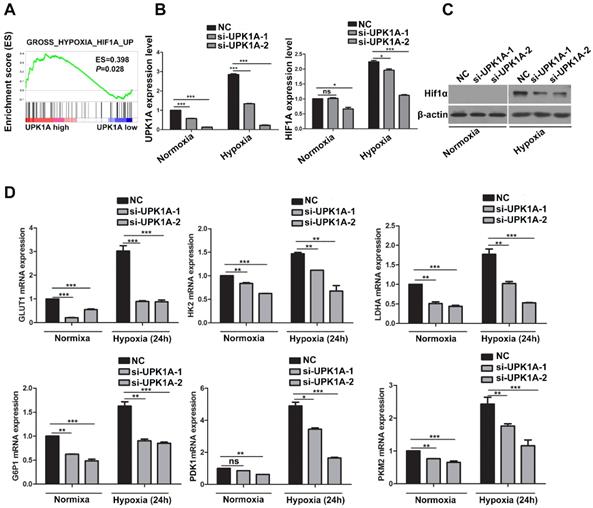
We next performed GSEA analysis on tumor samples from TCGA dataset to explore the mechanisms underlying UPK1A involved in HCC glycolysis and proliferation. The results demonstrated that high expression of UPK1A positively correlated with gene signature involved in hypoxia and HIF-1α signaling (Figure 5A). We thus assumed that UPK1A might regulate HIF-1α or its downstream targets. To test our hypothesis, we detected the expression of HIF-1α using qRT-PCR and western blot. The results revealed that downregulation of UPK1A remarkably reduced the expression of HIF-1α in both mRNA and protein level (Figure 5B-C), which suggested that UPK1A might regulate HIF-1α at transcriptional level in HCC cells. However, downregulation of UPK1A did not alter the expression of HIF-1α in MIHA and A549 cells (Figure S 2D). HIF-1α transactivates hypoxia-inducible glycolysis enzymes by binding to the HREs in promoters of these genes. Our results revealed that downregulation of UPK1A significantly decreased the expression of HIF-1α targets genes involved in glycolysis including GLUT1, HK2, LDHA G6PI, PDK1, and PKM2 (Figure 5D). Taken together, our results suggest that downregulation of UPK1A inhibits HCC cell glycolysis via regulating HIF-1α signaling.
Downregulation of UPK1A inhibits HCC cell glycolysis via regulating HIF-1α signaling. (A) GSEA analysis indicated that UPK1A positively correlated with gene signature involved in hypoxia and HIF-1α signaling. (B) mRNA expression level of UPK1A and HIF-1α after silencing UPK1A in SK-Hep-1 cell were detected using qRT-PCR. (C) HIF-1α expression level after silencing UPK1A in SK-Hep-1 cell was evaluated using western blot. (D) Expression level of HIF-1α target genes after silencing UPK1A in SK-Hep-1 cell were detected using qRT-PCR.
Discussion
UPK1A, an integral protein which belongs to the transmembrane 4 superfamily (TM4SF), is thought to be specific to normal urothelium [22, 23]. Some of the TM4SF superfamily members have been reported to play crucial roles in the regulation of cell proliferation, differentiation, motility and invasion [24], the physiological and biological function of UPK1A remains largely unrevealed. In the present study, we found that down-regulation of UPK1A significantly reduced HCC cell proliferation and glycolysis. Further investigation revealed UPK1A inhibits HCC cell proliferation and glycolysis via regulating HIF-1α signaling.
It has been reported that UPK1A was dysregulated in various cancer. However, the potential role of UPK1A in cancer development and progression remains quite contradictory. UPK1A was down-regulated and identified as a tumor suppressor in esophageal squamous cell carcinoma [6], colorectal cancer [25], and gastric cancer [10]. UPK1A was found to be up-regulated in urinary bladder transitional cell carcinoma instead [12]. There seemed to be more complex roles for UPK1A regarding development and progression in different cancer types. In esophageal squamous cell carcinoma, hypermethylation in the promoter region of UPK1A accounts for the inactivation and down-regulation of UPK1A [6]. The mechanisms underlying the up-regulation of UPK1A in cancer like urinary bladder transitional cell carcinoma is largely unknown. Through genomic analysis we found that genomic mutations especially amplification of UPK1A gene was usually observed in various cancers, including uterine carcinosarcoma, ovarian serous cystadenocarcinoma, lung cancer, pancreatic adenocarcinoma. Consistently, cancers with high UPK1A gene amplification frequency exhibited high expression of UPK1A in lung squamous cell carcinoma, lung adenocarcinoma, uterine corpus endometrial carcinoma, pancreatic adenocarcinoma, which indicates UPK1A gene amplification may account for upregulation of UPK1A in these cancers. Interestingly, patients with alterations in UPK1A gene showed poorer OS and Disease-free survival than those without alterations in UPK1A gene. Taken together, dysregulations of UPK1A may play crucial roles in cancers.
Hypoxia, commonly seen in solid tumor, is considered to play crucial roles in HCC progression and development [20]. Cancers cells inside solid tumor suffer from hypoxia due to abnormal growth of tumor, rapidly increased tumor size, abnormal growth of tumor vasculature, and so on [26]. Cancer cells prefer to glycolysis instead of oxidative phosphorylation under hypoxic condition [27]. HIF-1α, a major transcription factor which regulates a majority of glycolytic genes via binding to the HRE of glycolytic gene promoter, is highly expressed in HCC and predicts poor prognosis for HCC patients [28-30]. Here, we found HIF-1α directly bound to the HREs of UPK1A promoter region by ChIP assay, leading to the upregulation of UPK1A under hypoxic condition, which might account for the upregulation of UPK1A in HCC. GSEA analysis form TCGA dataset revealed that upregulation of UPK1A correlated with HIF-1α signaling pathway, which inspired us to determine whether UPK1A regulated HIF-1α signaling. Notably, down-regulation of UPK1A significantly decreased mRNA and protein expression level of HIF-1α, suggesting UPK1A regulated HIF-1α at transcriptional level. Consistently, UPK1A silencing significantly decreased the expression of HIF-1α targets genes involved in glycolysis including GLUT1, HK2, LDHA G6PI, PDK1, and PKM2. These results indicate that HIF-1α upregulates UPK1A which in turn increases HIF-1α and its downstream targets to promotes HCC cell glycolysis and proliferation under hypoxia.
Despite advance treatment strategies have been improved, the survival rate remains unsatisfactory for patients with HCC [31]. Therefore, novel risk markers to guide disease managements of HCC patients for the improvement of their survival are urgently needed. Upregulation of UPK1A in HCC patients was significantly related with poor prognosis by Kaplan-Meier survival analysis, suggesting that UPK1A may become a novel marker to predict OS for HCC patients. Univariable and multivariable Cox proportional hazard regression analysis show that upregulation of UPK1A was an independent factor in predicting OS for patients with HCC. In conclusion, our results indicate that UPK1A, significantly upregulated in HCC, predicts poor prognosis for patients with HCC. In addition, we provide a new mechanistic insight into the existence of a positive feedback loop between HIF-1α and UPK1A that modulates glycolysis and proliferation under hypoxia in HCC cells.
Abbreviations
AUM: asymmetric unit membrane; CBTCC: Cell Bank of Type Culture Collection; ChIP: Chromatin immunoprecipitation; DMEM: Dubecco's modified Eagle's medium; DAB: diaminobenzidine; EdU: 5-ethynyl-20-deoxyuridine; GEO: Gene expression Omnibus; GSEA: Gene Set Enrichment Analysis; GTEx: Genotype-Tissue Expression; HCC: hepatocellular carcinoma; HIF-1α: hypoxia-inducible factor 1α; HRE: hypoxia response elements; HC: immunohistochemistry; OS: overall survival; PBS: phosphate-buffered saline; qRT-PCR: Quantitative Real-Time PCR; siRNA: Small interfering RNAs; SEM: mean ± standard error of the mean; TBST: Tris-Buffered Saline Tween-20; TCGA: The Cancer Genome Atlas project; TM4SF: transmembrane 4 superfamily; UPK1A: Uroplakin 1A.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant Nos. 81902478, 81672756), China Postdoctoral Science Foundation funded project (Grant No. 2019M652975), and the Natural Science Foundation of Guangdong Province (Grant No. 2019A1515011436).
Author contributions
Conception and design of the study were provided by Dong-Yan Zhang and Yang Song. The experiments were performed by Dong-Yan Zhang, Yang Song, Hui Wang, Xue-Jing Zou and Ze-Qin Guo. Analysis of data: Yang Song, Li Liu and De-Hua Wu. Dong-Yan Zhang wrote the paper, Dong-Yan Zhang, Yang Song and De-Hua Wu coordinated and conceived the study. Dong-Yan Zhang and De-Hua Wu are responsible for its revision.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Allen AE, Locasale JW. Glucose Metabolism in Cancer: The Saga of Pyruvate Kinase Continues. Cancer cell. 2018;33:337-9
2. Yang F, Zhang H, Mei Y, Wu M. Reciprocal regulation of HIF-1alpha and lincRNA-p21 modulates the Warburg effect. Molecular cell. 2014;53:88-100
3. Shih JW, Chiang WF, Wu ATH, Wu MH, Wang LY, Yu YL. et al. Long noncoding RNA LncHIFCAR/MIR31HG is a HIF-1alpha co-activator driving oral cancer progression. Nature communications. 2017;8:15874
4. Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95:912-9
5. Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Current opinion in genetics & development. 2001;11:293-9
6. Kong KL, Kwong DL, Fu L, Chan TH, Chen L, Liu H. et al. Characterization of a candidate tumor suppressor gene uroplakin 1A in esophageal squamous cell carcinoma. Cancer research. 2010;70:8832-41
7. Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1997;11:428-42
8. Olsburgh J, Harnden P, Weeks R, Smith B, Joyce A, Hall G. et al. Uroplakin gene expression in normal human tissues and locally advanced bladder cancer. The Journal of pathology. 2003;199:41-9
9. Ogawa K, St John M, Luiza de Oliveira M, Arnold L, Shirai T, Sun TT. et al. Comparison of uroplakin expression during urothelial carcinogenesis induced by N-butyl-N-(4-hydroxybutyl)nitrosamine in rats and mice. Toxicologic pathology. 1999;27:645-51
10. Zheng Y, Wang DD, Wang W, Pan K, Huang CY, Li YF. et al. Reduced expression of uroplakin 1A is associated with the poor prognosis of gastric adenocarcinoma patients. PloS one. 2014;9:e93073
11. Zhu H, Tang Y, Zhang X, Jiang X, Wang Y, Gan Y. et al. Downregulation of UPK1A suppresses proliferation and enhances apoptosis of bladder transitional cell carcinoma cells. Medical oncology (Northwood, London, England). 2015;32:84
12. Kageyama S, Yoshiki T, Isono T, Tanaka T, Kim CJ, Yuasa T. et al. High expression of human uroplakin Ia in urinary bladder transitional cell carcinoma. Japanese journal of cancer research: Gann. 2002;93:523-31
13. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394-424
14. Yuan P, Yang T, Mu J, Zhao J, Yang Y, Yan Z. et al. Circadian clock gene NPAS2 promotes reprogramming of glucose metabolism in hepatocellular carcinoma cells. Cancer letters. 2020;469:498-509
15. De Matteis S, Ragusa A. Aberrant Metabolism in Hepatocellular Carcinoma Provides Diagnostic and Therapeutic Opportunities. Oxid Med Cell Longev. 2018;2018:7512159
16. Chen C, Lou T. Hypoxia inducible factors in hepatocellular carcinoma. Oncotarget. 2017;8:46691-703
17. Singh AK, Aryal B, Zhang X, Fan Y, Price NL, Suarez Y. et al. Posttranscriptional regulation of lipid metabolism by non-coding RNAs and RNA binding proteins. Seminars in cell & developmental biology. 2018;81:129-40
18. Cao C, Zhang T, Zhang D, Xie L, Zou X, Lei L. et al. The long non-coding RNA, SNHG6-003, functions as a competing endogenous RNA to promote the progression of hepatocellular carcinoma. Oncogene. 2017;36:1112-22
19. Choi SH, Park JY. Regulation of the hypoxic tumor environment in hepatocellular carcinoma using RNA interference. Cancer Cell Int. 2017;17:3
20. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Molecular cell. 2010;40:294-309
21. Chen R, Zhu S, Fan XG, Wang H, Lotze MT, Zeh HJ 3rd. et al. High mobility group protein B1 controls liver cancer initiation through yes-associated protein -dependent aerobic glycolysis. Hepatology (Baltimore, Md). 2018;67:1823-41
22. Xie B, Zhou G, Chan SY, Shapiro E, Kong XP, Wu XR. et al. Distinct glycan structures of uroplakins Ia and Ib: structural basis for the selective binding of FimH adhesin to uroplakin Ia. The Journal of biological chemistry. 2006;281:14644-53
23. Yu J, Lin JH, Wu XR, Sun TT. Uroplakins Ia and Ib, two major differentiation products of bladder epithelium, belong to a family of four transmembrane domain (4TM) proteins. The Journal of cell biology. 1994;125:171-82
24. Finch JL, Miller J, Aspinall JO, Cowled PA. Cloning of the human uroplakin 1B cDNA and analysis of its expression in urothelial-tumor cell lines and bladder-carcinoma tissue. International journal of cancer. 1999;80:533-8
25. He Y, Kong F, Du H, Wu M. Decreased expression of uroplakin Ia is associated with colorectal cancer progression and poor survival of patients. International journal of clinical and experimental pathology. 2014;7:5031-7
26. Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nature reviews Cancer. 2011;12:9-22
27. Choudhry H, Harris AL, McIntyre A. The tumour hypoxia induced non-coding transcriptome. Molecular aspects of medicine. 2016;47-48:35-53
28. Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer cell. 2007;12:108-13
29. Yang SL, Liu LP, Jiang JX, Xiong ZF, He QJ, Wu C. The correlation of expression levels of HIF-1alpha and HIF-2alpha in hepatocellular carcinoma with capsular invasion, portal vein tumor thrombi and patients' clinical outcome. Japanese journal of clinical oncology. 2014;44:159-67
30. Dai CX, Gao Q, Qiu SJ, Ju MJ, Cai MY, Xu YF. et al. Hypoxia-inducible factor-1 alpha, in association with inflammation, angiogenesis and MYC, is a critical prognostic factor in patients with HCC after surgery. BMC cancer. 2009;9:418
31. Rimassa L, Pressiani T, Merle P. Systemic Treatment Options in Hepatocellular Carcinoma. Liver cancer. 2019;8:427-46
Author contact
![]() Corresponding authors: Dong-Yan Zhang, E-mail: zdy233smu.edu.cn, Fax: +86-02061642131, and De-Hua Wu, E-mail: 18602062748com, Fax: +86-02061642131.
Corresponding authors: Dong-Yan Zhang, E-mail: zdy233smu.edu.cn, Fax: +86-02061642131, and De-Hua Wu, E-mail: 18602062748com, Fax: +86-02061642131.

 Global reach, higher impact
Global reach, higher impact