Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(8):2273-2282. doi:10.7150/jca.38613 This issue Cite
Research Paper
InvivoPen: A novel plasma source for in vivo cancer treatment
1. Engineering Research Center of IoT Technology Applications (Ministry of Education), Department of Electronic Engineering, Jiangnan University, Wuxi, China
2. Wuxi School of Medicine, Jiangnan University, Wuxi, China
3. Department of Oncology, Affiliated Hospital of Jiangnan University, Wuxi, China
4. School of Mechanical Engineering, Jiangnan University ,Jiangsu Wuxi 214122, China
5. Laboratory of Advanced Food Manufacturing Technology of Jiangsu Province, Jiangnan University, Jiangsu Wuxi 214122, China
6. School of Biotechnology, Jiangnan University, Wuxi, China
7. School of Chemistry, Physics and Mechanical Engineering, Queensland University of Technology, Brisbane, Queensland 4059, Australia
8. Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Queensland 4059, Australia
9. The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, 710061, China
*These authors contributed equally to this work.
Received 2019-7-22; Accepted 2020-1-4; Published 2020-2-10
Abstract
Background: With the anti-cancer efficacies of cold atmospheric plasma being increasingly recognized in vitro, a demand on creating an effective tool feasible for in vivo animal treatment has emerged.
Methods: Through the use of co-axial needles with different calibers in diameter, we designed a novel in situ ejection source of cold atmospheric plasma, namely invivoPen, for animal experiments. It punches just a single pinhole that could considerably ease the complexity of operating with small animals such as mouse.
Results: We showed that invivoPen could deliver similar efficacies as plasma activated medium with reduced cost in suppressing cell proliferation and migration as well as potentially boosting the viabilities of mice receiving invivoPen treatment. Blood test, renal and liver functionalities tests all suggest that physical plasma could effectively return tumor-carrying mice to the healthy state without harm to body conditions, and invivoPen slightly outweighs PAM in boosting animal immunity and reducing inflammation.
Conclusion: Our study contributes to the community in providing a minimal invasive in situ plasma source, having partly explained the efficacies of cold atmospheric plasma in treating triple negative breast cancers, and proposing the potential synergies between physical plasma and conventional drugs for cancer treatment.
Keywords: triple negative breast cancer, plasma jet, invivoPen, plasma activated medium, cancer treatment
Introduction
Physical plasma is the fourth state matter following solid, liquid and gas. It is an ionized gaseous electroneutral material composed of positively charged ions, electrons and neutral particles [1]. Physical plasma can be artificially generated by subjecting a neutral gas to a strong electromagnetic field to the point where ionized gaseous substances become increasingly electrically conductive. Cold atmospheric plasma (CAP) refers to non-equilibrium ionized gases under atmospheric pressure, with the temperature being, in general, between 25°C and 45°C [2].
CAP has been widely adopted in many biological applications such as disinfection/ sterilization [3], chronic wound healing and ulceration treatment [4]. Ever since the first report on the anti-cancer property of CAP in 2007 [5], its potential as an emerging oncotherapy has been gaining increased attention, with efficacies observed in cells of many tumors including, e.g., brain [6], skin [7], breast [8], colorectal [9], lung [10, 11], cervical [12], leukemia [13], liver [14], and head and neck [15, 16] cancers.
Typical configuration of include dielectric barrier discharge (DBD), plasma jet, and plasma torch [17]. One of the commonly used medically-relevant plasma source is kINPen MED [18] which, however, cannot penetrate through the skin and can only be used to treat cells or surface diseases. Mirpour et al. have established a micro-sized plasma jet for in vivo animal treatment [19] that requires an extra hole in the tissue for exhaust gas exhalation. However, in most cases, tumors grown in animal models such as mouse are small (<1 cm in diameter) and fragile; thus building a gas path within tumors might impose extra challenges to people who conduct the experiments. Also, if applied in clinics, generating two holes for a gas flow within solid tumors may create too invasive to patients that largely reduces their life qualities before achieving any desirable CAP therapeutic efficacy. As far as we know, in vivo CAP treatment is largely achieved using plasma activated medium (PAM) that preserves the activities of CAP within a short period of time such as a week [20, 21], and no in vivo CAP ejection tool with minimal invasion is so far available.
We found from our previous studies that CAP can selectively target triple negative breast cancer (TNBC) cells both in vitro and in vivo, a subtype of breast cancers lacking effective therapeutic modalities with little side effect. We are motivated to establish an in vivo CAP ejection platform feasible for solid tumor treatment to further investigate the therapeutic efficacies of CAP in treating TNBC tumors in vivo. We designed a CAP ejection source for minimal invasive in situ treatments, namely invivoPen, and demonstrated that it could convey similar efficacies in cancer proliferation and migration control but more favorable effects in preserving mice viabilities than PAM in treating TNBC-inoculated mice. We also proposed that invivoPen delivered its selective therapeutic efficacies on TNBCs through inducing luminal-like features of such cancers and selectively targeting cancer stem cells.
Materials and methods
Cell culture
A human breast cancer cell line, MDA-MB-231, purchased from American Type Culture Collection (Manassas, VA, USA), was used in this study. The cell line was cultured following supplier's recommendation. Cells were grown at 37 °C and 5% (v/v) CO2 in a humidified incubator. All media were purchased from Hyclone Laboratories, Inc. (United States).
Characterization of invivoPen
The temperatures of invivoPen at different peak-to-peak voltages were measured by the thermometer attached to the invivoPen outlet. A human impedance analog circuit [22] (Figure 2A) was used to simulate the current and voltage of invivoPen when it contacts with human body. The electrical properties of invivoPen were measured in 'skin resistance' given that invivoPen functions by puncturing through the skin. AC (Alternating Current) contact current and AC contact voltage between nodes A and B was measured by a multimeter.
UV-visible-NIR, with the wavelengths ranging from 200 nm to 800 nm, of the CAP generated by invivoPen was measured using Andor's Mechelle ME5000 spectrograph and iStar334T ICCD to detect reactive species such as nitrogen [N2], nitric oxide [-NO], [N+2], atomic oxygen [O], and hydroxyl radical [-OH].
Optical emission spectra measurement
The spectrometer and the detection probe were purchased from Andor Technology. The optical probe was placed 2 cm above the plasma jet nozzle. Integration time for data collection was set to 100 ms, frequency was set to 8.8 kHz, peak-to-peak output electrode voltage was set to 11 kV, and gas flow was controlled at 1 L·min-1.
In vivo tumor treatment
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Laboratory Center of Jiangnan University.
3×106 MDA-MB-231 cells suspended in 100 μL phosphate buffer solution (PBS) were injected subcutaneously in the right forelimbs of 16 female BALB/c mice aged 3-4 weeks with the weights of 16 ± 2 g on the first day. 15 MDAMB231 cell inoculated BALB/c mice were evenly divided into 3 groups, i.e., invivoPen group (receiving invivoPen treatment), PAM group (receiving PAM injection), and null group (no treatment). The first treatment was performed when tumor sizes reached 5 ± 0.5 mm, which were calculated following Equation 1:
Equation 1
where '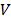 ', '
', '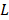 ' and '
' and '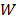 ' each represents the volume, largest diameter and smallest diameter of the tumor, respectively. Tumor diameters were measured using vernier caliper. Mice were anesthetized with ketamine (concentration is 10 mg·ml-1) intraperitoneally before each treatment. The injection volume was 10 μl·g-1 of the mouse body weight. Two approaches 'invivoPen treatment' and 'PAM injection' were conducted following procedures below:
' each represents the volume, largest diameter and smallest diameter of the tumor, respectively. Tumor diameters were measured using vernier caliper. Mice were anesthetized with ketamine (concentration is 10 mg·ml-1) intraperitoneally before each treatment. The injection volume was 10 μl·g-1 of the mouse body weight. Two approaches 'invivoPen treatment' and 'PAM injection' were conducted following procedures below:
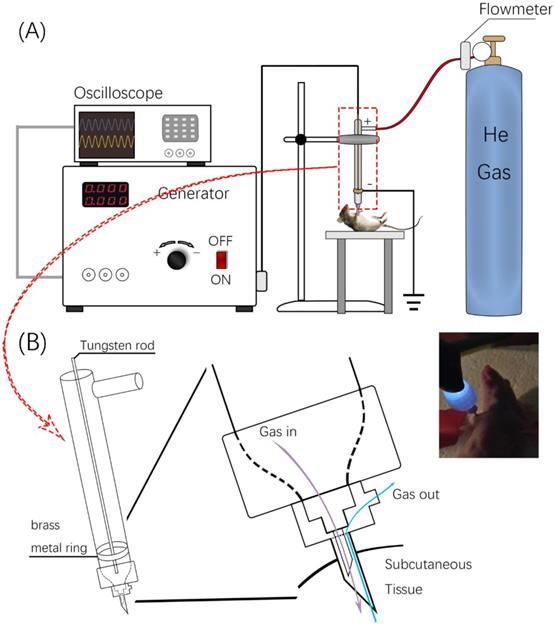
invivoPen treatment: a mouse was paralyzed following fixation on its back onto a liftable-insulation board. The generator of invivoPen was turned on, the peak-to-peak electrode voltage was set to 5 kV, the sinusoidal wave frequency was set to 8.8 kHz, and the Helium gas flow rate was set to 0.2 L·min-1. The nozzle of invivoPen was punched into mouse tumor through lifting the insulation board following 5 min CAP exposure. The heartbeat of mouse was monitored throughout the treatment process.
PAM injection: PAM was prepared by fixing the distance between invivoPen and the medium surface as 13 mm and treating each well of a 12-well plate for 10 min where each well was filled with 2 ml PBS. Parameters were set the same as in 'invivoPen treatment' and in optical emission spectra measurement. PAM was subcutaneously injected at two slots of the tumor for each mouse with 100 uL/slot following the same animal operation procedure as in 'invivoPen treatment'.
These treatments were repeated every 72 hours for each mouse until its death or the end of this study (30 days). Tumors were dissected after the sacrifice of the mice.
Animal blood test
Mice were intraperitoneally anesthetized before the blood was taken each time using 150μL 1% pentobarbital sodium solution. Four drops of blood were taken using a capillary tube through inserting into the mouse eye from the inner canthus and puncturing the posterior orbital vein. The blood was stored in centrifugal tubes infiltrated with 0.1% heparin sodium to prevent blood clotting. The blood routine analysis was operated by Mindray automatic blood analyzer.
Before the sacrifice of the mice, eyeball extraction was operated to collect blood for liver and renal functionality tests. For each mouse, the blood was placed in centrifuge tubes for 4 h following centrifugation at 3000 rpm for 10 min, and the extracted serum was used to test liver and renal functions. Indexes of liver and renal functions were measured by Mindray fully automatic biochemical analyser.
Immunohistochemistry staining (IHC)
The 5 μm thick paraffin sections were deparaffinized in xylene and rehydrated in ethanol at different gradients (100%, 100%, 95%, 70% in sequence). Tissue slices were incubated in 3% H2O2 for 20 min to inactivate endogenous peroxidase. After being heated in 10 mM citrate buffer for 15 min, tissue sections were incubated with primary antibodies ER (21244-1-AP, 1:200; Proteintech, Rosemont, IL, USA), HER2 (60311-1-Ig, 1:200; Proteintech), ALDH1 (#54135, 1:200; Cell Signaling Technology, St Louis, MO, USA) and E-Cadherin (#14472, 1:200; Cell Signaling Technology) overnight at 4°C. Corresponding secondary antibody (#8114; Cell Signaling Technology) was added and incubated for 1 hour at the room temperature. Images were observed with Pannoramic MIDI (3DHISTECH Ltd, Budapest, Hungary).
TUNEL assay
TUNEL assay was detected using the One Step TUNEL Apoptosis Assay Kit (C1086, Beyotime Biotechnology; Shanghai, PRC). After being deparaffinized, tissue slices were incubated at 37°C for 25 min in none-DNase proteinase-K working liquid (20μg·ml-1). TUNEL detection solution was added on the tissue slices and incubated at 37°C for 1 hour following dehydration. After dehydration, the tablet was sealed with the Antifade Mounting Medium with DAPI (H-1200, VECTOR Laboratories; Burlingame, CA). Samples were observed under a fluorescence microscope (ZEISS, Axio Imager Z2; Oberkochen, GER), where the green and blue fluorescence were observed under a 520 nm and a 460 nm laser, respectively.
Results
Configuration of InvivoPen
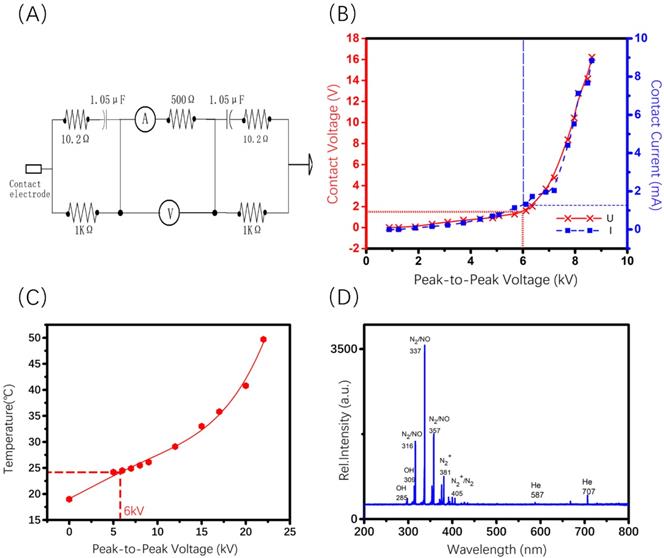
The invivoPen was developed as a dielectric block discharge (DBD) based CAP device for in vivo ejection, which is comprised of a high-voltage radio frequency power supply plasma generator (Coronalab, CTP-2000K), a Helium rotameter (KROHNE), and an oscilloscope (Tektronix, TBS 1102B), connected by two gas pipes, two high voltage wires, and a custom quartz tubes as the blocking medium (Figure 1).
The inner and outer diameters of the quartz tube are 3 mm and 9 mm, respectively. The length of invivoPen is 150 mm. A gas inlet with 30mm in length is 30 mm away from the upper end. The high voltage electrode is a tungsten steel rod with 1mm in diameter, and is fixed along the axis of the quartz dielectric tube in the middle. The negative electrode is a brass metal ring wrapping around the quartz tube, which is located 10 mm above the bottom, and the positive electrode is the tungsten rod inside the quartz tube. The space between the positive and negative electrodes is 1 mm (Figure 1A).
To execute a minimally invasive intratumoral treatment, a unique design of two co-axial needles with different calibers was fabricated in the pinhead. The diameters of the inner and outer needles are 0.25 mm and 0.5 mm, respectively, with the inclined face of the inner needle being in the opposite direction with that of the outer needle. The space in-between the inner and outer needles is the outlet for exhaust gas (Figure 1B).
Characterization of invivoPen
To maximize the treatment efficacy while ensuring the safety, the Peak-to-Peak voltage was set to 6 kV for invivoPen in the experiments throughout all in vivo experiments. The AC contact current was below 2 mA under 6 kV Peak-to-Peak voltage, which is safe to human [23]; and the AC contact voltage was below 2 V (Figure 2B), much lower than the International Electrotechnical Commission standard voltage, i.e., 50 V (IEC 60038-2009). Accordingly, the temperature at this voltage was below 30 °C (Figure 2C), which generated no burn. The Peak-to-Peak voltage was between 10 to 13 kV when generating PAM according to [24].
Reactive species produced from invivoPen were comprised of largely nitrogen oxides and hydroxide ions, with nitrogen oxides being the most abundant and almost no helium ions (Figure 2D).
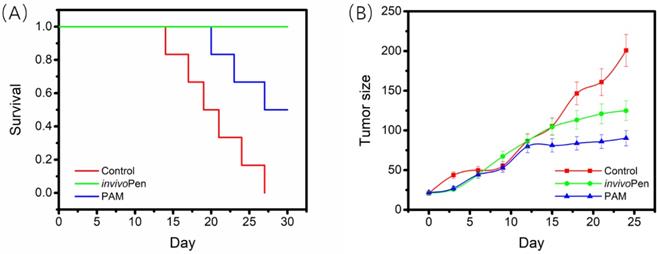
Characterization of treatment efficacy of invivoPen
All mice in the control group died within 27 days, 3 out of 5 mice from the TNBC-PAM group died within 30 days, and all mice from the TNBC-invivoPen group survived to the last day with relatively good viabilities. The 30-day survival of mice in the TNBC-invivoPen group was significantly higher than that of the TNBC-PAM group (p=4.9E-4, Figure 3A). Both invivoPen and PAM significantly inhibited tumor growth with statistical significance (p=0.044 for invivoPen, p=0.017 for PAM), yet the growth of tumors in the TNBC-PAM group was more suppressed than that in the invivoPen group (Figure 3B).
Design and usage of invivoPen. (A) Schematic illustration of invivoPen design. (B) In situ mouse treatment using invivoPen.
Charaterization of the components generated using invivoPen. (A) Human body impedance analog circuit, (B) Electrical characterization, (C) operating temperature examination, and (D) Optical emission spectra analysis of invivoPen.
Comparisons on invivoPen and PAM treatments in preserving mice viability and suppressing tumor growth. (A) The 30-day survival curves of nude mice after receiving CAP treatment. (B) The 30-day tumor growth curves after CAP treatment.
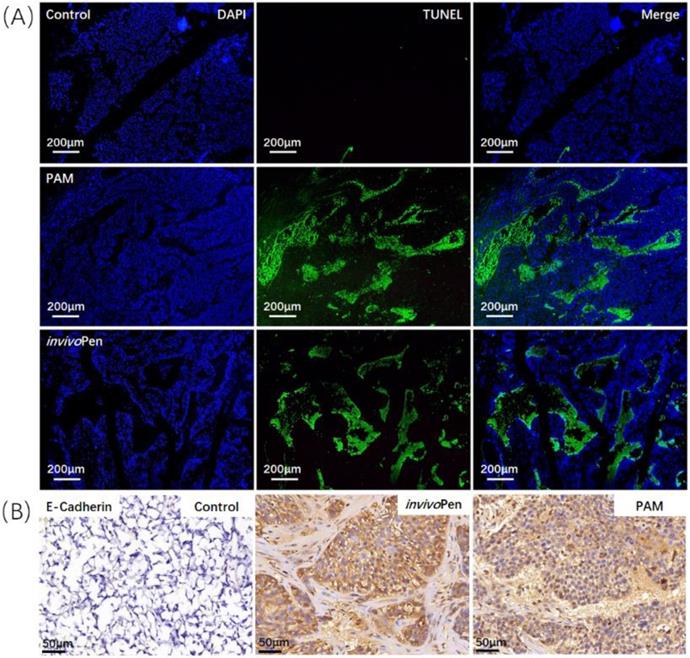
Both PAM and invivoPen treatments showed similar efficacies in inducing tumor cell apoptosis and suppressing tumor migrative abilities. A large number of cells undergone apoptosis in PAM- or invivoPen-treated tumor samples (Figure 4A). E-cadherin expression, a clinical immunohistochemistry marker with suppressive role on tumor migration [25, 26], was considerably induced in PAM or invivoPen-treated tumor samples (Figure 4B).
Safety assessment of invivoPen
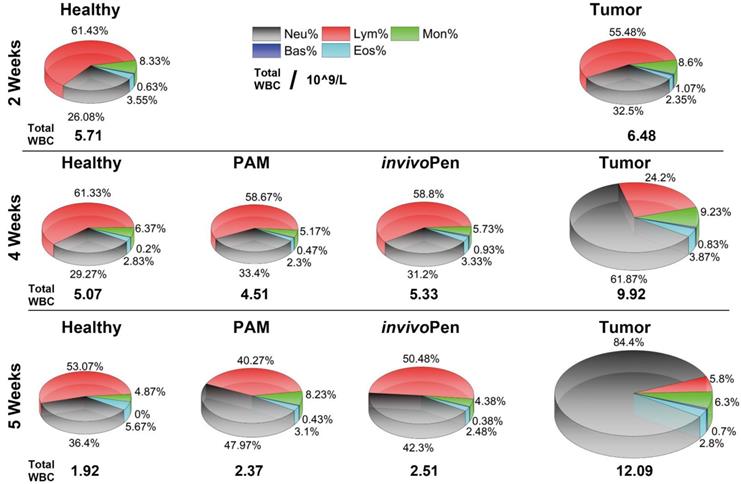
Blood test results showed that different types of white blood cells were all considerably altered in mice inoculated with tumor cells, and such differences were amplified with time (i.e., from 1.13 times at 2 weeks to 1.95 times at 4 weeks, and to 6.33 times at 5 weeks for the total while blood cells, Table 1), and indexes on red blood cells did not alter with statistical significance between healthy and tumor-carrying mice (Supplementary Table 1). Both PAM and invivoPen could effectively reduce the total white blood cell level and the fractions of different white blood cell types back to normal (Figure 5), and invivoPen slightly outweighs PAM in a few indexes such as monocytes, neutrophil granulocyte, eosinophil granulocyte, and basophil granulocyte (Table 1).
Creatine, uric acid, and urea (tested at the 5 weeks) were considerably increased in mice inoculated with tumor cells (Table 2), suggesting a damage on renal functionalities when tumor developed. PAM and invivoPen could both effectively reduce levels of these indexes back to normal (Table 2).
Comparisons on invivoPen and PAM treatments in inducing in vivo tumor apoptosis and reducing tumor migrative abilities. (A) Tumor cell apoptosis examined using TUNEL staining. (B) Tumor migrative ability examined using immunohistochemistry staining of tumor migration suppressor E-Cadherin. These assays were conducted on tumor samples from mice inoculated with TNBC cells MDBMA231.
Leukocyte components Analysis from the blood test.
| 2 Weeks | 4 Weeks | 5 Weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy | Tumor | Healthy | Tumor | PAM | invivoPen | Healthy | Tumor | PAM | invivoPen | |||
| WBC | 5.71 | 6.48 | 5.07 | 9.92 | 4.51 | 5.33 | 1.92 | 12.09 | 2.37 | 2.51 | ||
| Neu | 1.44 | 2.14 | 1.48 | 7.39 | 1.59 | 1.61 | 0.70 | 10.2 | 1.12 | 1.06 | ||
| Lym | 3.58 | 3.53 | 3.11 | 1.29 | 2.57 | 3.24 | 1.027 | 0.70 | 0.98 | 1.27 | ||
| Mon | 0.46 | 0.57 | 0.32 | 0.85 | 0.23 | 0.29 | 0.09 | 0.76 | 0.19 | 0.11 | ||
| Eos | 0.19 | 0.16 | 0.16 | 0.29 | 0.11 | 0.16 | 0.09 | 0.34 | 0.07 | 0.065 | ||
| Bas | 0.04 | 0.08 | 0.01 | 0.1 | 0.01 | 0.037 | 0 | 0.09 | 0.0067 | 0.005 | ||
The unit is 109/L. WBC, Neu, Lym, Mon, Eos and Bas each represents white blood cell, neutrophil granulocyte, lymphocyte, monocytes, eosinophil granulocyte and basophil granulocyte number, respectively.
Comparisons on white blood cells between healthy, tumor and tumor receiving invivoPen and PAM treatment in vivo at different testing time points.
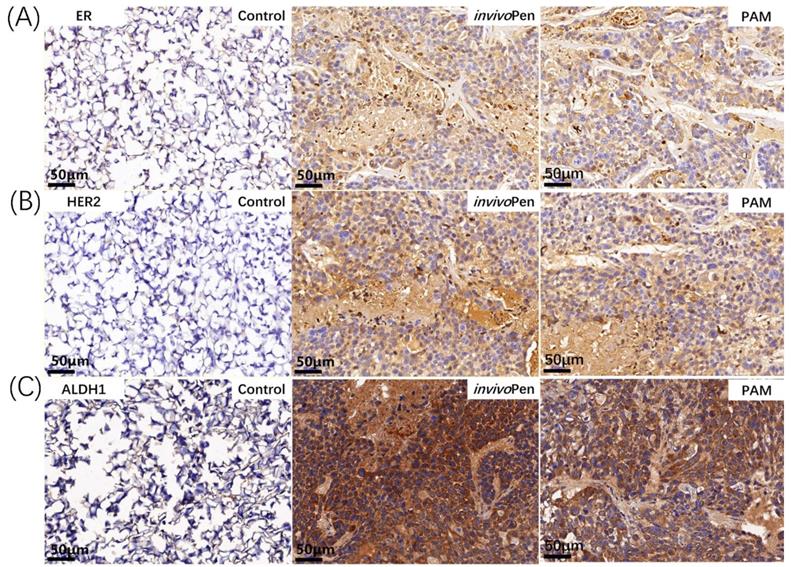
Immunohistochemistry staining of breast cancer subtyping and cancer stem cell markers in tumor samples. (A) ER and (B) HER2 are canonical breast cancer subtyping markers, and (C) ALDH1 is a typical cancer stem cell marker. The staining was conducted on tumor samples from mice inoculated with TNBC cells MDBMA231.
Renal function analysis from the urine test.
| CREA-S(μmol/L) | UA(μmol/L) | UREA(μmol/L) | |
|---|---|---|---|
| Healthy1 | 10.60 | 67.00 | 12.26 |
| Healthy2 | 13.4 | 58.90 | 15.80 |
| Healthy3 | 12.4 | 59.60 | 14.55 |
| Healthy4 | 11.9 | 81.40 | 10.61 |
| Tumor1 | 28.4 | 127.5 | 20.57 |
| Tumor2 | 36.9 | 146.5 | 23.35 |
| PAM1 | 21.4 | 94.00 | 12.60 |
| PAM2 | 13.3 | 56.80 | 17.92 |
| PAM3 | 12.7 | 77.90 | 14.53 |
| invivoPen1 | 13.1 | 83.60 | 18.55 |
| invivoPen2 | 22.2 | 112.6 | 17.98 |
| invivoPen3 | 17.1 | 56.40 | 16.25 |
| invivoPen4 | 17.4 | 68.90 | 21.02 |
CREA-S, UA and UREA each represents creatinine, uric acid and urea, respectively.
Indexes on liver functionalities such as alanine aminotransferase, aspartic transaminase, alkaline phosphatase, gamma-glutamyltransferase did not show significant between-group alterations but exhibited large within-group fluctuations (supplementary Table 2).
Characterization of the mechanism leading to CAP treatment efficacies
Both PAM- and invivoPen- treated TNBC tumors showed increased expression of ER and HER2 (Figure 6A, 6B), which are two primary canonical markers for breast cancer subtyping [27].
ALDH1 status changed from negative to positive after either PAM- or invivoPen-treatment (Figure 6C).
Discussion
Substances generated from invivoPen are largely comprised of nitrogen oxides and hydroxide ions, with little helium ions, indicating that a large amount of nitrogens and oxygens as well as some water vapor from the air are effectively reassembled to form different reactive oxygen and nitrogen species (RONS) capable of conferring multi-modal efficacies.
The invivoPen exhibited similar effects as PAM on suppressing cancer cell proliferation and migration, demonstrating the success of CAP ejection in situ without being clogged by the surrounding tissues through the use of invivoPen. Interestingly, mice treated with invivoPen delivered better viabilities and survival rates than those administrated with PAM, suggesting that direct CAP treatment in vivo could avoid any potential side effects caused by the cytotoxic substances generated from reactions between components in the medium and RONS. Also, it is indicative of a more stimulated immune system in mice by invivoPen than PAM. CAP has been reported capable of stimulating the activities of immune cells such as macrophages[28] and inducing immunogenic cell death[29-32] that enable its killing effect on distant cancer cells. Whether direct in situ CAP ejection such as using invivoPen can generate different in vivo effects on the immune system than PAM administration worths further investigations.
The animal blood test showed that after being exposed to CAP, either using PAM or invivoPen, indexes on white blood cells such as lymphocyte, monocytes, neutrophil granulocyte, eosinophil granulocyte, and basophil granulocyte returned to normal, and invivoPen showed higher efficacy in reducing monocyte and neutrophil granulocyte number than PAM. As a higher lymphocyte level is suggestive of a better immune response, and lower levels on monocytes, neutrophil granulocyte, eosinophil granulocyte, and basophil granulocyte implicate less inflammation, we would deduce from these results that physical plasma could boost animal immunity and confer anti-inflammation efficacy besides selectively killing cancer cells. Further, the slightly better performance observed on invivoPen in returning indexes on white blood cells back to the healthy state indicates the advantage of direct in vivo treatment over indirect PAM in cancer control and the necessity of designing and fabricating in vivo plasma ejection sources translating physical plasma into clinics.
Indexes on red blood cells including red blood cell, average corpuscular volume, average corpuscular hemoglobin, average corpuscular hemoglobin concentration were not significantly altered between healthy and tumor-bearing mice, and physical plasma did not cause significant fluctuations to them, suggesting that red blood cells are not relevant indexes here and being exposed to physical plasma, either in the form of PAM or invivoPen would not cause side effects on animal red blood cell levels.
Renal functionalities were dampened in mice inoculated with tumor cells, and both PAM and invivoPen treatments could effectively rescue these experimental animals from renal dysfunctionality, suggesting the efficacy of physical plasma in cancer control. The liver functionality assay did not show significant alterations among healthy mice, mice carrying tumors and tumors receiving PAM or invivoPen treatment overall, despite the large within-group variations that might be caused by the assay sensitivity to animal conditions at the time of test, further demonstrating the safety of physical plasma as an onco-therapy.
The cost of invivoPen is lower than PAM as it does not need liquid as the media to confer CAP efficacy, and the cost of fabricating invivoPen nozzle is equivalent to that of the needle used for PAM injection.
Both invivoPen and PAM treatment could induce ER and HER2 expression, suggesting a state transition of tumors from the triple negative to the luminal-like phenotype after CAP treatment. Being a less malignant type of breast cancers with known targeted therapies (e.g. Tamoxifen and Herceptin), such tumor state transition not only partially explains the more favorable outcome of mice observed in the experiments but also implicates potential synergies between CAP and Tamoxifen and/or Herceptin in targeting TNBCs.
Though the expression of ALDH1, a stem cell marker [33], increased after invivoPen or PAM treatment, ALDH1-high spots exhibited increased cell death, suggesting that cells with higher cancer stemness have higher sensitivities to CAP treatment. This is consistent with our observation that CAP could selectively target TNBC cells that have higher cancer stemness than the other breast cancer cells [24].
We present in this paper a novel in situ ejection source of CAP, namely invivoPen, for animal experiments or human treatments if applied in clinics. It can deliver similar efficacies as PAM with reduced cost and improved safety in suppressing cancer cell proliferation and migration, and is more protective on the viabilities of tested mice. This proposed invivoPen may serve as the prototype of the minimal invasive source for physical plasma ejection applied in clinics in the future.
Supplementary Material
Supplementary tables.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant No. 81972789), the National Science and Technology Major Project (Grant No. 2018ZX10302205-004-002), the Six Talent Peaks Project in Jiangsu Province (Grant No. SWYY-128), Technology Development Funding of Wuxi (Grant No. WX18IVJN017), Major Project of Science and Technology in Henan Province (Grant No.161100311400). These funding sources have no role in the writing of the manuscript or the decision to submit it for publication.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Goldston RJ, Rutherford PH. Introduction to plasma physics. Bristol, UK: Institute of Physics Publishing. 1995
2. Lee HJ, Shon CH, Kim YS, Kim S, Kim GC, Kong MG. Degradation of adhesion molecules of G361 melanoma cells by a non-thermal atmospheric pressure microplasma. New J Phys. 2009;11:115026
3. Mohd Nasir N, Lee BK, Yap SS, Thong KL, Yap SL. Cold plasma inactivation of chronic wound bacteria. Arch Biochem Biophys. 2016;605:76-85
4. Poor AE, Ercan UK, Yost A, Brooks AD, Joshi SG. Control of multi-drug-resistant pathogens with non-thermal-plasma-treated alginate wound dressing. Surg Infect (Larchmt). 2014;15:233-43
5. Yan D, Sherman JH, Keidar M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget. 2017;8:15977-95
6. Vandamme M, Robert E, Lerondel S, Sarron V, Ries D, Dozias S. et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int J Cancer. 2012;130:2185-94
7. Kaushik NK, Attri P, Kaushik N, Choi EH. A preliminary study of the effect of DBD plasma and osmolytes on T98G brain cancer and HEK non-malignant cells. Molecules. 2013;18:4917-28
8. Sun JK, Chung TH, Bae SH, Leem SH. Induction of apoptosis in human breast cancer cells by a pulsed atmospheric pressure plasma jet. Appl Phys Lett. 2010;97:023702-3
9. Plewa JM, Yousfi M, Frongia C, Eichwald O, Ducommun B, Merbahi N. et al. Low-temperature plasma-induced antiproliferative effects on multi-cellular tumor spheroids. New J Phy. 2014;16:043027
10. Sun JK, Joh HM, Chung TH. Production of intracellular reactive oxygen species and change of cell viability induced by atmospheric pressure plasma in normal and cancer cells. Appl Phys Lett. 2013;103:153705-5
11. Kim JY, Ballato J, Foy P, Hawkins T, Wei Y, Li J. et al. Apoptosis of lung carcinoma cells induced by a flexible optical fiber-based cold microplasma. Biosens Bioelectron. 2011;28:333-8
12. Kangil K, Hak Jun A, Jae Hyeok L, Jae Ho K, Sang Sik Y, Jong Soo K. Cellular membrane collapse by atmospheric-pressure plasma jet. Appl Phys Lett. 2014;104:013701-3
13. Thiyagarajan M, Anderson H, Gonzales XF. Induction of apoptosis in human myeloid leukemia cells by remote exposure of resistive barrier cold plasma. Biotechnol Bioeng. 2014;111:565-74
14. Xianhui Z, Maojin L, Rouli Z, Kecheng F, Size Y. Ablation of liver cancer cells in vitro by a plasma needle. Appl Phys Lett. 2008;93:3
15. Guerrero-Preston R, Ogawa T, Uemura M, Shumulinsky G, Valle BL, Pirini F. et al. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int J Mol Med. 2014;34:941-6
16. Kang SU, Cho JH, Chang JW, Shin YS, Kim KI, Park JK. et al. Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014;5:e1056
17. Xu X, Dai X, Xiang L, Cai D, Xiao S, Ostrikov K. Quantitative assessment of cold atmospheric plasma anti-cancer efficacy in triple-negative breast cancers. Plasma Processes and Polymers. 2018;15:1800052
18. Bekeschus S, Schmidt A, Weltmann K-D, von Woedtke T. The plasma jet kINPen - A powerful tool for wound healing. Clinical Plasma Medicine. 2016;4:19-28
19. Mirpour S, Piroozmand S, Soleimani N, Jalali Faharani N, Ghomi H, Fotovat Eskandari H. et al. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci Rep. 2016;6:29048
20. Mohades S, Barekzi N, Razavi H, Maruthamuthu V, Laroussi M. Temporal evaluation of the anti-tumor efficiency of plasma-activated media. Plasma Processes and Polymers. 2016;13:1206-11
21. Mohades S, Laroussi M, Sears J, Barekzi N, Razavi H. Evaluation of the effects of a plasma activated medium on cancer cells. Phys Plasmas. 2015;22:122001
22. Biegelmeier G. New Knowledge on the Impedance of the Human Body. Electrical Shock Safety Criteria. 1985 p. 115-32
23. Kouwenhoven WB. Effects of electricity on the human body. Electr Eng. 2013;68:199-203
24. Liangjian X, Xiaoyu X, Shuo Z, Dongyan C, Xiaofeng D. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radical Bio Med. 2018;124:205-13
25. Huntsman DG, Caldas C. Assignment1 of the E-cadherin gene (CDH1) to chromosome 16q22.1 by radiation hybrid mapping. Cytogenet Cell Genet. 1998;83:82-3
26. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Reviews Cancer. 2009;9:265-73
27. Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. 2009;15:593-602
28. Lee CB, Seo IH, Chae M-W, Park JW, Choi EH, Uhm HS. et al. Effects of Non-thermal Plasma Activated Water on the Anti-cancer Immune Activities of Macrophages. Clinical Plasma Medicine. 2018;9:31
29. Miller V, Lin A, Fridman AJPC, Processing P. Why Target Immune Cells for Plasma Treatment of Cancer. Springer. 2016;36:259-68
30. Lin A, Gorbanev Y, De Backer J, Van Loenhout J, Van Boxem W, Lemiere F. et al. Non-Thermal Plasma as a Unique Delivery System of Short-Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv Sci (Weinh). 2019;6:1802062
31. Lin A, Truong B, Patel S, Kaushik N, Choi EH, Fridman G. et al. Nanosecond-Pulsed DBD Plasma-Generated Reactive Oxygen Species Trigger Immunogenic Cell Death in A549 Lung Carcinoma Cells through Intracellular Oxidative Stress. Int J Mol Sci. 2017:18
32. Lin AG, Xiang B, Merlino DJ, Baybutt TR, Sahu J, Fridman A. et al. Non-thermal plasma induces immunogenic cell death in vivo in murine CT26 colorectal tumors. Oncoimmunology. 2018;7:e1484978
33. Resetkova E, Reis-Filho JS, Jain RK, Mehta R, Thorat MA, Nakshatri H. et al. Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res Treat. 2010;123:97-108
Author contact
![]() Corresponding author: Xiaofeng Dai, Email: xiaofeng.daiedu.cn, Mobile: +86 18168870169
Corresponding author: Xiaofeng Dai, Email: xiaofeng.daiedu.cn, Mobile: +86 18168870169

 Global reach, higher impact
Global reach, higher impact