Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(25):6191-6198. doi:10.7150/jca.36707 This issue Cite
Research Paper
The clinicopathological characteristic associations of long non-coding RNA gene H19 polymorphisms with uterine cervical cancer
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
2. Department of Obstetrics and Gynecology, Hsinchu MacKay Memorial Hospital, Hsinchu, Taiwan
3. Mackay Medicine, Nursing, and Management College, Taipei, Taiwan
4. Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung, Taiwan
5. Department of Radiation Oncology, Chung Shan Medical University Hospital, Taichung, Taiwan
6. Department of Obstetrics and Gynecology, Chung Shan Medical University Hospital, Taichung, Taiwan
7. School of Medicine, Chung Shan Medical University, Taichung, Taiwan
8. Department of Obstetrics and Gynecology, Chi-Mei Foundation Medical Center, Tainan, Taiwan
9. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
Received 2019-5-15; Accepted 2019-9-18; Published 2019-10-15
Abstract
The purposes of the current study were conducted to explore the relationships among long non-coding RNA gene H19 (LncRNA H19) polymorphisms and clinicopathological characteristics of uterine cervical cancer, and patient prognosis in Taiwan. Five genetic variants of LncRNA H19 rs3024270, rs2839698, rs3741219, rs2107425 and rs217727 were recruited from one hundred and thirty-four patients with invasive cancer, 101 with high-grade cervical intraepithelial neoplasia (CIN) of uterine cervix and 325 controls and their genetic distributions were determined. It indicated no associations of these LncRNA H19 genetic variants with development of cervical cancer. CC/CT in LncRNA H19 rs2839698 exhibited less risk to have pelvic lymph node metastasis [Odds ratio (OR): 0.19, 95% Confidence interval (CI):0.04-0.82, p=0.028)], as compared with TT. Meanwhile, cervical cancer patients with AA/AG in rs3741219 also had less risk to develop pelvic lymph node metastasis (OR: 0.17, 95% CI: 0.05-0.63, p=0.008), large tumor (OR: 0.17, 95% CI: 0.04-0.82, p=0.014) as well as parametrium (OR: 0.26, 95% CI: 0.07-0.95, p=0.045) and vagina invasion (OR: 0.25, 95% CI: 0.07-0.91, p=0.041, as compared to those with GG. However, only positive pelvic lymph node metastasis was related to worse recurrence-free survival and poor overall survival. Conclusively, it indicated no association of LncRNA H19 SNPs with cervical carcinogensis in Taiwanese women. Although genotypes TT in LncRNA H19 rs2839698 and GG in rs3741219 are related to some poor clinicopathological parameters of cervical cancer, only pelvic lymph node status could predict 5 year patient survival significantly.
Keywords: long non-coding RNA gene H19, uterine cervical cancer, clinicopathological characteristics, lymph node metastasis, 5 year survival
Introduction
Uterine cervical cancer was regarded as the most common gynecological cancer, if carcinoma in situ (CIS) was recruited, based on 2013 cancer registry annual report in Taiwan. Cervical carcinogenesis is a multistep process of neoplastic transition from cervical intraepithelial neoplasia (CIN), which was considered as precancerous lesions, to invasive cancer, which was found to be the end of this progressive intraepithelial dysplastic atypia [1, 2]. The histological diagnoses include CIN1 (low-grade CIN or mild dysplasia; mitoses and immature cells in the lower one-third of cervical epithelium) as well as high-grade CIN including CIN 2 (moderate dysplasia) and CIN 3 (severe dysplasia and CIS), which define involvement of mitoses and immature cells in middle and upper third respectively, were in accordance with their cytological counterparts, low-grade squamous cell intraepithelial lesions (LSIL) and high-grade squamous cell intraepithelial lesions (HSIL) [3].
Long non-coding RNA gene H19 (LncRNA gene H19), a 2.7 kb gene which expresses maternally and imprints paternally, is situated near the telomeric region of chromosome 11p15.5 and encodes a 2.3 kb long, capped, spliced, and polyadenylated noncoding RNA [4, 5]. The transcript of this gene LncRNA H19 was found by Brannan and is one of the first discovered LncRNAs [4, 6]. It indicated that H19 displays critical roles in embryonic growth and development tissues [7, 8] and is overexpressed in a variety of cancers, including gastric and breast cancers [9, 10]. MiR-675, which is known as one of the most important transcripts in the H19 locus, is located at exon 1 and implicated in the carcinogenesis and tumor invasiveness [11]. LncRNA H19 has been reported to be predictive of clinicopathological characteristics and H19 expression was associated with histological grades, clinical stages and lymph node metastasis status using a meta-analysis [12, 13].
Single nucleotide polymorphism (SNP) variants mean the form of DNA variation in human [14]. When genetic variants present in exon, promoter regions or 3'-untranslated region of a gene, they may exert the impacts on gene expression and biological processes [14]. Some SNPs within LncRNA genes have been identified to affect the expression and function of LncRNAs, which are known as regulatory RNAs longer than 200 nucleotides without protein-coding potential, and therefore have an influence on individual cancer susceptibility and patient prognosis [13, 15-17]. Recently, some studies demonstrated that LncRAN H19 SNPs was related to cancer development. To our knowledge, few studies investigate the clinical implication of H19 SNPs in uterine cervical cancer in Taiwan. Then, we inferred that H19 SNPs was associated with the development and progression of cervical cancer. We therefore conducted this study to investigate the relationships among H19 SNPs, cervical carcinogenesis and clinicopathological variables and patient prognosis.
Materials and Methods
Data source and participants
The individuals, enrolling 134 invasive cancer and 101 high-grade CIN of uterine cervix as well as 325 normal controls, were recruited consecutively from the Department of Obstetrics and Gynecology in Taichung Chung Shan Medical University Hospital, Taiwan from February 1994 to December 2014 in this retrospective study. Invasive cancer patients underwent the standard treatment protocols that were revised from National Comprehensive Cancer Network guidelines.
Cervical punch biopsy under colposcopy was performed for patients suspected to have invasive cancer or precancerous lesions and the diagnoses for these patients were clarified by pathology report. Normal controls were enrolled when they received Papanicolaou smears at outpatient department for general examination in same hospital and the normal cytologic diagnosis was compatible with and confirmed by normal colposcopic findings. Chung Shan Medical University Hospital institutional review board approved this study (CSMUH No: CS18208).
Selection of H19 genetic variants and DNA extraction from all participants
Five genetic polymorphisms of LncRNA H19 were selected based on the International HapMap Project dbSNP database for the current study. Furthermore, these SNPs of LncRNA H19 gene were selected since these SNPs were associated with the progression of the various cancers [18-21]. Genetic variants rs3024270, rs2839698, rs3741219, rs2107425 and rs217727 were included for genotypic determination. Hardy-Weinberg equilibrium value ≥ 0.05 was necessary.
Standard venipuncture technique was performed by the staffs to collect blood samples from all subjects and they were deposed into Vacutainer tubes merged with ethylenediaminetetraacetic acid. The specimens were immediately stored at 4℃. DNA was extracted from leukocytes based on previous protocols [22]. After extraction, DNA was dissolved into pH 7.8 TE buffer. Thereafter, it was quantified by the measurement of OD260. The OD260/OD280 ratio was then determined and the range of 1.8-2.0 met our criteria and thought to be pure to prevent its cross reactivity from the present homologous RNA in the specimens. The final products were then stored at -20°C and were applied as templates for the polymerase chain reaction (PCR).
Statistical analysis
Analysis of variance (ANOVA) was performed to compare the age distribution of the studied subjects with Tukey test for post hoc analysis. Hardy-Weinberg equilibrium was applied to detect the genotype frequencies of rs3024270, rs2839698, rs3741219, rs2107425 and rs217727 in normal controls [degree of freedom (d.f.)=2]. Chi-square or Fisher exact tests were used to investigate the associations between various H19 SNPs frequencies and the development of cervical neoplasia (comprising including invasive cancer and precancerous lesions) or among H19 distributions, cervical invasive cancer and precancerous lesions and normal controls. These tests were also used to associate H19 SNPs distributions with various clinicopathological characteristics.
A Cox proportional hazard model was used to assess the effects of H19 SNPs on the recurrence-free survival or overall survival after adjusting various clinicopathological characteristics in relation to follow-up intervals. In addition, Kaplan-Meier curves were used to plot the effect of H19 SNPs and significant clinicopathological variables for 5 year survival. The log-rank test was used to detect the differences between these curves. Hazard ratios (HRs) and their 95% confidence intervals (CI) were examined by the SPSS, version 12.0. A significant difference was defined if p value <0.05.
Results
Age distribution and Hardy-Weinberg equilibrium
Patients with cervical neoplasias exhibited significantly different age distribution from normal control women (50.3 ± 13.6 vs. 44.2 ± 10.4, p<0.001). It indicated that the age distribution was statistically different between patients with cervical cancer and control women (55.0 ± 12.2 vs. 44.2 ± 10.4, p<0.001) as well as between patients with cervical invasive cancer and those with precancerous lesions (55.0 ± 12.2 vs. 44.1 ± 12.9, p<0.001), but not statistically different between those with precancerous lesions and control women (44.1 ± 12.9 vs. 44.2 ± 10.4, p= 0.995), applied by the ANOVA using Tukey test for post hoc analysis.
The minor allele frequencies of H19 genetic polymorphisms rs3024270, rs2839698, rs3741219, rs2107425 and rs217727 in control women were all ≥5 %. In these controls group, genotypic frequency of H19 SNP rs3024270 satisfy the Hardy-Weinberg equilibrium [χ2 value, 1.020, p= 0.601; d.f.=2]. The distributions of H19 SNPs rs2839698, rs3741219, rs2107425 and rs217727 were also conform to the Hardy-Weinberg equilibrium (χ2 value, 0.015, p= 0.993; χ2 value, 0.361, p= 0.835; χ2 value, 0.450, p= 0.799 and χ2 value, 0.154, p= 0.926, respectively).
Association of H19 genetic polymorphisms with development of cervical cancer
Table 1 shows the genotypic distributions of LncRNA H19 SNPs in the Taiwanese women with cervical neoplasias and the controls. No significantly different frequencies of rs3024270, rs2839698, rs3741219, rs2107425 and rs217727 were exhibited between patients with cervical neoplasias and normal controls. Even after controlling for the age, no significant differences were still present for these SNPs between patients with cervical neoplasias and controls.
Although the cervical neoplasia group was subdivided into invasive cancer and precancerous lesion subgroups, no significant differences existed for the distributions of H19 rs3024270, rs2839698, rs3741219, rs2107425 and rs217727 among patients with invasive cancer and precancerous lesions as well as normal controls (Table 2). Even controlling for age, no significant differences were present for these SNPs.
Relationships of LncRNA H19 with clinicopathological characteristics
Patients with cervical invasive cancer, who present with CC/CT in LncRNA H19 rs2839698, had less risk to develop pelvic lymph node metastasis, as compared to those with TT (OR: 0.19, 95% CI: 0.04-0.82, p=0.028; Table 3). Meanwhile, cervical cancer patients with AA/AG in rs3741219 also exhibited less risk to develop pelvic lymph node metastasis, as compared to those with GG (OR: 0.17, 95% CI: 0.05-0.63, p=0.008; Table 3). In addition, these patients exerted less risk to have large tumor (OR: 0.17, 95% CI: 0.04-0.82, p=0.014), parametrium invasion (OR: 0.26, 95% CI: 0.07-0.95, p=0.045) and vagina invasion (OR: 0.25, 95% CI: 0.07-0.91, p=0.041; Table 3). However, there were no significant associations of the distribution of LncRNA H19 rs3024270, rs2107425 and rs217727 with clinicopathological characteristics.
Impact of H19 genetic polymorphisms and recurrence-free survival and overall survival of the patients with cervical invasive cancer
In correlating LncRNA H19 rs2839698 and rs3741219 with patient prognosis, Cox proportional hazard model was applied. However, rs2839698 and rs3741219 were not associated with recurrence-free survival of cervical cancer patients (p=0.932 and 0.935 respectively; Table 4). H19 rs2839698 and rs3741219 also could not predict patient overall survival (p=0.961 and 0.959 respectively). Only positive pelvic lymph node metastasis was related to worse recurrence-free survival (HR: 2.81, 95% CI: 1.09-7.26, p=0.033) and poor overall survival (HR: 4.79, 95% CI: 1.81-12.66, p=0.002; Table 4).
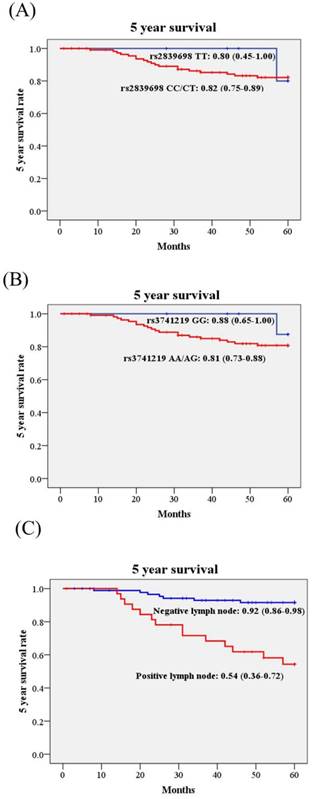
Kaplan-Meier curves were used to plot the influence of H19 SNPs and clinicopathological variables on 5 year survival rate in cervical cancer patients. Although H19 rs3741219 were associated with some clinicopathological parameters, the 5 year survival rates were not different in patients with different genotypes. The 5 year survival rate of patients with AA/AG was 0.81 (95% CI: 0.73-0.88), while those with GG 0.88 (95%: 0.65-1.00; Figure 1). Similar effect was noted in rs2839698. Only patients with different lymph node status present different 5 year survival rate significantly (Figure 1C). The 5 year survival rate of cervical cancer patients with positive lymph node metastasis was only 0.54 (95% CI: 0.36-0.72) as compared to those with negative lymph node metastasis 0.92 (95% CI: 0.86-0.98; Figure 1C).
Genetic variant frequencies of the H19 gene in Taiwanese women with uterine cervical neoplasias and normal controls
| Genetic polymorphisms | Normal controls (n =325) | Cervical neoplasiasa (n=235) | ORs (95% CIs) | p values | AORs (95% CIs)b | Adjusted p values |
|---|---|---|---|---|---|---|
| rs3024270 | 0.512 | 0.371 | ||||
| CCc | 71 | 51 | 1.00 | 1.00 | ||
| CG | 150 | 120 | 1.11 (0.72-1.72) | 1.11 (0.71-1.74) | 0.651 | |
| GG | 95 | 60 | 0.88 (0.54-1.43) | 0.82 (0.50-1.36) | 0.445 | |
| CCc | 71 | 51 | 1.00 | 0.914 | 1.00 | 0.984 |
| CG/GG | 245 | 180 | 1.02 (0.68-1.54) | 1.00 (0.65-1.52) | ||
| GGc | 95 | 60 | 1.00 | 0.295 | 1.00 | 0.183 |
| CC/CG | 221 | 171 | 1.23 (0.84-1.79) | 1.31 (0.88-1.94) | ||
| rs2839698 | 0.936 | 0.981 | ||||
| CCc | 154 | 115 | 1.00 | 1.00 | ||
| CT | 134 | 99 | 0.99 (0.69-1.41) | 1.04 (0.72-1.50) | 0.849 | |
| TT | 30 | 20 | 089 (0.48-1.65) | 1.00 (0.72-1.50) | 0.991 | |
| CCc | 154 | 115 | 1.00 | 0.868 | 1.00 | 0.866 |
| CT/TT | 164 | 119 | 0.97 (0.69-1.36) | 1.03 (0.73-1.46) | ||
| TTc | 30 | 20 | 1.00 | 0.720 | 1.00 | 0.967 |
| CC/CT | 288 | 214 | 1.12 (0.62-2.02) | 1.01 (0.55-1.87) | ||
| rs3741219 | 0.875 | 0.895 | ||||
| AAc | 152 | 112 | 1.00 | 1.00 | ||
| AG | 130 | 100 | 1.04 (0.73-1.49) | 1.09 (0.75-1.58) | 0.649 | |
| GG | 32 | 21 | 0.89 (0.49-1.63) | 1.00 (0.54-1.88) | 0.989 | |
| AAc | 152 | 112 | 1.00 | 0.937 | 1.00 | 0.692 |
| AG/GG | 162 | 121 | 1.01 (0.72-1.42) | 1.07 (0.76-1.53) | ||
| GGc | 32 | 21 | 1.00 | 0.645 | 1.00 | 0.906 |
| AA/AG | 282 | 212 | 1.15 (0.64-2.04) | 1.04 (0.57-1.89) | ||
| rs2107425 | 0.684 | 0.474 | ||||
| CCc | 109 | 88 | 1.00 | 1.00 | ||
| CT | 155 | 107 | 0.86 (0.59-1.24) | 0.79 (0.54-1.17) | 0.236 | |
| TT | 48 | 38 | 0.98 (0.59-1.63) | 0.94 (0.56-1.59) | 0.823 | |
| CCc | 109 | 88 | 1.00 | 0.496 | 1.00 | 0.308 |
| CT/TT | 203 | 145 | 0.89 (0.62-1.26) | 0.83 (0.57-1.19) | ||
| TTc | 48 | 38 | 1.00 | 0.770 | 1.00 | 0.762 |
| CC/CT | 264 | 195 | 0.93 (0.59-1.48) | 0.93 (0.58-1.50) | ||
| rs217727 | 0.875 | 0.855 | ||||
| CCc | 135 | 102 | 1.00 | 1.00 | 0.632 | |
| CT | 139 | 103 | 0.98 (0.68 (1.41) | 0.91 (0.63-1.33) | 0.663 | |
| TT | 39 | 28 | 0.95 (0.55-1.65) | 0.88 (0.50-1.56) | ||
| CCc | 135 | 102 | 1.00 | 0.880 | 1.00 | 0.584 |
| CT/TT | 178 | 131 | 0.97 (0.69-1.37) | 0.91 (0.63-1.29) | ||
| TTc | 39 | 28 | 1.00 | 0.876 | 1.00 | 0.771 |
| CC/CT | 274 | 205 | 1.04 (0.62-1.75) | 1.08 (0.63-1.85) |
Statistical analysis: logistic regression model or chi-square test. aCervical neoplasias included precancerous lesions and invasive cancer of the uterine cervix. bThe adjusted p values as well as adjusted odds ratios and their 95% confident intervals were examined by logistic regression model after controlling for age. cUsed as a reference for comparison to determine the odds ratios of other genotypes. 95% CIs, 95% confidence intervals.
Genetic variant frequencies of H19 in Taiwanese women with invasive cancer or precancerous lesions of uterine cervix and normal controls
| Genetic polymorphisms | Normal controls (n =325) | Pre-cancerous lesions (n =101) | Invasive cancer (n =134) | p values | AORs (95% CIs)a | Ad. p values | AORs (95% CIs)b | Ad. p values |
|---|---|---|---|---|---|---|---|---|
| rs3024270 | ||||||||
| CCc | 71 | 25 | 26 | 0.629 | 1.00 | 1.00 | ||
| CG | 150 | 51 | 69 | 1.41 (0.81-2.45) | 0.228 | 1.31 (0.78-2.20) | 0.299 | |
| GG | 95 | 23 | 37 | 1.46 (0.77-2.78) | 0.252 | 0.98 (0.51-1.86) | 0.945 | |
| CCc | 71 | 25 | 26 | 0.601 | 1.00 | 1.00 | ||
| CG/GG | 245 | 74 | 106 | 0.86 (0.51-1.45) | 0.565 | 1.22 (0.70-2.11) | 0.490 | |
| GGc | 95 | 23 | 37 | 0.419 | 1.00 | 1.00 | ||
| CC/CG | 221 | 76 | 95 | 1.42 (0.84-2.41) | 0.188 | 1.20 (0.74-1.96) | 0.455 | |
| rs2839698 | ||||||||
| CCc | 154 | 46 | 69 | 0.820 | 1.00 | 1.00 | ||
| CT | 134 | 45 | 54 | 1.01 (0.46-2.22) | 0.988 | 1.11 (0.47-2.60) | 0.815 | |
| TT | 30 | 10 | 10 | 0.89 (0.41-1.97) | 0.781 | 1.15 (0.50-2.67) | 0.739 | |
| CCc | 154 | 46 | 69 | 0.622 | 1.00 | 1.00 | ||
| CT/TT | 164 | 55 | 64 | 1.12 (0.72-1.76) | 0.611 | 0.94 (0.61-1.47) | 0.798 | |
| TTc | 30 | 10 | 10 | 0.769 | 1.00 | 1.00 | ||
| CC/CT | 288 | 91 | 123 | 0.95 (0.45-2.01) | 0.886 | 1.13 (0.50-2.56) | 0.764 | |
| rs3741219 | ||||||||
| AAc | 152 | 44 | 68 | 0.869 | 1.00 | 1.00 | ||
| AG | 130 | 46 | 54 | 1.22 (0.76-1.97) | 0.407 | 0.98 (0.62-1.56) | 0.928 | |
| GG | 32 | 9 | 12 | 0.97 (0.43-2.20) | 0.947 | 1.01 (0.46-2.22) | 0.988 | |
| AAc | 152 | 44 | 68 | 0.634 | 1.00 | 1.00 | ||
| AG/GG | 162 | 55 | 66 | 1.17 (0.75-1.85) | 0.489 | 0.98 (0.63-1.53) | 0.939 | |
| GGc | 32 | 9 | 12 | 0.899 | 1.00 | 1.00 | ||
| AA/AG | 282 | 90 | 122 | 1.13 (0.52-2.47) | 0.752 | 0.99 (0.46-2.12) | 0.969 | |
| rs2107425 | ||||||||
| CCc | 109 | 39 | 49 | 0.751 | 1.00 | 1.00 | ||
| CT | 155 | 43 | 64 | 0.77 (0.47-1.27) | 0.310 | 0.83 (0.51-1.36) | 0.461 | |
| TT | 48 | 19 | 19 | 1.10 (0.58-2.10) | 0.770 | 0.85 (0.43-1.67) | 0.633 | |
| CCc | 109 | 39 | 49 | 0.771 | 1.00 | 1.00 | ||
| CT/TT | 203 | 62 | 83 | 0.85 (0.53-1.35) | 0.492 | 0.84 (0.53-1.33) | 0.449 | |
| TTc | 48 | 19 | 19 | 0.629 | 1.00 | 1.00 | ||
| CC/CT | 264 | 82 | 113 | 0.79 (0.44-1.41) | 0.421 | 1.06 (0.57-1.96) | 0.853 | |
| rs217727 | ||||||||
| CCc | 135 | 45 | 57 | 0.900 | 1.00 | 1.00 | ||
| CT | 139 | 41 | 62 | 0.88 (0.54-1.44) | 0.617 | 0.96 (0.60-1.54) | 0.875 | |
| TT | 39 | 14 | 14 | 1.07 (0.53-1.26) | 0.842 | 0.75 (0.36-1.57) | 0.447 | |
| CCc | 135 | 45 | 57 | 0.937 | 1.00 | |||
| CT/TT | 178 | 55 | 76 | 0.93 (0.59-1.46) | 0.736 | 0.91 (0.58-1.43) | 0.695 | |
| TTc | 39 | 14 | 14 | 0.739 | 1.00 | 1.00 | ||
| CC/CT | 274 | 86 | 119 | 0.88 (0.45-1.69) | 0.692 | 1.30 (0.65-2.61) | 0.452 |
aAdjusted p values and adjusted odds ratios with their 95% CIs were examined using multiple and multinomial logistic regression models after controlling for age between patients with uterine cervical precancerous lesions and control women. bAdjusted p values and adjusted odds ratios with their 95% CIs were examined using multiple and multinomial logistic regression models after controlling for age between patients with uterine cervical invasive cancer and control women. cUsed as a reference for comparison to determine the odds ratios of other genotypes. AORs, adjusted odds ratios; 95% CIs, 95% confidence intervals; Ad. p, adjusted p.
Relationships of genotypic distribution of H19 rs2839698 and rs3741219 with clinicopathological characteristics of the patients with uterine cervical invasive cancer
| Characteristicsa | rs2839698 | ORs (95% CIs) | p value | rs3741219 | ORs (95% CIs) | p value | |||
|---|---|---|---|---|---|---|---|---|---|
| TTb | CC/CT | GGb | AA/AG | ||||||
| Clinical stage | 0.122 | 0.091 | |||||||
| stage Ib | 2 | 73 | 1.00 | 3 | 72 | 1.00 | |||
| ≥ stage II | 5 | 47 | 0.26 (0.05-1.38) | 7 | 46 | 0.27 (0.07-1.11) | |||
| Pathologic type | 0.634 | 1.00 | |||||||
| squamous cell carcinomab | 6 | 99 | 1.00 | 9 | 97 | 1.00 | |||
| adenocarcinoma | 2 | 21 | 0.64 (0.12-3.37) | 2 | 21 | 0.97 (0.20-4.84) | |||
| Cell grading | 0.617 | 1.00 | |||||||
| well (grade 1)b | 2 | 19 | 1.00 | 2 | 19 | 1.00 | |||
| moderate & poor (grades 2/3) | 6 | 101 | 1.77 (0.33-9.45) | 9 | 99 | 1.16 (0.23-5.79) | |||
| Stromal invasion depth | 0.724 | 0.591 | |||||||
| ≤10 mmb | 3 | 55 | 1.00 | 6 | 53 | 1.00 | |||
| >10 mm | 5 | 62 | 0.68 (0.15-2.96) | 5 | 62 | 1.40 (0.41-4.86) | |||
| Tumor diameterb | 0.142 | 0.014c | |||||||
| ≤ 4cm | 2 | 67 | 1.00 | 2 | 67 | 1.00 | |||
| >4cm | 6 | 53 | 0.26 (0.05-1.36) | 9 | 51 | 0.17 (0.04-082) | |||
| Parametrium | 0.117 | 0.045c | |||||||
| no invasionb | 3 | 80 | 1.00 | 4 | 79 | 1.00 | |||
| invasion | 5 | 37 | 0.28 (0.06-1.22) | 7 | 36 | 0.26 (0.07-0.95) | |||
| Vagina | 0.117 | 0.041c | |||||||
| no invasionb | 3 | 82 | 1.00 | 4 | 82 | 1.00 | |||
| invasion | 5 | 38 | 0.28 (0.06-1.22) | 7 | 36 | 0.25 (0.07-0.91) | |||
| Pelvic lymph node | 0.028c | 0.008c | |||||||
| no metastasisb | 3 | 91 | 1.00 | 4 | 90 | 1.00 | |||
| metastasis | 5 | 28 | 0.19 (0.04-0.82) | 7 | 27 | 0.17 (0.05-0.63) | |||
Statistical analyses: chi-square or Fisher's exact tests, cp<0.05. aSome clinicopathological data could not be obtained from the patients with cervical invasive cancer due to incomplete medical charts or records. bAs a reference. ORs, odds ratios; 95% CIs, 95% confidence intervals.
Analysis for the associations of H19 genetic variants rs2839698 and rs3741219 as well as various clinicopatholgical characteristics with the recurrence-free survival and overall survival of the patients with uterine cervical cancer
| Recurrence-free survival | Overall survival | ||||
|---|---|---|---|---|---|
| Variables | p value | HR & 95% CIb | p value | HR & 95% CIb | |
| H19 genetic polymorphisms | 0.932 | - | 0.961 | - | |
| rs2839698 CC/CT vs. TTa | |||||
| rs3741219 AA/AG vs. GGa | 0.935 | - | 0.959 | - | |
| Clinicopathological characteristics | |||||
| Pelvic lymph node | |||||
| metastasis vs. no metastasisa | 0.033 | 2.81 (1.09-7.26) | 0.002 | 4.79 (1.81-12.66) | |
Statistical analyses: Cox proportional hazard model. aAs a comparison reference. bHR, hazard ratio and 95% CI, 95% confidence interval for H19 genetic variants rs2839698 and rs3741219 and clinicopathological variables, compared to their respective controls.
Discussion
It indicated that there were no significantly different frequencies of LncRNA H19 SNPs rs3024270, rs2839698, rs3741219, rs2107425 and rs217727 between patients with cervical neoplasias and controls in Taiwan in the current study. Even after the cervical neoplasia group was classified into invasive cancer and precancerous lesion subgroups, it still displayed no significant differences for the genotypic frequencies of these H19 SNPs among patients with invasive cancer and precancerous lesions as well as normal controls. They were not involved in cervical carcinogenesis. Using meta-analysis, Lu et al. found that H19 SNP rs217727 was not significantly correlated with cancer risk in dominant (TT + CT vs. CC) and recessive (TT vs. CT + CC) genetic models [23]. However, genotypes CT/TT have been reported to be correlated with reduced risk of breast cancer [24] and elevated risk of gastric and bladder cancers [25, 26]. Guo et al. found that H19 SNP rs217727 is significantly associated with the susceptibility of oral squamous cell carcinoma in Chinese population [27]. In contrast to our findings, Jin et al. demonstrated that the genotypes CT/TT in H19 SNP rs217727 carried the elevated risk of cervical cancer (OR: 1.62, 95% CI: 1.15-2.29; p=0.0059) in Chinese population as compared to CC [28]. H19 rs217727 is located on the exon 5 [29]. SNPs in the exon or promoter area of H19 gene may influence the expression of H19 or the structure and function of the H19 RNA [19, 21, 29]. This is supported by the cumulative evidence to reveal that abnormal H19 expression is found in bladder cancer and hepatocellular carcinoma, and increases cancer cell proliferation, indicating an oncogenic function [7, 30, 31]. H19 SNP rs2839698 mutation has been reported to reduce the risk of bladder cancer [32]. However, Li et al. demonstrated that H19 SNP rs2839698 was related to increased risk of colorectal cancer in Chinese population [29]. Moreover, H19 rs210742 was found to be associated with the risk of lung cancer significantly [33]. But, H19 SNP rs3741219 was not found to be associated with the risk of breast cancer [24].
Kaplan-Meier curves for 5 year survival rate based on long non-coding RNA gene H19 polymorphisms and pelvic lymph node status. (A), rs2839698 CC/CT vs. TT, 5 year survival rate: 0.82, 95% CI: 0.75-0.89 vs. 0.80, 95% CI: 0.45-1.00; p=0.722); (B), rs3741219 AA/AG vs. GG, 5 year survival rate: 0.81, 95% CI: 0.73-0.88 vs. 0.88, 95% CI: 0.65-1.00; p=0.429); (C), pelvic lymph node status, positive vs. negative, 5 year survival rate: 0.54, 95% CI: 0.36-0.72 vs. 0.92, 95% CI: 0.86-0.98; p<0.001). Log-rank test was applied to detect statistical significance. 95% CI, 95% confidence interval.
H19 SNP rs2839698 is located within the 3ʹ untranslated region (3ʹ UTR) of exon 1 of LncRNA gene H19 and probably affects the function of H19 gene to some extents through the changes of crucial folding structures and targeting miRNA [29]. Variants in 3ʹ UTR of H19 SNP rs2839698 leads to the loss of hsa-miR-24-1-5p and hsa-miR-24-2-5p function, which present as tumor suppressors and therefore their reduced expressions may promote invasiveness in some cancers such as colorectal and bladder cancers [34, 35]. Xia et al. revealed that the base A mutated to G in H19 SNP rs3741219, which is located within exon 5, may create hsa-miR-1539 miRNA binding sites on H19 and probably changes the LncRNA structure, influences its stability and affects miRNA-LncRNA interaction [24]. The functions of H19 can be dissected into two main actions; in addition to presenting as a modulator of micro-RNAs or proteins via their binding, H19 is also the mainly precursor of miR-675 and may determine its level, which possesses multiple targets in diverse signaling pathways, and then regulates a variety of biological processes via target inhibition by miR-675 [36, 37]. Reduced miR-675 expression has been reported to be correlated with invasion of hepatocellular carcinoma [38]. Furthermore, H19 may be involved in epithelial to mesenchymal transition and promotes invasion and metastasis of some solids cancers such as pancreatic cancer and hepatocellular carcinoma [38-43].
The elevated expression of LncRNA H19 was reported to be associated with poor prognosis in gastric cancer, cervical cancer and lung cancer [9, 44, 45]. Our study revealed that LncRNA H19 SNPs rs2839698 and rs3741219 were related to pelvic lymph node metastasis, the most critical prognostic parameter for the survival of cervical cancer patients [46, 47]. However, they could not predict patient survival significantly in the present study. Yang et al. demonstrated no significant association of H19 SNPs with overall survival of patients with hepatocellular carcinoma [48]. Until now, no study presents a significant association of H19 SNPs with survival of cervical cancer patients.
There were some limitations in the current research. Firstly, we only studied the central Taiwan population and did not recruit residents of other areas. Secondly, the sample size was relatively small especially for the invasive cancer and precancerous groups. This may restrict the possible subgroup analysis. Thirdly, because the controls were enrolled from outpatient department for general examination in our hospital and there was no information of the human papillomavirus infection, which could not be analyzed for the impact of this factor.
In conclusion, the present research reveals no association of LncRNA H19 SNPs with cervical carcinogensis in Taiwanese women. However, genotypes TT in LncRNA H19 rs2839698 and GG in rs3741219 are related to some poor clinicopathological parameters of cervical cancer. But they cannot predict patient survival significantly.
Acknowledgements
The research is supported by grants from Taiwan Ministry of Science and Technology (MOST 107-2314-B-040-017-MY3) and Chung Shan Medical University Hospital (CSH-2019-D-003). This study was also supported by Chung Shan Medical University and Hsinchu MacKay Memorial Hospital (CSMU-HCMMH-107-01).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bharti AC, Shukla S, Mahata S, Hedau S, Das BC. Anti-human papillomavirus therapeutics: facts & future. Indian J Med Res. 2009;130:296-310
2. Baak JP, Kruse AJ, Robboy SJ, Janssen EA, van Diermen B, Skaland I. Dynamic behavioural interpretation of cervical intraepithelial neoplasia with molecular biomarkers. J Clin Pathol. 2006;59:1017-1028
3. Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR. et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072-1079
4. Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10:28-36
5. Lecerf C, Le Bourhis X, Adriaenssens E. The long non-coding RNA H19: an active player with multiple facets to sustain the hallmarks of cancer. Cell Mol Life Sci. 2019
6. Ma Y, Zhang P, Wang F, Yang J, Yang Z, Qin H. The relationship between early embryo development and tumourigenesis. J Cell Mol Med. 2010;14:2697-2701
7. Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu-lail R, Hochberg A. et al. The H19 non-coding RNA is essential for human tumor growth. PLoS One. 2007;2:e845
8. Gabory A, Jammes H, Dandolo L. The H19 locus: role of an imprinted non-coding RNA in growth and development. Bioessays. 2010;32:473-480
9. Li H, Yu B, Li J, Su L, Yan M, Zhu Z. et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318-2329
10. Berteaux N, Aptel N, Cathala G, Genton C, Coll J, Daccache A. et al. A novel H19 antisense RNA overexpressed in breast cancer contributes to paternal IGF2 expression. Mol Cell Biol. 2008;28:6731-6745
11. Huang JL, Zheng L, Hu YW, Wang Q. Characteristics of long non-coding RNA and its relation to hepatocellular carcinoma. Carcinogenesis. 2014;35:507-514
12. Liu FT, Pan H, Xia GF, Qiu C, Zhu ZM. Prognostic and clinicopathological significance of long noncoding RNA H19 overexpression in human solid tumors: evidence from a meta-analysis. Oncotarget. 2016;7:83177-83186
13. Aalijahan H, Ghorbian S. Long non-coding RNAs and cervical cancer. Exp Mol Pathol. 2019;106:7-16
14. Shastry BS. SNPs: impact on gene function and phenotype. Methods Mol Biol. 2009;578:3-22
15. Weng SL, Wu WJ, Hsiao YH, Yang SF, Hsu CF, Wang PH. Significant association of long non-coding RNAs HOTAIR genetic polymorphisms with cancer recurrence and patient survival in patients with uterine cervical cancer. Int J Med Sci. 2018;15:1312-1319
16. Tao R, Hu S, Wang S, Zhou X, Zhang Q, Wang C. et al. Association between indel polymorphism in the promoter region of lncRNA GAS5 and the risk of hepatocellular carcinoma. Carcinogenesis. 2015;36:1136-1143
17. Lv Z, Xu Q, Yuan Y. A systematic review and meta-analysis of the association between long non-coding RNA polymorphisms and cancer risk. Mutat Res. 2017;771:1-14
18. Cui P, Zhao Y, Chu X, He N, Zheng H, Han J. et al. SNP rs2071095 in LincRNA H19 is associated with breast cancer risk. Breast Cancer Res Treat. 2018;171:161-171
19. Li XF, Yin XH, Cai JW, Wang MJ, Zeng YQ, Li M. et al. Significant association between lncRNA H19 polymorphisms and cancer susceptibility: a meta-analysis. Oncotarget. 2017;8:45143-45153
20. Wu ER, Chou YE, Liu YF, Hsueh KC, Lee HL, Yang SF. et al. Association of lncRNA H19 Gene Polymorphisms with the Occurrence of Hepatocellular Carcinoma. Genes (Basel). 2019:10
21. Yang PJ, Hsieh MJ, Hung TW, Wang SS, Chen SC, Lee MC. et al. Effects of Long Noncoding RNA H19 Polymorphisms on Urothelial Cell Carcinoma Development. Int J Environ Res Public Health. 2019:16
22. Wu HH, Liu YF, Yang SF, Lin WL, Chen SC, Han CP. et al. Association of single-nucleotide polymorphisms of high-mobility group box 1 with susceptibility and clinicopathological characteristics of uterine cervical neoplasia in Taiwanese women. Tumour Biol. 2016
23. Lu Y, Tan L, Shen N, Peng J, Wang C, Zhu Y. et al. Association of lncRNA H19 rs217727 polymorphism and cancer risk in the Chinese population: a meta-analysis. Oncotarget. 2016;7:59580-59588
24. Xia Z, Yan R, Duan F, Song C, Wang P, Wang K. Genetic Polymorphisms in Long Noncoding RNA H19 Are Associated With Susceptibility to Breast Cancer in Chinese Population. Medicine (Baltimore). 2016;95:e2771
25. Yang C, Tang R, Ma X, Wang Y, Luo D, Xu Z. et al. Tag SNPs in long non-coding RNA H19 contribute to susceptibility to gastric cancer in the Chinese Han population. Oncotarget. 2015;6:15311-15320
26. Hua Q, Lv X, Gu X, Chen Y, Chu H, Du M. et al. Genetic variants in lncRNA H19 are associated with the risk of bladder cancer in a Chinese population. Mutagenesis. 2016;31:531-538
27. Guo QY, Wang H, Wang Y. LncRNA H19 polymorphisms associated with the risk of OSCC in Chinese population. Eur Rev Med Pharmacol Sci. 2017;21:3770-3774
28. Jin T, Wu X, Yang H, Liu M, He Y, He X. et al. Association of the miR-17-5p variants with susceptibility to cervical cancer in a Chinese population. Oncotarget. 2016;7:76647-76655
29. Li S, Hua Y, Jin J, Wang H, Du M, Zhu L. et al. Association of genetic variants in lncRNA H19 with risk of colorectal cancer in a Chinese population. Oncotarget. 2016;7:25470-25477
30. Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Upregulated H19 contributes to bladder cancer cell proliferation by regulating ID2 expression. FEBS J. 2013;280:1709-1716
31. Zhang L, Yang F, Yuan JH, Yuan SX, Zhou WP, Huo XS. et al. Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis. 2013;34:577-586
32. Verhaegh GW, Verkleij L, Vermeulen SH, den Heijer M, Witjes JA, Kiemeney LA. Polymorphisms in the H19 gene and the risk of bladder cancer. Eur Urol. 2008;54:1118-1126
33. Yin Z, Cui Z, Li H, Li J, Zhou B. Polymorphisms in the H19 gene and the risk of lung Cancer among female never smokers in Shenyang, China. BMC Cancer. 2018;18:893
34. Gao Y, Liu Y, Du L, Li J, Qu A, Zhang X. et al. Down-regulation of miR-24-3p in colorectal cancer is associated with malignant behavior. Med Oncol. 2015;32:362
35. Inoguchi S, Seki N, Chiyomaru T, Ishihara T, Matsushita R, Mataki H. et al. Tumour-suppressive microRNA-24-1 inhibits cancer cell proliferation through targeting FOXM1 in bladder cancer. FEBS Lett. 2014;588:3170-3179
36. Keniry A, Oxley D, Monnier P, Kyba M, Dandolo L, Smits G. et al. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol. 2012;14:659-665
37. Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13:313-316
38. Lv J, Ma L, Chen XL, Huang XH, Wang Q. Downregulation of LncRNAH19 and MiR-675 promotes migration and invasion of human hepatocellular carcinoma cells through AKT/GSK-3beta/Cdc25A signaling pathway. J Huazhong Univ Sci Technolog Med Sci. 2014;34:363-369
39. Ma C, Nong K, Zhu H, Wang W, Huang X, Yuan Z. et al. H19 promotes pancreatic cancer metastasis by derepressing let-7's suppression on its target HMGA2-mediated EMT. Tumour Biol. 2014;35:9163-9169
40. Yan L, Zhou J, Gao Y, Ghazal S, Lu L, Bellone S. et al. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene. 2015;34:3076-3084
41. Raveh E, Matouk IJ, Gilon M, Hochberg A. The H19 Long non-coding RNA in cancer initiation, progression and metastasis - a proposed unifying theory. Mol Cancer. 2015;14:184
42. Wang L, Cai Y, Zhao X, Jia X, Zhang J, Liu J. et al. Down-regulated long non-coding RNA H19 inhibits carcinogenesis of renal cell carcinoma. Neoplasma. 2015;62:412-418
43. Orom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G. et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46-58
44. Chen B, Yu M, Chang Q, Lu Y, Thakur C, Ma D. et al. Mdig de-represses H19 large intergenic non-coding RNA (lincRNA) by down-regulating H3K9me3 and heterochromatin. Oncotarget. 2013;4:1427-1437
45. Peng L, Yuan XQ, Liu ZY, Li WL, Zhang CY, Zhang YQ. et al. High lncRNA H19 expression as prognostic indicator: data mining in female cancers and polling analysis in non-female cancers. Oncotarget. 2017;8:1655-1667
46. Kamura T, Tsukamoto N, Tsuruchi N, Saito T, Matsuyama T, Akazawa K. et al. Multivariate analysis of the histopathologic prognostic factors of cervical cancer in patients undergoing radical hysterectomy. Cancer. 1992;69:181-186
47. Choi KH, Kim JY, Lee DS, Lee YH, Lee SW, Sung S. et al. Clinical impact of boost irradiation to pelvic lymph node in uterine cervical cancer treated with definitive chemoradiotherapy. Medicine (Baltimore). 2018;97:e0517
48. Yang ML, Huang Z, Wang Q, Chen HH, Ma SN, Wu R. et al. The association of polymorphisms in lncRNA-H19 with hepatocellular cancer risk and prognosis. Biosci Rep. 2018:38
Author contact
![]() Corresponding author: Po-Hui Wang, MD, PhD, Institute of Medicine, Chung Shan Medical University, 110, Section 1, Chien-Kuo North Road, Taichung, 40201, Taiwan. Tel.: 886-4-24739595 ext. 21721; Fax: 884-4-24738493; E-mail: ginhow84921344com.tw
Corresponding author: Po-Hui Wang, MD, PhD, Institute of Medicine, Chung Shan Medical University, 110, Section 1, Chien-Kuo North Road, Taichung, 40201, Taiwan. Tel.: 886-4-24739595 ext. 21721; Fax: 884-4-24738493; E-mail: ginhow84921344com.tw

 Global reach, higher impact
Global reach, higher impact