Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(22):4128-4138. doi:10.7150/jca.26885 This issue Cite
Research Paper
Identification of Integrin β1 as a Novel PAG1-Interacting Protein Involved in the Inherent Radioresistance of Human Laryngeal Carcinoma
1. Department of pharmacology, School of Basic Medical Sciences, Hubei University of Medicine, Shiyan, Hubei 442000, P.R. China
2. Department of Clinical Oncology, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei 442000, P.R. China
Received 2018-4-25; Accepted 2018-8-19; Published 2018-10-18
Abstract
Inherent radioresistance plays a crucial role in the failure of radiotherapy. Using the inherent radioresistant (Hep-2max) and radiosensitive (Hep-2min) cell lines established from the parental cell line Hep-2, we previously reported that phosphoprotein associated with glycosphingolipid-enriched microdomains 1(PAG1) overexpression in laryngeal carcinoma cells was correlated with inherent radioresistant phenotypes. However, the underlying mechanisms of this effect remain unknown. In the present study, we performed a proteomic screen to investigate the interactome of PAG1 in Hep-2max cells resulting in the identification of several interaction partners. Bioinformatic analysis and immunofluorescence experiments indicated the integrin β1 to be a crucial interaction partner of PAG1. PAG1 was also highly expressed in laryngeal carcinoma radioresistant tissues and showed co-localization with integrin β1. In addition, we demonstrated that integrin β1's binding to PAG1 could be interrupted by MβCD, an inhibitor of lipid rafts formation. Moreover, knockdown of integrin β1 by RNA interference sensitized radioresistant cells to irradiation. Importantly, we identified 2 potential interaction sites (Pro216-Arg232 and Asn356-Gly377) in the cytoplasmic domain of PAG1 using high throughput peptide arrays. Taken together, these results suggest that the binding of PAG1 to integrin β1 in lipid rafts is essential for inherent radioresistance of human laryngeal carcinoma.
Keywords: PAG1, integrin β1, inherent radioresistance, laryngeal carcinoma, interaction partner
Introduction
Laryngeal carcinoma is a common malignant tumor of head and neck in both more and less economically developed countries [1]. The incidence and mortality rates of laryngeal carcinoma have remarkably increased in recent decades. An estimated 13,360 cases will be diagnosed with laryngeal carcinoma and 3,660 cases will die from this disease in the United States in 2017 [2]. In 2015, it is predicted that there will be about 264,000 newly diagnosed cases and 14,500 deaths occurred in China [3]. Patients with laryngeal carcinoma usually present late leading to the reduced treatment efficacy and a high rate of recurrence [4]. Radiotherapy is one of the most important treatment strategies for laryngeal carcinoma. Unfortunately, many patients do not benefit from this existing therapy due to the inherent or acquired resistance to radiotherapy.
Acquired radioresistance of tumor cells is induced by fractionated ionizing radiation which leads to radiation tolerance and adaptive response [5]. Inherent radioresistance is developed because it exists a subpopulation of clonogenic cells within the tumor[6]. Also, differentially expressed genes were reported to play important roles in acquired radioresistance of laryngeal carcinoma [7-9]. But few reports have investigated the genes contributing to the inherent radioresistance of laryngeal carcinoma. In our previous study, we established the inherent radioresistant (Hep-2max) and radiosensitive (Hep-2min) cell lines from the parental laryngeal carcinoma cell line Hep-2[10].Interestingly, we found that phosphoprotein associated with glycosphingolipid-enriched microdomains 1(PAG1) overexpression in laryngeal carcinoma cells was correlated with inherent radioresistant features. However, the pathways that contribute to this event remain largely unknown.
PAG1 is a ubiquitously expressed transmembrane adaptor protein that localizes exclusively in lipid rafts [11]. It interacts with a number of important cytoplasmic and plasma membrane-associated proteins in various experimental systems and different cellular contexts[12]. Human PAG1 protein contains a 16 amino acids (aa) extracellular region, 20 aa transmembrane segments and 393 aa cytoplasmic domains. Therefore, in this study, we investigated the interaction partners of PAG1 in laryngeal carcinoma cells. Our results reveal for the first time that integrin β1 serves as a binding partner for PAG1 involved in the inherent radioresistance of laryngeal carcinoma.
Materials and Methods
Patients and ethics
30 laryngeal carcinoma tissues were collected from patients who underwent radiotherapy at the Taihe Hospital, Hubei University of Medicine. This study was approved by the Research Ethics Committee of Hubei University of medicine (Shiyan, Hubei, China).The written informed consent was obtained from all patients. The experimental procedures conformed to the standards set by the Declaration of Helsinki. Samples were divided into “sensitive” (complete response or partial response) and “insensitive” (stable disease or progressive disease) groups according to the patient's responses assessed via medical image analysis and detection of serum tumor markers after radiotherapy[13].
Cell culture
The human inherent radioresistant (Hep-2max) and radiosensitive (Hep-2min) laryngeal carcinoma cell lines were preserved in our laboratory. Both of cell lines were cultured in DMEM medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum and incubated at 37°C in a humidified incubator.
Immunoprecipitation (IP) and Immunoblotting
The cells were lysed in lysis buffer as previously described [14]. IP assays were performed by incubating total cell lysates (1 mg) with the indicated antibodies at 4°C for 12 h. Then protein A/G agarose beads (Thermo Fisher, Rockford, IL, USA) were added to the above complex and incubated for another 4 h at 4°C. After washing with lysis buffer, the immunoprecipitates were separated on a 10% SDS-PAGE, blotted on PVDF membranes, and probed with the indicated antibodies. Quantification was carried out using ImageJ software.
LC-MS/MS analysis
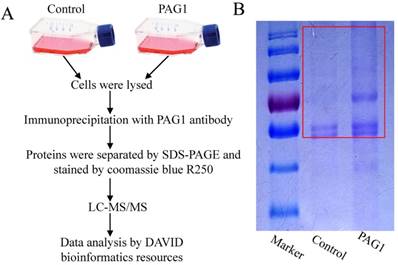
The procedure for LC-MS/MS was performed as described previously[15]. The PAG1 immunoprecipitates were resolved by SDS-PAGE. Protein bands visible after coomassie blue R250 (Sigma, St. Louis, MO, USA) staining were subjected to in-gel reduction, carboxyamidomethylation and tryptic digestion. Digested peptides were measured on a Dionex Ultimate 3000 nano-LC system coupled to a linear quadrupole ion trap-Orbitrap mass spectrometer equipped with a nanoelectrospray ion source [16]. Protein identification was performed by searching the data against the databases using Mascot Deamon(Matrix Science, London, UK; version 2.3.01). Subcellular location of the identified proteins was determined using DAVID bioinformatics resources.
Immunofluorescence
Immunofluorescence staining was performed as previously reported[17].Briefly, cultured cells on glass coverslips or frozen tissue sections (8 μM thickness) were fixed in 4% paraformaldehyde for 10 min and blocked with blocking buffer (0.1% Triton X-100 and 10% normal serum in PBS) for 1 h. Fixed cells or tissue sections were then incubated with the indicated primary antibodies for 2 h followed by fluorochrome-conjugated secondary antibodies. After being washed with PBS, the slides were incubated with DAPI (Sigma) for nuclear staining. Images were acquired with a fluorescence confocal microscope (Zeiss, Thornwood, NY, USA).
Isolation of Lipid Rafts
Preparation of lipid rafts was performed as described previously [18,19]. Briefly, the lysates were homogenized with 10 strokes in a Dounce homogenizer. Then the homogenates were repeatedly passed through a 22-gauge needle (30 times) and centrifuged at 1000 × g for 10 min to remove mitochondria, nuclei and other large debris. To generate the density gradients, the homogenates (1 ml) were mixed 1: 1 with 80% sucrose in MNE buffer. Then 2 ml 35% and 1 ml 5% sucrose were added dropwise. After ultracentrifugation for 18 h at 200,000×g in a Beckman MLS50 rotor, 12 fractions (400 μl/fraction) were collected from the top to bottom. Fractions 1-12 were separated by SDS-PAGE, blotted on PVDF membranes, and probed with the appropriate antibodies or with cholera toxin B-subunit (to identify the ganglioside GM1, Sigma).
siRNA transfection
Hep-2max cells were transfected with siRNA targeting integrin β1 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The following sequences were used: integrant β1 siRNA, sense 5'-GCA AAUUCUAGCAAUGUAATT-3', anti-sense 5'-GACGUAAGAUCGUUACAUU-3' and negative control (NC) siRNA, sense 5'-UUCUCCGAACGUGUCACGUTT-3', anti-sense 5'-ACGUGACACGUUCGGAGAATT. The siRNA duplex oligoribonucleotides were purchased from GenePharma (Shanghai, China).
Quantitative real-time polymerase chain reaction (qPCR) and western blotting
Total RNA was extracted from cells and tissues using Trizol reagent (Invitrogen).qPCR was performed with the SYBR-Green Real-Time PCR Master Mix kit (Toyobo, Osaka, Japan). Primers were as follows: GAPDH (for internal control), 5′-CCAACCGCGAGAAGATGA-3′ (upper) and 5′-CCAGAGGCGTACAGGGATAG-3′ (lower); integrin β1, 5′-GACGCCGCGCGGAAAAGATG-3′(upper) and 5′-GCACCACCCACAATTTGGCCC-3′ (lower); and PAG1, 5′-GAGTCCACCTACACCTCCATTC-3′ (upper) and 5′-GCCTTTTCTTCCTCTCTGTTGA-3′ (lower). Total protein was extracted and subjected to 10% SDS-PAGE and Western blotting, as previously described [16]. The following antibodies were used: anti-integrin β1 (ab24693; 1:1000), anti-PAG1 (ab155100; 1:1000), and anti-GAPDH (ab128915; 1:2000) (All from Abcam; Cambridge, MA, USA).
Radiosensitivity analysis
The radiosensitivity of cells was determined by cell proliferation, colony formation and flow cytometry assays. For the cell proliferation assay, cells were seeded into 96-well plates for 24 h. After being exposed to different doses of irradiation as indicated, CCK-8 solution (Beyotime, Jiangsu, China) was added to each well according to the manufacturer's instructions. For the colony formation assay, cells were treated with various doses of radiation and plated in 6-well plates. After being incubated for 14 days, the surviving colonies were fixed, stained and counted as published previously [10]. For the flow cytometry assay, the cells treated with or without irradiation were collected and stained with a combination of Annexin V-FITC and propidium iodide (PI) according to the manufacturer's manual(Beyotime). Irradiations were performed using an X-ray machine (X-RAD 320, Precision X-ray) at 320 kV, 10 mA with a 2-mm aluminum filter, and the dose rate was 2 Gy/min[20]. All cell irradiations were carried out at the Department of Clinical Oncology, Taihe Hospital, Hubei University of Medicine.
Synthetic peptide arrays for protein-protein interaction
Eighty-five peptides were synthesized on a cellulose membrane in an array format by POPCHEM Company (Beijing, China) using the previously reported methods [21]. The synthetic peptide contained 7 overlapping amino acids(aa) with a length of 12 aa. All the peptides were derived from and walked downstream with 5 aa frameshift along the aa sequence of PAG1.Then the array was activated and blocked with Arrayit's Protein Microarray Kit (Sunnyvale, CA, USA) for 4 h at room temperature. After incubation with the human integrin β1 recombinant protein (Sino Biological Inc., Beijing, China) overnight at 4°C, the array was probed with the anti-integrin β1 mAb (1:1000) and washed in PBST (PBS with 0.5% Tween-20).Finally, the membrane was incubated with HRP-labelled secondary antibody and visualized with enhanced chemiluminescence detection system (Beyotime).The image was captured using a ChemiDoc TM XRS+ system (Bio-Rad, Hercules, California, USA). The optical density of each point was analyzed using TotalLab software and calculated with Spot Edge Average algorithm.
Statistical analysis
All data were analyzed by the SPSS 14.0 software. All values were expressed as means ±standard deviation (SD). Student's t-tests were performed to evaluate statistical differences between groups. P < 0.05 was considered statistically significant.
Results
Identification of interaction partners of PAG1 by proteomic analysis
Since PAG1 has been described to be predominantly expressed in Hep-2max cells and involved in the inherent radioresistance, we wanted to identify novel key interaction partners of PAG1 in Hep-2max cells. We first applied a proteomic approach by IP of PAG1 followed by LC-MS/MS analysis. Hep-2max cells were lysed and the lysates were incubated with PAG1 antibody prior to adding protein A/G agarose beads. Then PAG1 immunoprecipitates in the gel were cut out and examined (Fig.1A and B).We thereby identified several proteins, which were immunoprecipitated together with PAG1 but not with the control IgG. To investigate the subcellular location and function of the identified proteins, bioinformatics analysis was performed using DAVID bioinformatics resources (Table 1). Among these interaction partners, integrin β1 was the only one that localized in lipid rafts and regulated apoptosis. We therefore selected it for further investigation.
Co-localization of PAG1 and integrin β1 in the cell membrane
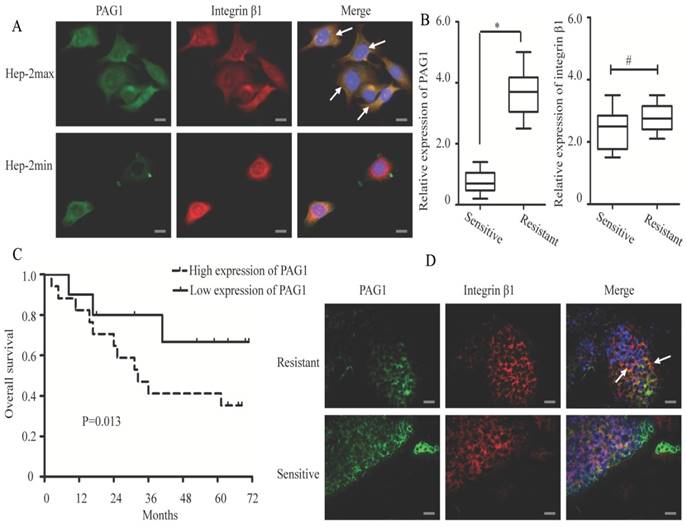
To further explore the interaction between PAG1 and integrin β1, we examined whether these two proteins showed the co-localized expression in Hep-2max and Hep-2min cells. In Hep-2max cells, we observed localization of PAG1 in the cell membrane (Fig. 2A, green staining). In contrast, integrin β1 showed different patterns of localization, either in the cytoplasm or at the cell membrane (red staining). The co-localization of PAG1 and integrin β1 was seen by yellow staining in the overlay. In Hep-2min cells, immunofluorescence staining showed that these two proteins were partially and weakly co-localized in the cell membrane.
Then a total of 30 clinical tissue samples collected from patients with laryngeal carcinoma were divided into “sensitive” and “resistant” groups according to the patient's response to radiotherapy. As shown in Fig.2B, PAG1 mRNA was significantly up-regulated in the “resistant” group tissues (n = 20) compared with that in the “sensitive” group ones (n = 10). However, integrin β1 mRNA expression level was not significantly changed between the two groups. We also noted that patients with high PAG1 expression showed significantly shorter overall survival than those with low PAG1 expression (Fig.2C). To further confirm our results in cells, we analyzed the co-localization between PAG1 and integrin β1 in the tissue samples. Merged images of immunofluorescence staining demonstrated that PAG1 was mainly localized at the cell membrane in the “resistant” group tissues and showed the co-localization with integrin β1 (Fig.2D). In addition, merged images of PAG1 and integrin β1 showed a lack of co-localization in the “sensitive” group tissues. Taken together, these results suggested the integrin β1 to be an essential interaction partner of PAG1.
Identification of interaction proteins of PAG1 in Hep-2max cells. (A) Schematic illustration of the strategy used to detect PAG1-interacting proteins. (B) The immunoprecipitates in the red box were cut out and analyzed by LC-MS/MS analysis.
List of potential interaction partners of PAG1 identified by LC-MS/MS analysis
| UniProt ID | Protein name | Number of unique peptides identified | Subcellular location (part) | Molecular function (part) |
|---|---|---|---|---|
| Q09666 | AHNAK | 23 | Cytoplasm , lipid rafts | Calcium-dependent membrane repair |
| P27824 | Calnexin | 17 | Endoplasmic reticulum | Protein folding |
| P06748 | Nucleophosmin | 15 | Cytoplasm, nucleus | DNA repair |
| P05556 | Integrin β1 | 18 | Cytoplasm , lipid rafts | Cell apoptosis, proliferation |
| P11021 | 78 kDa glucose- regulated protein | 44 | endoplasmic reticulum | Protein folding |
| P08670 | Vimentin | 30 | Cytoplasm,cytosol | Movement of cell component |
| P14618 | Pyruvate kinase | 14 | Cytoplasm, nucleus | Response to hypoxia |
| P19338 | Nucleolin | 3 | Nucleus | Angiogenesis |
| P62826 | GTP-binding nuclear protein | 7 | Cytoplasm, nucleus | DNA metabolic process |
| P35232 | Prohibitin | 13 | Cytoplasm, nucleus | Regulation of transcription |
| P13010 | X-ray repair cross- complementing protein 5 | 10 | Nucleus | Double-strand break repair |
| P46940 | Ras GTPase- activating-like protein | 14 | Cytoplasm , lipid rafts | Regulation of GTPase activity |
| P12956 | X-ray repair cross- complementing protein 6 | 19 | Nucleus | Telomere maintenance |
| Q06830 | Peroxiredoxin-1 | 8 | Cytoplasm, nucleus | Response to reactive oxygen species |
Immunofluorescence co-localization of PAG1 and integrin β1 in cultured cells and tissue samples. (A) Immunostaining of endogenous PAG1 and endogenous integrin β1 in cells were performed for PAG1 using a FITC-labelled secondary antibody, or integrin β1 using a Cy3-labelled secondary antibody. (B) Relative expression levels of PAG1 and integrin β1 mRNA (normalized to GAPDH) were detected in radiosensitive (n =10) and radioresistante (n = 20) laryngeal carcinoma tissues via qRT-PCR (*P < 0.05, # P >0.05). (C) Kaplan-Meier survival curve indicated that the overall survival rate in patients with high PAG1 expression was significantly lower than that in patients with low PAG1 expression (log-rank test, P< 0.05). (D) Immunofluorescence staining of PAG1 (green) and integrin β1(red) in the clinical tissue samples.The arrows indicated that PAG1 and integrin β1 co-localized at the cell membrane. Cell nuclei were stained with DAPI. Scale bar is 50 μm.
Lipid rafts are necessary for interaction between PAG1 and integrin β1
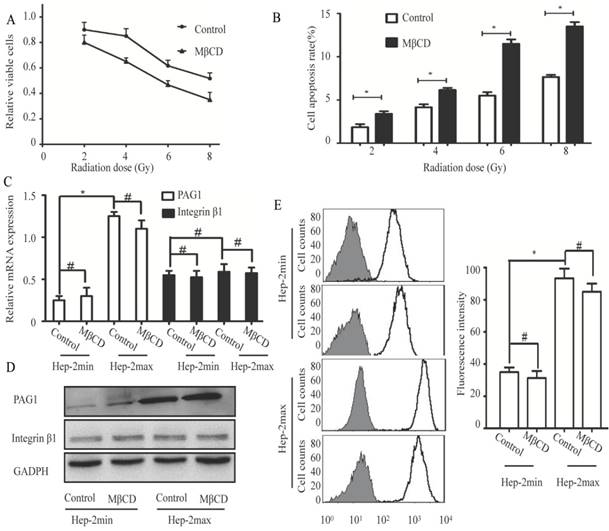
The cell membrane has specialized microdomains called lipid rafts. PAG1 has been shown to be co-localized with integrin β1 in the cell membrane. Moreover, localization of PAG1 and integrin β1 is both lipid rafts-dependent [22, 23]. On the basis of these evidences, we hypothesized that lipid rafts contributed to inherent radioresistance by modulating the interaction between PAG1 and integrin β1. We first treated Hep-2max cells with 5 mM MβCD (Methyl-beta-cyclodextrin), which can effectively disrupt the integrity of lipid rafts[24]. Cell proliferation assay showed that MβCD treatment displayed an enhanced radiation-induced inhibition compared with the control cells (Fig.3A). Consistent with this, MβCD treatment markedly increased radiation-induced apoptosis as detected by flow cytometry (Fig.3B).These results indicated that the integrity of lipid rafts was responsible for the inherent radioresistance of Hep-2max cells.
Next, we investigated the influence of lipid rafts on the expression of PAG1 and integrin β1. As determined by qPCR and Western blotting, the mRNA and protein levels of PAG1 were significantly higher in Hep-2max cells than in Hep-2min cells. The disruption of lipid rafts by MβCD did not alter the expression of PAG1 at both mRNA and protein levels (Fig.3C and D). In addition, the mRNA and protein expression levels of integrin β1 were not significantly different between Hep-2max and Hep-2min cells, which displayed the similar trend with that of integrin β1 in the tissue samples. We also found that MβCD did not affect the expression of integrin β1 (Fig.3C and D).We then detected the activity of integrin β1 using an activated integrin β1-specific antibody (HUTS-21) by flow cytometry [25]. Analysis of the fluorescence intensity confirmed that there was no significant difference in the activated integrin β1 between control and MβCD-treated cells (Fig.3E).The effect of lipid rafts on inherent radioresistance cannot be accomplished by regulating the expression of PAG1 or integrin β1.
Disruption of lipid rafts increases the radiosensitivity but does not influence the expression of PAG1 and integrin β1. Hep-2max or Hep-2min cells were treated with 5 mM MβCD for 2 h before different doses of irradiation. (A)The relative viable cells in each group were analyzed by the CCK-8 assay. (B) The cell apoptotic rate in each group was analyzed by flow cytometry. (C) Relative expression levels of PAG1 and integrin β1 mRNA (normalized to GAPDH) in each group were detected via qRT-PCR. (D) The protein expression of PAG1 and integrin β1 in each group was detected via western blotting. (E) The amount of cell surface-activated integrin β1 was analyzed using flow cytometry. Non-specific mouse IgG was used as a background fluorescence control (*P < 0.05, # P >0.05).
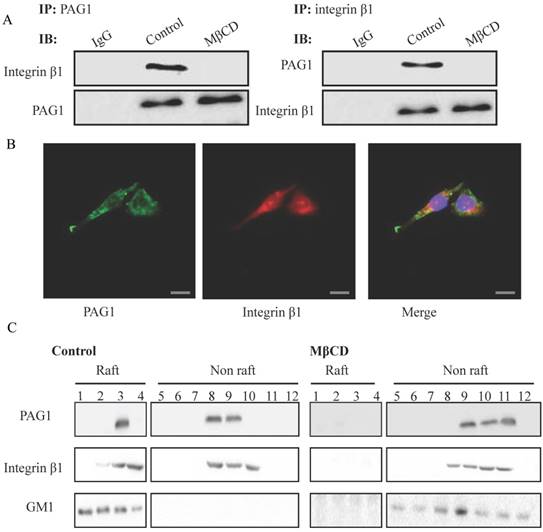
To further validate the interaction between PAG1 and integrin β1, we performed co-IP assays in Hep-2max cells using PAG1 or integrin β1 antibody, respectively. As shown in Fig. 4A, endogenous PAG1 was co-precipitated with integrin β1, and endogenous integrin β1 could be pulled down by PAG1 antibody. However, the protein-protein complex was interrupted by MβCD treatment. Immunofluorescence experiments also showed that the co-localization of PAG1 and integrin β1 almost disappeared (Fig.4B).On the other hand, we explored the distribution of PAG1 and integrin β1 in lipid raft fractions. As illustrated in Fig. 4B, fractions 1-12 were subjected to SDS-PAGE and probed to detect PAG1, integrin β1, and GM1. The ganglioside (GM1) is widely used as a marker of lipid rafts. We found that fractions 1 and 4 were observed to contain lipid rafts, whereas fractions 8-12 contained the cytosolic components. Notably, the relative proportion of PAG1 and integrin β1 was substantially higher in the lipid raft fraction compared with that in the cytosolic component (Fig.4C). MβCD treatment resulted in their delocalization from lipid raft fractions, suggesting that the location of PAG1 and integrin β1 in the membrane depends on intact lipid rafts. Thus, lipid rafts may serve as platforms in which PAG1-integrin β1 interaction took place.
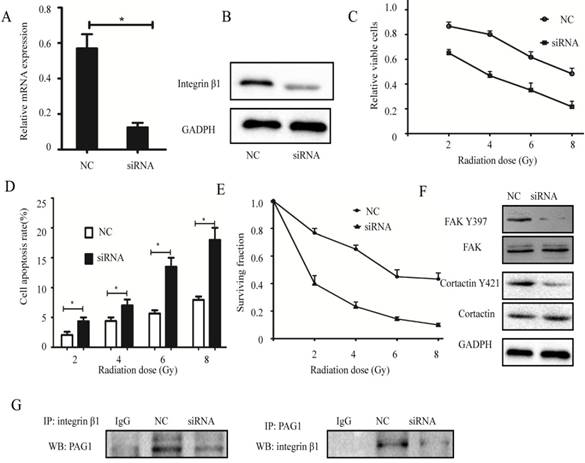
Knockdown of integrin β1 enhances the radiosensitivity of laryngeal carcinoma cells
To investigate whether the association between PAG1 and integrin β1 plays an important role in the inherent radioresistance, we knocked down endogenous integrin β1 using specific siRNA. Results of qPCR and Western blotting showed that the mRNA and protein expression levels of integrin β1 in Hep-2max cells transfected with siRNA were strongly suppressed (Fig.5A and B). In addition, cells expressing low levels of integrin β1 led to a marked decrease in the relative cell number (Fig. 5C) but more promotion of apoptosis rate (Fig. 5D) with radiation. Consistent with this, the result of the colony formation assay revealed that knockdown of integrin β1 in Hep-2max cells showed fewer survival fractions when exposed to various doses of irradiation compared with NC cells (Fig. 5E). Interestingly, downregulation of integrin β1 in Hep-2max cells caused a dephosphorylation of FAK Y397 and cortactin Y421 (Fig. 5F). IP experiments showed PAG1/ integrin β1 interaction was inhibited in integrin β1 knockdown cells (Fig. 5G). These results indicated that integrin β1 was indispensable for PAG1-mediated inherent radioresistance.
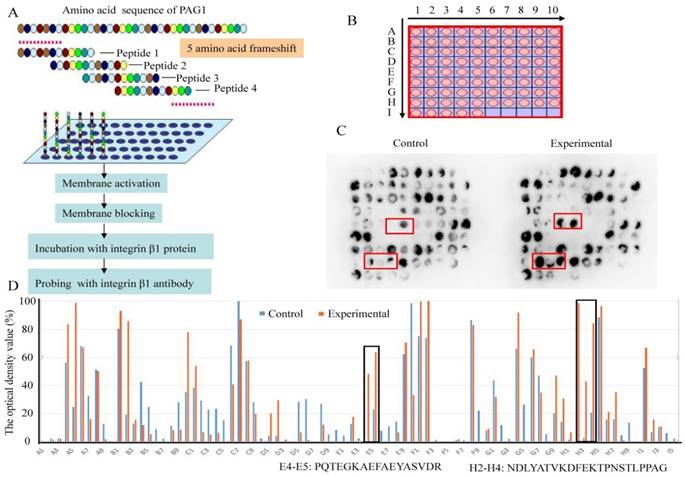
Prediction of protein-protein interaction sites with synthetic peptide arrays
To identify the interaction sites between PAG1 and integrin β1, the SPOT synthesis technique was used. This method has the potential to produce the required peptide arrays, which provides a novel and powerful tool for gaining insight into the basis of specific protein-protein interactions [26]. Thus, a library of overlapping peptides (12-mers), each shifted by 5 amino acids across the entire sequence of PAG1, was SPOT-synthesized on cellulose membranes (Fig.6A and B). The sequence for the peptide library was shown in Table 2. Then the immobilized membrane incubated with recombinant integrin β1 protein served as an experimental group. The membrane without integrin β1 protein incubation was used as a control group. Probing the peptide arrays with integrin β1 antibody, the binding map was generated (Fig.6C) and evaluated immunologically using the TotalLab software [27]. The optical density over 30% in the experimental group and less than 30% in the control group was identified as the positive interaction spots (Fig.6D). Positive reactions were obtained with the two regions (spot E4-E5 and H2-H4) in the cytoplasmic domain of PAG1. One of these binding sites was within Pro216-Arg232 and the other within Asn356-Gly377. These results confirmed that PAG1 interacted with integrin β1 owing to the presence of binding sites.
Discussion
Inherent radioresistance is a major barrier to effective treatment for laryngeal carcinoma. In laryngeal carcinoma cells, PAG1 was differentially upregulated in a subtype resistant to radiotherapy, and knockdown of PAG1 in inherent radioresistant cells rendered the cells sensitive to radiotherapy[10]. PAG1 is a ubiquitous protein, but its expression is variable in different types of cancer cells. For example, overexpression of PAG1 was observed in acute lymphoblastic leukemia [28] and renal cell carcinoma [11]. But in neuroblastoma[29], esophageal carcinoma [30] and colon cancer[31], PAG1 may function as a potential tumor suppressor. Therefore, PAG1 expression was either downregulated or upregulated, which involved in a number of interaction partners. Indeed, PAG has been shown to interact with a number of proteins such as Lck, Fyn, Lyn, Csk, Shc, Vav, GAP, PI3K, ZAP-70 and Syk[12]. In this study, we performed a proteomic screen in inherent radioresistant laryngeal carcinoma cells to identify novel interaction partners of PAG1 potentially providing further insights into the function of this multi-faceted protein. We found that integrin β1 was a critical lipid-raft-dependent PAG1-interacting protein.
The sequence of peptide arrays for PAG1
| Spot | Sequence | Spot | Sequence | Spot | Sequence | Spot | Sequence |
|---|---|---|---|---|---|---|---|
| A1 | MGPAGSLLGSGQ | C3 | SASDLLDSQDST | E5 | KAEFAEYASVDR | G7 | GQSLTVPESTYT |
| A2 | SLLGSGQMQITL | C4 | LDSQDSTGKPKC | E6 | EYASVDRNKKCR | G8 | VPESTYTSIQGD |
| A3 | GQMQITLWGSLA | C5 | STGKPKCHQSRE | E7 | DRNKKCRQSVNV | G9 | YTSIQGDPQRSP |
| A4 | TLWGSLAAVAIF | C6 | KCHQSRELPRIP | E8 | CRQSVNVESILG | G10 | GDPQRSPSSCND |
| A5 | LAAVAIFFVITF | C7 | RELPRIPPESAV | E9 | NVESILGNSCDP | H1 | SPSSCNDLYATV |
| A6 | IFFVITFLIFLC | C8 | IPPESAVDTMLT | E10 | LGNSCDPEEEAP | H2 | NDLYATVKDFEK |
| A7 | TFLIFLCSSCDR | C9 | AVDTMLTARSVD | F1 | DPEEEAPPPVPV | H3 | TVKDFEKTPNS T |
| A8 | LCSSCDREKKPR | C10 | LTARSVDGDQGL | F2 | APPPVPVKLLDE | H4 | EKTPNSTLPPAG |
| A9 | DREKKPRQHSGD | D1 | VDGDQGLGMEG P | F3 | PVKLLDENENLQ | H5 | STLPPAGRPSEE |
| A10 | PRQHSGDHENLM | D2 | GLGMEGPYEVLK | F4 | DENENLQEKEGG | H6 | AGRPSEEPEPDY |
| B1 | GDHENLMNVPSD | D3 | GPYEVLKDSSSQ | F5 | LQEKEGGEAEES | H7 | EEPEPDYEAIQT |
| B2 | LMNVPSDKEMFS | D4 | LKDSSSQENMVE | F6 | GGEAEESATDTT | H8 | DYEAIQTLNREE |
| B3 | SDKEMFSRSVTS | D5 | SQENMVEDCLYE | F7 | ESATDTTSETNK | H9 | QTLNREEEKATL |
| B4 | FSRSVTSLATDA | D6 | VEDCLYETVKEI | F8 | TTSETNKRFSSL | H10 | EEEKATLGTNGH |
| B5 | TSLATDAPASSE | D7 | YETVKEIKEVAA | F9 | NKRFSSLSYKSR | I1 | TLGTNGHHGLVP |
| B6 | DAPASSEQNGAL | D8 | EIKEVAAAAHLE | F10 | SLSYKSREEDPT | I2 | GHHGLVPKENDY |
| B7 | SEQNGALTNGDI | D9 | AAAAHLEKGHSG | G1 | SREEDPTLTEEE | I3 | VPKENDYESISD |
| B8 | ALTNGDILSEDS | D10 | LEKGHSGKAKST | G2 | PTLTEEEISAMY | I4 | DYESISDLQQGR |
| B9 | DILSEDSTLTCM | E1 | SGKAKSTSASKE | G3 | EEISAMYSSVNK | I5 | SDLQQGRDITRL |
| B10 | DSTLTCMQHYEE | E2 | STSASKELPGPQ | G4 | MYSSVNKPGQLV | ||
| C1 | CMQHYEEVQTSA | E3 | KELPGPQTEGKA | G5 | NKPGQLVNKSGQ | ||
| C2 | EEVQTSASDLLD | E4 | PQTEGKAEFAEY | G6 | LVNKSGQSLTVP |
Lipid rafts are necessary for the interaction between PAG1 and integrin β1. (A) Confirmation of the interaction between PAG1 and integrin β1 by co-IP. (B) Immunofluorescence staining of PAG1 (green) and integrin β1 (red) in Hep-2max cells treated with MβCD. Cell nuclei were stained with DAPI. Bar is 50 μm. (C) Location of PAG1 and integrin β1 in lipid raft fractions. Gradient fractions of Hep-2max cells treated with or without MβCD were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with specific antibodies.
Mass spectrometry (MS) is an analytical chemistry technique that helps identify co-precipitated proteins from immunoprecipitated samples. A major advantage of immunoprecipitated samples is their reduced complexity and therefore MS analysis enables faster scan speeds, better mass accuracy and allows identification of proteins at low concentrations[32]. Samples from the immunoprecipitation are separated by SDS- PAGE, stained with Coomassie blue and digested with enzyme. The resulting peptides are chromatographically separated and analyzed by tandem mass spectrometry (MS/MS) [33]. To identify the corresponding protein, experimental tandem mass spectra is usually compared with theoretically generated spectra through a searchable database such as Mascot. Using this approach, Han et al. have investigated the interactome of FMNL1 in primary hematopoietic cells resulting in the identification of numerous interaction partners [15]. By proteomic analysis, Bi et al. have demonstrated that nucleolin could interact with integrin β1 and led to melanoma cell migration on fibronectin [34]. Hernychova et al. also performed MS analysis using epithelial cancer samples and finally identified 23 proteins as potential ΔNp63 binding partners[32]. Here, we analyzed PAG1 interacting proteins by IP and LC-MS/MS in inherent radioresistant laryngeal carcinoma cells. A total of 14 proteins were found in our experiments. We further performed bioinformatics analysis to investigate the function and subcellular location of these identified proteins. Interestingly, AHNAK, integrin β1, Ras GTPase-activating-like protein (rasGAP) are located in lipid rafts. However, integrin β1 has been shown to be involved in cell survival, proliferation, and cancer therapy resistance [35, 36]. It has not been previously identified as an interaction partner of PAG1. In addition, we confirmed interaction of PAG1 and integrin β1 by immunofluorescence staining and subsequent co-IP. We also found that the binding localization of PAG1 to integrin β1 was correlated with radioresistance of laryngeal carcinoma patients. However, a systematic analysis of the in vitro and in vivo functions of integrin β1 and other identified proteins are necessary to clarify the molecular mechanisms of PAG1 in inherent radioresistant cells.
Effects of integrin β1 knockdown on the radiosensitivity. Hep-2max cells were transiently transfected with integrin β1 siRNA or negative siRNA (NC). (A) The efficiency of integrin β1 knockdown was determined by qPCR. GADPH was used as a loading control. (B) The efficiency of integrin β1 knockdown was determined by western blotting. (C)The relative viable cells were analyzed by the CCK-8 assay. (D) The cell apoptotic rate was analyzed by flow cytometry. (E)The survival fraction value was detected by colony formation assay. (F) The expression of FAK, FAK Y397, cortactin, and cortactin Y421 in each group was detected by western blotting. (G) Confirmation of the interaction between PAG1 and integrin β1 by co-IP. (*P < 0.05)
Identification of interaction sites between PAG1 and integrin β1 using high throughput peptide arrays. (A) Schematic illustration of the strategy used to detect the interacting sites. (B) A library of 85 peptides was synthesized in an array format on a cellulose membrane using SPOT synthesis. (C) The images of peptide array binding patterns. Positively interacting peptides generate dark spots, while those that do not interact leave white (blank) spots. (D) The optical density value was assessed using the TotalLab software. Spot numbers relate to peptides in the scanned array and whose sequence was given as indicated.
In the plasma membrane, PAG1 is localized almost exclusively to the raft membrane microdomains. The concept of lipid rafts is based on the segregation of lipids into liquid-ordered and liquid-disordered phases [37]. Lipid rafts contain both protein and lipid components, and are thought to exist in continuity with non-raft regions of membrane [38]. Lipid rafts serve as platforms in which protein-protein or protein-lipid interactions take place, and facilitate efficient signal transduction and membrane dynamics[34, 39]. For example, helicobacter pylori could activate HMGB1 expression and recruited RAGE into lipid rafts in gastric epithelial cells to promote inflammation [40]. Lipid rafts were able to enhance liver cancer cell proliferation and migration by up-regulation of TLR7 expression[41]. In the present study, we have clearly demonstrated that PAG1 and integrin β1 coexisted in the same coimmunoprecipitates and lipid rafts disruption by MβCD suppressed their association. It is generally accepted that the expression and activation of integrin β1 and its interaction with other proteins is responsible for its function. Our results showed that MβCD did not directly influence the expression and activation of integrin β1.But MβCD treatment resulted in the translocation of PAG1 and integrin β1 from membrane to cytosol fraction. In addition, knockdown of integrin β1 sensitized radioresistant cells to irradiation by targeting FAK/cortactin signaling pathway, which was in accordance with the previous study[42]. Recently, a direct involvement of PAG1 in integrin β1 signaling via dioxin receptor/arylhydrocarbon receptor has been reported[43]. Therefore, PAG1-mediated inherent radioresistance in laryngeal carcinoma might be explained by the formation of raft-resident PAG1-based oncogenic signalosome with integrin β1.
Synthetic peptide arrays, especially SPOT synthesis, are widely used in protein interaction domain recognition studies[44]. SPOT synthesis can be carried out fully automatically in an analytical or preparative mode. Comparing intra-array spot signal intensities results in ranking peptide-binding preferences (good binders, medium binders, non-binders) towards a single sample[21]. Using this novel technology, Baillie et al. employed a library of overlapping peptides (25-mers) immobilized on cellulose membranes to identify the interaction sites on β-arrestin 2 for binding of PDE4D5 [26]. Importantly, we also identified 2 potential interaction sites (Pro216-Arg232 and Asn356- Gly377) in the cytoplasmic domain of PAG1 using high throughput peptide arrays. However, the question of how the interaction of the two molecules influences inherent radioresistance remains to be answered due to the conformational differences between recombinant protein and natural protein. Mutants, which are mutant for the interaction sites, or synthetic peptides, which inhibit the interaction between two proteins will be employed in our further study.
In conclusion, the binding of PAG1 to integrin β1 in lipid rafts is essential for inherent radioresistance of laryngeal carcinoma. Further understanding of PAG1 and integrin β1 interaction in lipid rafts may prevent the recurrence and progression of laryngeal carcinoma after radiotherapy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81502666), the Initial Project for Post-Graduates of Hubei University of Medicine (2016QDJZR10), the Natural Science Foundation of Hubei Province (2015CFA076) and the Free Exploration Foundation of Hubei University of Medicine (FDFR201802).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30
3. Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
4. Wong TS, Gao W, Li ZH. et al. Epigenetic dysregulation in laryngeal squamous cell carcinoma. J Oncol. 2012;2012:739461
5. Shimura T, Kakuda S, Ochiai Y. et al. Targeting the AKT/GSK3beta/cyclin D1/Cdk4 survival signaling pathway for eradication of tumor radioresistance acquired by fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:540-8
6. Li Z, Yang X, Xia N. et al. PTOP and TRF1 help enhance the radio resistance in breast cancer cell. Cancer Cell Int. 2014;14:7
7. Zhou FX, Xiong J, Luo ZG. et al. cDNA expression analysis of a human radiosensitive-radioresistant cell line model identifies telomere function as a hallmark of radioresistance. Radiat Res. 2010;174:550-7
8. Kim JS, Chang JW, Park JK. et al. Increased aldehyde reductase expression mediates acquired radioresistance of laryngeal cancer cells via modulating p53. Cancer Biol Ther. 2012;13:638-46
9. Choe MH, Min JW, Jeon HB. et al. ERp57 modulates STAT3 activity in radioresistant laryngeal cancer cells and serves as a prognostic marker for laryngeal cancer. Oncotarget. 2015;6:2654-66
10. Ke Q, Wu J, Ming B. et al. Identification of the PAG1 gene as a novel target of inherent radioresistance in human laryngeal carcinoma cells. Cancer Biother Radiopharm. 2012;27:678-84
11. Feng X, Lu X, Man X. et al. Overexpression of Csk-binding protein contributes to renal cell carcinogenesis. Oncogene. 2009;28:3320-31
12. Hrdinka M, Horejsi V. PAG-a multipurpose transmembrane adaptor protein. Oncogene. 2014;33:4881-92
13. Li J, Wang Y, Song Y. et al. miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol Cancer. 2014;13:193
14. Chavez JD, Schweppe DK, Eng JK. et al. Quantitative interactome analysis reveals a chemoresistant edgotype. Nat Commun. 2015;6:7928
15. Han Y, Yu G, Sarioglu H. et al. Proteomic investigation of the interactome of FMNL1 in hematopoietic cells unveils a role in calcium-dependent membrane plasticity. J Proteomics. 2013;78:72-82
16. Shen L, Dong X, Yu M. et al. beta3GnT8 Promotes Gastric Cancer Invasion by Regulating the Glycosylation of CD147. J Cancer. 2017;8:314-22
17. Shen Y, Stanislauskas M, Li G. et al. Epigenetic and genetic dissections of UV-induced global gene dysregulation in skin cells through multi-omics analyses. Sci Rep. 2017;7:42646
18. Vences-Catalan F, Rajapaksa R, Levy S. et al. The CD19/CD81 complex physically interacts with CD38 but is not required to induce proliferation in mouse B lymphocytes. Immunology. 2012;137:48-55
19. Hawash IY, Kesavan KP, Magee AI. et al. The Lck SH3 domain negatively regulates localization to lipid rafts through an interaction with c-Cbl. J Biol Chem. 2002;277:5683-91
20. Shen L, Dong XX, Wu JB. et al. Radiosensitisation of human glioma cells by inhibition of beta1,6-GlcNAc branched N-glycans. Tumour Biol. 2016;37:4909-18
21. Volkmer R, Tapia V, Landgraf C. Synthetic peptide arrays for investigating protein interaction domains. FEBS Lett. 2012;586:2780-6
22. Kanou T, Oneyama C, Kawahara K. et al. The transmembrane adaptor Cbp/PAG1 controls the malignant potential of human non-small cell lung cancers that have c-src upregulation. Mol Cancer Res. 2011;9:103-14
23. Leitinger B, Hogg N. The involvement of lipid rafts in the regulation of integrin function. J Cell Sci. 2002;115:963-72
24. Wang R, Bi J, Ampah KK. et al. Lipid raft regulates the initial spreading of melanoma A375 cells by modulating beta1 integrin clustering. Int J Biochem Cell Biol. 2013;45:1679-89
25. Liu CH, Hu RH, Huang MJ. et al. C1GALT1 promotes invasive phenotypes of hepatocellular carcinoma cells by modulating integrin beta1 glycosylation and activity. PLoS ONE. 2014;9:e94995
26. Baillie GS, Adams DR, Bhari N. et al. Mapping binding sites for the PDE4D5 cAMP-specific phosphodiesterase to the N- and C-domains of beta-arrestin using spot-immobilized peptide arrays. Biochem J. 2007;404:71-80
27. De-Simone SG, Napoleao-Pego P, Teixeira-Pinto LA. et al. IgE and IgG epitope mapping by microarray peptide-immunoassay reveals the importance and diversity of the immune response to the IgG3 equine immunoglobulin. Toxicon. 2014;78:83-93
28. Svojgr K, Burjanivova T, Vaskova M. et al. Adaptor molecules expression in normal lymphopoiesis and in childhood leukemia. Immunol Lett. 2009;122:185-92
29. Agarwal S, Ghosh R, Chen Z. et al. Transmembrane adaptor protein PAG1 is a novel tumor suppressor in neuroblastoma. Oncotarget. 2016;7:24018-26
30. Zhou D, Dong P, Li YM. et al. Overexpression of Csk-binding protein decreases growth, invasion, and migration of esophageal carcinoma cells by controlling Src activation. World J Gastroenterol. 2015;21:1814-20
31. Sirvent A, Benistant C, Pannequin J. et al. Src family tyrosine kinases-driven colon cancer cell invasion is induced by Csk membrane delocalization. Oncogene. 2010;29:1303-15
32. Hernychova L, Nekulova M, Potesil D. et al. A combined immunoprecipitation and mass spectrometric approach to determine deltaNp63-interacting partners. Klin Onkol. 2012;25(Suppl 2):2S64-9
33. Washburn MP, Ulaszek R, Deciu C. et al. Analysis of quantitative proteomic data generated via multidimensional protein identification technology. Anal Chem. 2002;74:1650-7
34. Bi J, Wang R, Zhang Y. et al. Identification of nucleolin as a lipid-raft-dependent beta1-integrin-interacting protein in A375 cell migration. Mol Cells. 2013;36:507-17
35. Poschau M, Dickreuter E, Singh-Muller J. et al. EGFR and beta1-integrin targeting differentially affect colorectal carcinoma cell radiosensitivity and invasion. Radiother Oncol. 2015;116:510-6
36. Eke I, Dickreuter E, Cordes N. Enhanced radiosensitivity of head and neck squamous cell carcinoma cells by beta1 integrin inhibition. Radiother Oncol. 2012;104:235-42
37. Pang DJ, Hayday AC, Bijlmakers MJ. CD8 Raft localization is induced by its assembly into CD8alpha beta heterodimers, Not CD8alpha alpha homodimers. J Biol Chem. 2007;282:13884-94
38. Korade Z, Kenworthy AK. Lipid rafts, cholesterol, and the brain. Neuropharmacology. 2008;55:1265-73
39. Sun X, Fu Y, Gu M. et al. Activation of integrin alpha5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proc Natl Acad Sci USA. 2016;113:769-74
40. Lin HJ, Hsu FY, Chen WW. et al. Helicobacter pylori Activates HMGB1 Expression and Recruits RAGE into Lipid Rafts to Promote Inflammation in Gastric Epithelial Cells. Front Immunol. 2016;7:341
41. Liu Y, Guo X, Wu L. et al. Lipid rafts promote liver cancer cell proliferation and migration by up-regulation of TLR7 expression. Oncotarget. 2016;7:63856-69
42. Eke I, Deuse Y, Hehlgans S. et al. beta(1)Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J Clin Invest. 2012;122:1529-40
43. Rey-Barroso J, Colo GP, Alvarez-Barrientos A. et al. The dioxin receptor controls beta1 integrin activation in fibroblasts through a Cbp-Csk-Src pathway. Cell Signal. 2013;25:848-59
44. Rao VS, Srinivas K, Sujini GN. et al. Protein-protein interaction detection: methods and analysis. Int J Proteomics. 2014;2014:147648
Author contact
![]() Corresponding author: Li Shen, Department of Clinical Oncology, Taihe Hospital, Hubei University of Medicine, 30 South Renmin Road, Shiyan, Hubei 442000, P.R.China. E-mail: shenlihbcom
Corresponding author: Li Shen, Department of Clinical Oncology, Taihe Hospital, Hubei University of Medicine, 30 South Renmin Road, Shiyan, Hubei 442000, P.R.China. E-mail: shenlihbcom

 Global reach, higher impact
Global reach, higher impact