Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(21):4009-4017. doi:10.7150/jca.20274 This issue Cite
Research Paper
Cause-specific mortality prediction model for patients with basaloid squamous cell carcinomas of the head and neck: a competing risk analysis
1. Institute of Otolaryngology, Department of Otolaryngology - Head and Neck Surgery, Chinese PLA General Hospital, China. 28 Fuxing Road, Beijing 100853, China;
2. Department of Epidemiology Research, Toho University, Japan. 4-16-20, Omori-Nishi Ota-ku, Tokyo 143-0015, Japan;
3. Division of Allergy, Department of Medical Subspecialties, National Center for Child Health and Development, 2-10-1 Okura, Setagaya-ku, Tokyo 157-8535, Japan.
4. Medical Support Center for Japan Environment and Children's Study, National Center for Child Health and Development, Japan. 2-10-1 Okura, Setagaya-ku, Tokyo 157-8535, Japan.
*These authors contributed equally
Received 2018-3-27; Accepted 2018-8-23; Published 2018-10-16
Abstract
Purpose: Basaloid squamous cell carcinoma (BSCC) is a rare, high-grade variant of squamous cell carcinoma (SCC). Most published studies based on population-based datasets focus on prognostic differences between SCC and BSCC. Competing risk analyses for this disease have not been performed. We used Surveillance Epidemiology and End Results (SEER) data to calculate and model the cumulative incidence of death for patients with head and neck BSCC (HNBSCC) with competing risk approaches, and built a model to predict probability of cause-specific death for these patients.
Methods: We analyzed data on 1163 patients who were diagnosed with primary lip and oral cavity, oropharynx, or hypopharynx and larynx BSCC and registered in the SEER program between 2004 and 2013. We calculated crude cumulative incidence function (CIF) for mortality after diagnosis of HNBSCC. We built a Fine and Gray's proportional sub-distribution hazard model and nomogram to predict their probability of cause-specific death. We calculated concordance indexes (c-index) and plotted calibration curves to evaluate model performance.
Results: Five-year cumulative incidence of cause-specific death after diagnosis of HNBSCC was 26.5% (95% CI: 23.4-29.8%); cumulative incidence of other causes of death was 11.8% (95% CI: 9.4-14.3%). Old age, large tumor size, hypopharynx and larynx sites, lymph node-positive, distant metastasis, and non-radiotherapy were significant factors for high probability of cause-specific death. The model was well calibrated. The bootstrap-corrected c-index for the model was 0.71.
Conclusions: We built the first competing risk nomogram for HNBSCC. The model performance was found to be good. This individualized prognostic predictive tool will aid physicians in clinical counseling, and will assist patients in planning for their future lives.
Keywords: basaloid squamous cell carcinoma, head and neck cancer, cumulative incidence function, censoring, prediction model.
Purpose
Basaloid squamous cell carcinoma (BSCC) is a rare, high-grade variant of squamous cell carcinoma (SCC) [1]. It was first described by Wain et al. in 1986 in a case series with 10 patients,[2] and recognized as a distinct clinicopathological entity in the 2005 World Health Organization (WHO) classification [3]. Its histopathological appearance is distinct from that of common SCC. BSCC tumors are composed of both basaloid and squamous components. Basaloid cells often have an ulcerating infiltrative growth pattern, peripheral nuclear palisading, increased mitotic activity, and small cystic spaces filled with mucinous material [4]. This tumor may be mistaken for adenoid cystic carcinoma.
BSCC tumors have a distinct predilection for the base of the tongue, hypopharynx, and supraglottic larynx. Other less common sites in upper aerodigestive tract areas include the mouth, oral mucosa palate, tonsils, sinonasal tract, nasopharynx, and trachea [5]; it has also been reported in the esophagus, thymus, lungs, uterine cervix, and anus.[6-9] Incidence rates were 0.45 per 100,000 for BSCC and 0.25 for head and neck BSCC (HNBSCC) in the United States over 2000-2013, based on estimates from the Surveillance Epidemiology and End Results (SEER) 9 registries research data [10].
BSCC tumors mostly occur in men in their sixties and seventies [11]. Alcohol and tobacco use are considered the main etiological agents associated with BSCC development [4]. Clinical signs and symptoms are related to tumor location [5]. BSCC is considered to be an aggressive tumor because patients are often diagnosed with advanced-stage disease, and it has a high risk of nodal and distant metastases [11]. The observed 5-year overall survival (OS) rate in the US was 58.6% for patients with HNBSCC during 2000-2013, according to the SEER database [10].
Due to its rarity, most studies have been limited to case reports or small, single-institution case series, which might not have enough power to detect significant differences in prognosis between groups. Although survival for patients with HNBSCC has been estimated in recent years based on population-based cohorts, all published studies using SEER data focus on evaluating prognostic differences between SCC and BSCC. Competing risk analyses for this disease have not been performed. To improve prognostic information, and to assess the relative burdens of cause-specific death and other causes of death, in this study, we used SEER data to calculate and model the cumulative incidence of death for HNBSCC with competing risk approaches, and built a nomogram to predict the probability of cause-specific death for patients with this disease.
Methods
The SEER program of the National Cancer Institute collects data for all cancer patients in 18 defined geographic regions across the United States, including information on cancer patients' demographics, primary tumor site, histological type, grade, cancer stage, and treatment. The SEER-18 registries include the San Francisco (SF)-Oakland standard metropolitan statistical area, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose-Monterey (SJM), Los Angeles (LA), Alaska Natives, Rural Georgia, California excluding SF/SJM/LA, Kentucky, Louisiana, New Jersey, and Greater Georgia registries. It is the largest population-based cancer registry in the US, and covers approximately 28% of the US population.[10] Institutional review board approval was not required because SEER Research Data is publicly available, and informed consent were not necessary because all patient information is de-identified. All authors have signed the authorization form and received permission from SEER program to access and use the dataset [12].
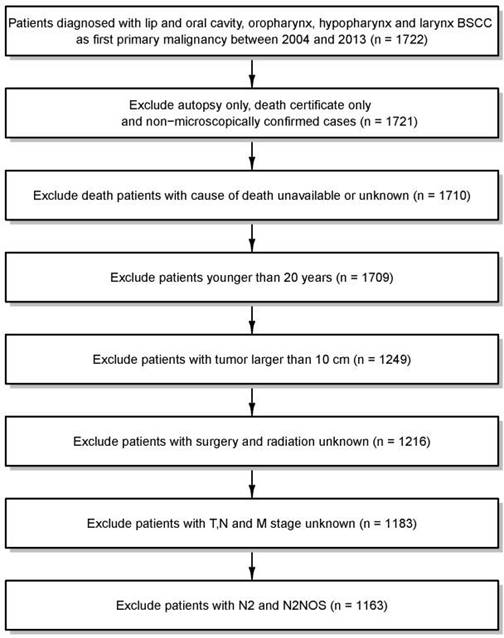
In this analysis, we used the April 2016 release of the SEER-18 database for case extraction [10]. All patients with diagnoses of primary lip and oral cavity, oropharynx, or hypopharynx and larynx BSCC (International Classification of Disease for Oncology, Third Edition, code 8083/3: basaloid squamous cell carcinoma) registered between 2004 and 2013 were extracted from the SEER database. From these patients, we excluded patients (a) who were only identified through death certificate or by autopsy; (b) whose cancers were diagnosed without histological conformation; (c) for whom the cause of death was unavailable or unknown; (d) who were younger than 20 years; (e) whose tumors were >10 cm; or (f) for whom TNM staging data or treatment information were missing. In the analysis, we regrouped N stage as N0, N1, N2a, N2b, N2c and N3. Thus, we omitted 20 cases of N2 or N2-NOS stage, as it was impossible to categorize them into these subgroups. After all exclusions, data from 1163 cases of HNBSCC diagnosed from 2004 through 2013 were available for analysis (Figure 1).
Patients were observed from diagnosis until death or through December 2013. We estimated median follow-up with the reverse Kaplan-Meier approach. Cause-specific death and other cause of death were the two failure events in this competing-risk setting. We used cumulative incidence function (CIF) to describe cause-specific mortality and other causes of death for patients with HNBSCC.[13] We also calculated crude CIF for mortality after a diagnosis of HNBSCC according to age at diagnosis, tumor size, tumor site, T classification at presentation, lymph node status, distant metastasis, AJCC stage, and treatment. When calculating crude CIF, we categorized patients by age at diagnosis (20-49, 50-59, 60-69, and ≥70 years) and by tumor size (<2, 2-3.9, and ≥4 cm). Tumor sites were regrouped as lip or oral cavity, oropharynx, and hypopharynx and larynx. Differences in CIF among the patient subgroups were tested with the Gray's test [13].
Data selection
We built a Fine and Gray's proportional sub-distribution hazard model to model CIF for cause-specific death after HNBSCC diagnosis [14]. Variables that have been shown to affect prognosis of patients with BSCC and those routinely available from the cancer registry were incorporated into the multivariable model. Based on the Fine and Gray's model, we also constructed a simple HNBSCC predictive nomogram to estimate the probability of cause-specific death. We calculated concordance indexes (c-index) and plotted calibration curves to evaluate model performance [15, 16]. If the model calibration is correct, dots on the calibration plot should be close to a 45° diagonal line [15]. The model was internally validated with 200 bootstrap samples.
The software SEER Stat 8.3.2 was used to extract the study cohort from the SEER dataset [10]. We used R (version 3.3.1) software [17] with its packages cmprsk, rms, and pec for analysis [18-20]. All P-values resulted from two-sided tests.
Results
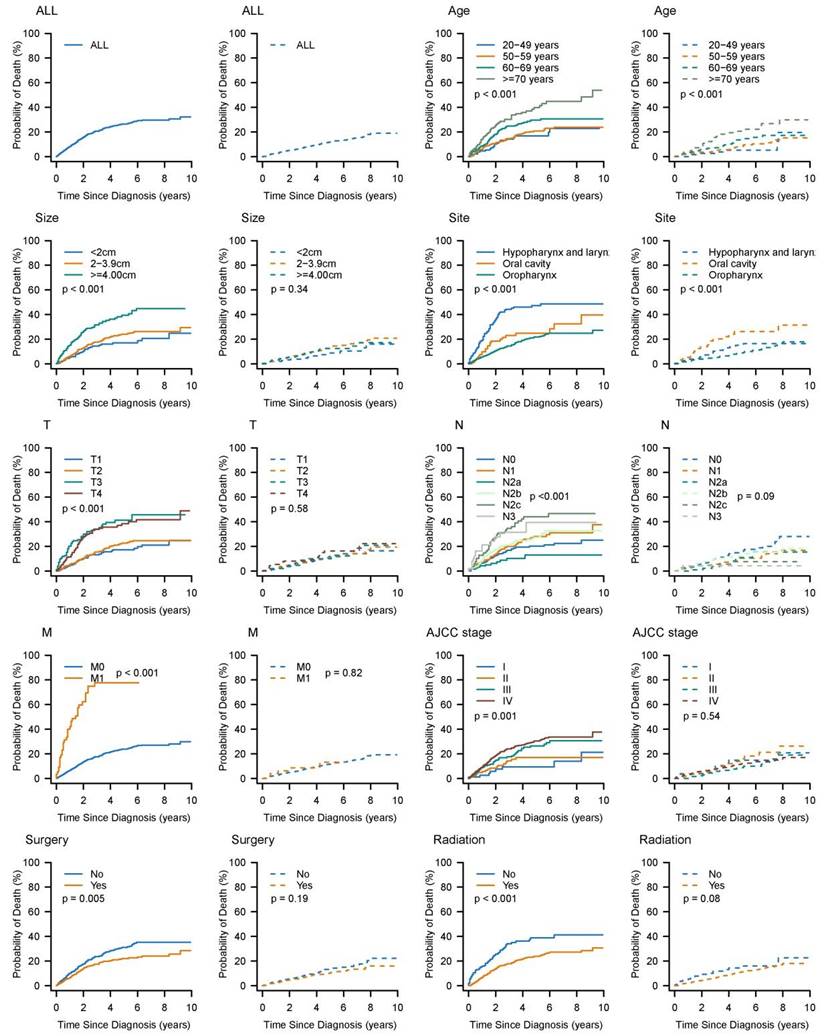
The characteristics of the 1163 HNBSCC cases and their 5-year cumulative incidence of death are summarized in Table 1. Cumulative incidence curves by age at diagnosis, tumor size, anatomic site, stage, and treatment, are plotted in Figure 2. Of these patients, approximately 70% were diagnosed at 50-70 years of age; 75% had tumors smaller than 4 cm; 75% had oropharynx BSCC; 74% had node-positive disease; 5% had distant metastasis; 80% had AJCC stage III or greater; 51% underwent surgery; and 83% received radiotherapy.
The median follow-up was 40 months (interquartile range: 18-73 months). A total of 336 HNBSCC patients died during the follow-up period: 232 from their cancers and 104 from other causes. The 5-year cumulative incidence of cause-specific death for patients with HNBSCC was 26.5% (95% CI: 23.4-29.8%); cumulative incidence of other causes of death was 11.8% (95% CI: 9.4-14.3%). All prognostic factors listed in Table 1 significantly influenced CIF for cause-specific mortality. CIF for both cause-specific death and other causes of death increased with age. Patients with larger tumors had higher cumulative incidence of cause-specific death. Patients with oropharynx BSCC, T1 stage, and N0 or M0 stage had lower CIFs for cause-specific death. Undergoing surgery or radiotherapy was associated with decreased probability of cause-specific death.
The results of the Fine and Gray's proportional sub-distribution hazard model for cause-specific mortality are listed in Table 2. Age at diagnosis and tumor size were significant predictors of cause-specific death, with sub-distribution hazard ratios (sdHR) of 1.04 (95% CI: 1.02-1.05, P<0.001) and 1.14 (95% CI: 1.04-1.26, P=0.004), respectively. The oropharynx BSCC subgroup had a better prognosis than the hypopharynx and larynx subgroups. Patients with positive lymph nodes or distant metastases were more likely to die of their cancers than those with no lymph node involvement or distant metastasis. Radiotherapy was also associated with better prognosis (sdHR =0.59, 95% CI: 0.40-0.88, P=0.008).
Cumulative incidence estimates of death for patients with head and neck BSCC by patient characteristics (solid line: cause-specific death; dotted line: other cause of death).
Five-year cumulative incidences of death among patients with head and neck BSCC.
| Cause-specific death (%) | Death from other causes (%) | |||||
|---|---|---|---|---|---|---|
| Characteristics | N | % | Death | % | 5-year (95%CI) | 5-year (95%CI) |
| Total | 1163 | 336 | 26.5 (23.4 to 29.8) | 11.8 (9.4 to 14.3) | ||
| Age (years) | ||||||
| 20-49 years | 140 | 12.0 | 27 | 8.0 | 17.0 (10.4 to 24.9) | 5.3 (1.9 to 11.4) |
| 50-59 years | 456 | 39.2 | 100 | 29.8 | 21.0 (16.4 to 26.0) | 8.4 (5.4 to 12.3) |
| 60-69 years | 366 | 31.5 | 111 | 33.0 | 29.6 (23.8 to 35.7) | 13.6 (9.1 to 19.0) |
| 70+ years | 201 | 17.3 | 98 | 29.2 | 39.8 (31.3 to 48.2) | 20.6 (14.1 to 28.1) |
| Size | ||||||
| <2 cm | 248 | 21.3 | 49 | 14.6 | 17.1 (11.8 to 23.4) | 8.9 (4.7 to 14.8) |
| 2-3.9 cm | 624 | 53.7 | 174 | 51.8 | 24.2 (20.0 to 28.5) | 12.6 (9.5 to 16.2) |
| >=4 cm | 291 | 25.0 | 113 | 33.6 | 39.4 (32.1 to 46.6) | 12.4 (7.9 to 18.0) |
| Site | ||||||
| HL | 191 | 16.4 | 99 | 29.5 | 47.3 (39.0 to 55.2) | 15.1 (9.6 to 21.9) |
| Lip or oral cavity | 99 | 8.5 | 43 | 12.8 | 24.9 (15.8 to 35.0) | 26.3 (16.3 to 37.5) |
| Oropharynx | 873 | 75.1 | 194 | 57.7 | 21.8 (18.3 to 25.6) | 8.9 (6.6 to 11.8) |
| T stage | ||||||
| T1 | 314 | 27.0 | 67 | 19.9 | 17.3 (12.4 to 22.9) | 11.8 (7.4 to 17.3) |
| T2 | 492 | 42.3 | 119 | 35.4 | 22.6 (18.0 to 27.7) | 10.5 (7.3 to 14.3 |
| T3 | 155 | 13.3 | 67 | 19.9 | 41.3 (31.7 to 50.6) | 10.0 (5.2 to 16.7) |
| T4 | 202 | 17.4 | 83 | 24.7 | 38.5 (30.1 to 46.8) | 16.3 (10.1 to 23.9) |
| N stage | ||||||
| N0 | 307 | 26.4 | 88 | 26.2 | 20.2 (15.0 to 25.9) | 14.9 (10.2 to 20.4) |
| N1 | 218 | 18.7 | 65 | 19.3 | 28.1 (20.9 to 35.8) | 10.4 (5.9 to 16.3) |
| N2a | 131 | 11.3 | 18 | 5.4 | 13.0 (6.3 to 22.1) | 11.0 (4.1 to 21.9) |
| N2b | 320 | 27.5 | 98 | 29.2 | 27.2 (21.2 to 33.5) | 13.0 (8.7 to 18.2) |
| N2c | 137 | 11.8 | 51 | 15.2 | 44.1 (33.3 to 54.5) | 7.6 (3.1 to 14.9) |
| N3 | 50 | 4.3 | 16 | 4.8 | 39.5 (19.6 to 59.0) | 4.3 (0.8 to 12.1) |
| M stage | ||||||
| M0 | 1109 | 95.4 | 295 | 87.8 | 23.9 (20.8 to 27.2) | 11.7 (9.3 to 14.4) |
| M1 | 54 | 4.6 | 41 | 12.2 | 77.7 (61.2 to 87.8) | 13.3 (4.0 to 28.1) |
| AJCC stage | ||||||
| I | 83 | 7.1 | 17 | 5.1 | 9.6 (3.8 to 18.5) | 14.9 (6.6 to 26.3) |
| II | 130 | 11.2 | 33 | 9.8 | 17.1 (10.3 to 25.5) | 13.9 (7.3 to 22.6) |
| III | 220 | 18.9 | 62 | 18.5 | 26.5 (19.5 to 34.0) | 8.8 (4.7 to 14.4) |
| IV | 730 | 62.8 | 224 | 66.7 | 30.4 (26.1 to 34.8) | 12.0 (9.0 to 15.4) |
| Surgery | ||||||
| No | 569 | 48.9 | 192 | 57.1 | 31.1 (26.3 to 34.9) | 13.2 (9.8 to 17.2) |
| Yes | 594 | 51.1 | 144 | 42.9 | 22.0 (18.0 to 26.4) | 10.3 (7.3 to 13.8) |
| Radiation | ||||||
| No | 197 | 16.9 | 84 | 25.0 | 38.8 (30.4 to 47.2) | 14.4 (9.0 to 21.1) |
| Yes | 966 | 83.1 | 252 | 75.0 | 23.9 (20.6 to 27.4) | 11.2 (8.7 to 14.1) |
BSCC, basaloid squamous cell carcinoma; HL, hypopharynx and larynx.
Proportional sub-distribution of probability of cancer-specific death for patients with head and neck BSCC.
| Characteristics | Coefficient | sdHR (95% CI) | P | |
|---|---|---|---|---|
| Age (years) | 0.04 | 1.04 (1.02to 1.05) | <0.001 | |
| Size (cm) | 0.14 | 1.14 (1.04 to 1.26) | 0.004 | |
| Site | ||||
| Oral cavity | -0.51 | 0.60 (0.35 to 1.03) | 0.065 | |
| Oropharynx | -0.85 | 0.43 (0.31 to 0.60) | <0.001 | |
| T stage | ||||
| T4 | -0.04 | 0.96 (0.66 to 1.39) | 0.817 | |
| N stage | ||||
| N1 | 0.61 | 1.83 (1.19 to 2.83) | <0.006 | |
| N2a | 0.01 | 1.01 (0.52 to 1.97) | 0.972 | |
| N2b | 0.53 | 1.69 (1.09 to 2.60) | 0.018 | |
| N2c+ | 1.04 | 2.82 (1.79 to 4.42) | <0.001 | |
| M1 | 1.15 | 3.14 (1.88 to 5.28) | <0.001 | |
| Surgery | -0.10 | 0.90 (0.67 to 1.21) | 0.498 | |
| Radiation | -0.52 | 0.59 (0.40 to 0.88) | 0.008 |
BSCC basaloid squamous cell carcinoma; sdHR sub-distribution hazard ratio.
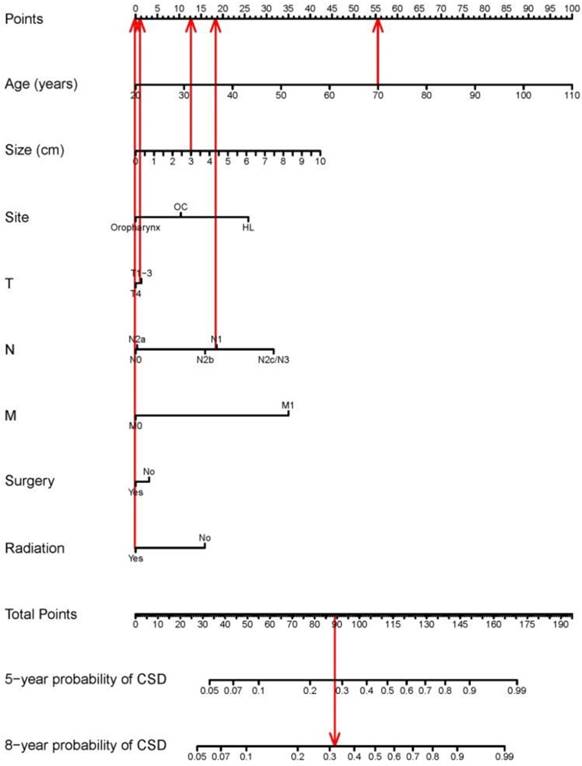
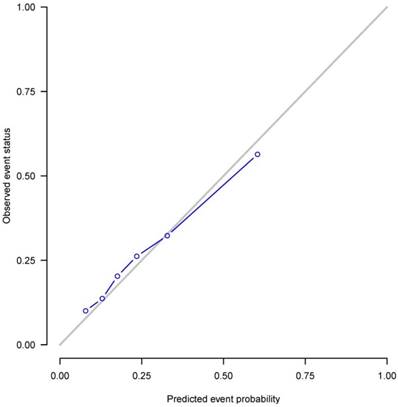
The nomogram based on the proportional sub-distribution hazard model we developed is shown in Figure 3. Five-year or 8-year probability of cause-specific mortality for patients with HNBSCC can be estimated from individual patient and tumor characteristics. To calculate the probability of cause-specific death for a specific HNBSCC patient, locate the patient's age on “Age (years)” row and draw a vertical line straight up to the “Points” row to obtain a value of points for age. Repeat the process for size, site, TNM stage, and treatment. Sum the value of points for each variable, locate this total of points on the “Total-points” axis, and draw a vertical line straight down to find the probability of cause-specific mortality for patients with HNBSCC. For example, a 70-year-old (56 points from the “Points” row) patient with oropharynx BSCC (0 point), with a tumor size of 3 cm (13 points), T2 (1 point), N1 (19 points), M0 (0 point), who underwent surgery (0 point) and radiotherapy (0 point), has a total of 89 points, which corresponds to the 5-year probability of cause-specific death of 28%. The calibration curve is plotted in Figure 4. Dots on the plot are close to the 45° diagonal line, which suggests that the model was well calibrated. Apparent c-index and bootstrap-corrected c-index were 0.73 and 0.71, respectively.
Discussion
In this study, we evaluated the prognosis for HNBSCC with competing risk approaches. We found 5-year CIFs of 26.5% and 11.8% for cause-specific death and other causes of death, respectively. The current analysis was based on 1163 patients from the SEER database diagnosed between 2004 and 2013. This is the newest and biggest series for BSCC in head and neck sites.
Most studies of prognosis in BSCC have compared OS or disease-specific survival (DSS) between BSCC and SCC. Thariat et al. reported 51 BSCC cases in 2008 with data from a cancer center [21], which compared OS between patients with BSCC and those with SCC. Due to the small sample size, multivariable analysis was not performed in their study. Reports using SEER have been published in recent years. Linton et al. summarized 642 cases of BSCC in the oral cavity, oropharynx, and larynx and hypopharynx. OS was the primary endpoint. They demonstrated that patients with oropharynx BSCC had a better prognosis than those with SCC [22]. Fritsch et al. conducted 4 studies with SEER data that estimated DSS for BSCC. Their results indicated that BSCC of the oral cavity carries a prognosis comparable to that of common oral SCC, and BSCC of the oropharynx has a more favorable prognosis than conventional-type oropharyngeal SCC, whereas BSCC of the larynx has a worse prognosis than SCC [23-25]. In 2014, Fritsch et al. performed an updated analysis of 1083 patients with HNBSCC and 66,929 patients with SCC, who were diagnosed between 2000 and 2008. Similar results were observed for tumors in the oral cavity, oropharynx, and larynx; they also added information on prognosis for tumors in other sites (sinonasal, nasopharyngeal, and hypopharyngeal). The authors concluded that survival outcomes from HNBSCC were similar or better than those from conventional-type SCC in most sites [26].
Nomogram for predicting probabilities of cause-specific death after diagnosis of head and neck BSCC. CSD: cause-specific death; HL: hypopharynx and larynx; OC: lip or oral cavity. As the red line showed in the figure, a 70-year-old (56 points from the “Points” row) patient with oropharynx BSCC (0 point), with a tumor size of 3 cm (13 points), T2 (1 point), N1 (19 points), M0 (0 point), who underwent surgery (0 point) and radiotherapy (0 point), has a total of 89 points, which corresponds to the 5-year probability of cause-specific death of 28%.
Calibration plot. X-axis: mean predicted probability of cause-specific death after diagnosis of head and neck BSCC, based on the model. Y-axis: Observed cumulative incidence for cause-specific death.
Unlike the above published reports, the current study focused on cause-specific death. Our study cohort only included patients with BSCC, as comparing BSCC with SCC was not our aim. We have presented the cumulative incidence of cause-specific death for patients with HNBSCC. In a competing risk setting, death from other causes were not censored, but treated as a competing risk failure event. CIF reflects the mortality patterns actually observed. It is an unbiased estimate for probability of failure [27-29]. To our knowledge, this is the first competing risk analysis to quantify the probability of death from cancer or other causes after a diagnosis of HNBSCC.
Our model shows that larger tumor size, advanced N stage, and metastasis significantly predicted high probability of cause-specific death. Although the TMN system is a good tool to indicate prognosis for patients with head and neck cancers, we found that age at diagnosis was another strong predictor of cause-specific death. A nomogram based on a model that includes patients' demographic and clinical characteristics could provide more accurate individualized prediction than does TNM staging.
Our results also associated radiotherapy with decreased probability of cause-specific death. Although the endpoint was different, radiosensitivity in BSCC was studied by Larner et al. based on observations of 15 BSCC patients who were treated with either definitive or postoperative RT [30]. The authors reported an 86% local control rate and 100% regional control rate among patients treated with RT only. In a review that summarized the role of HPV, and its implication in HNBSCC treatment and prognosis, the authors suggested that HPV status may partly explain radiosensitivity in BSCC [9].
Accurate estimation of prognosis for patients with cancer is extremely useful in patient counseling. Our nomogram affords individualized quantitated probability of cause-specific mortality after a diagnosis of HNBSCC. Competing risk nomograms have been established for other tumors, such as breast cancer, prostate cancer, thyroid cancer, kidney cancer, sarcoma, melanoma, and head and neck SCC cancer. [12, 29, 31-35] This is the first effort to build a nomogram for patients with HNBSCC based on Fine and Gray's proportional sub-distribution hazard model. The model performance was found to be good. This predictive tool is also easy to use because the variables incorporated in the model can be obtained from clinical work.
The strength of the current study is that the cohort has enough histologically confirmed BSCC patients from a population-based dataset. Due to its rarity, most published studies regarding HNBSCC are case reports. SEER data can provide a sufficiently large sample size to build reliable multivariable models, especially for modeling the prognosis of rare entities.[36] Moreover, whereas clinical trial studies often tend to select patients with better prognoses, the population-based study design allows us to generalize our results to a larger population.[12, 36]
Our study has several limitations. Weaknesses inherent to the SEER dataset include lack of some information on some prognosis factors that were not routinely collected by cancer registries such as biomarkers, smoking and alcohol use. HPV is considered to be an important prognostic factor for patients with HNBSCC. Although HPV information has been collected since 2004, such data cannot be obtained for head and neck sites from the current public-use dataset; thus, this study was unable to estimate the effects of HPV on HNBSCC prognosis. We also did not have detailed information on treatment variables, such as chemotherapy, relapse, or surgical margins. As more than 30% of sample had missing grade data, we did not adjust this variable when modeling CIF. In addition, the model did not incorporate comorbidity due to its absence in the SEER public-use dataset. We used the SEER cause-of death item to determine whether a failure event occurred or not. Cause of death in SEER is based on death certificate reporting. Although accuracy of death certificates is imperfect, studies have shown that causes of death from death certificates are comparable to those obtained from autopsy in patients with malignancies.[34, 37] Finally, the study cohort data, which are obtained from the USA, may not reflect the prognosis of patients in other countries very well. Although this model's performance was validated with the bootstrap approach, it still needs further validation with other populations.
In conclusion, this is the first effort to present CIFs for cause-specific mortality and competing-risk death for HNBSCC with competing risk analyses. We further modeled probability of cause-specific mortality after HNBSCC diagnosis with the proportional sub-distribution hazard approach, and built the first head and neck BSCC nomogram to estimate cause-specific death. This individualized prognostic predictive tool will aid physicians in clinical counseling, such as making a rational follow-up schedule, and will assist patients in planning for their future lives.
Abbreviations
BSCC: basaloid squamous cell carcinoma; CIF: cumulative incidence function; c-index: concordance indexes; HNBSCC: head and neck basaloid squamous cell carcinoma; LA: Los Angeles; OS: overall survival; SCC: squamous cell carcinoma; sdHR: subdistribution hazard ratios; SEER: Surveillance Epidemiology and End Results; SF: San Francisco; SJM: San Jose-Monterey; WHO: World Health Organization.
Acknowledgements
The authors would like to thank SEER for open access to their database. The opinions or views expressed in this paper are those of the authors and do not represent the opinions or recommendations of the National Cancer Institute. This project received no grant funding.
Conflicts of interest
The authors have no potential conflicts of interest.
References
1. Kuan EC, Peng KA, Bhuta S, Diaz MF, Zhang ZF, Abemayor E. et al. Basaloid squamous cell carcinoma of the maxilla: Report of a case and literature review. American journal of otolaryngology. 2015;36:402-7
2. Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: report of 10 cases. Human pathology. 1986;17:1158-66
3. Barnes C, Eveson J, Reichart P, Sidransky D. World Health Organization Classification of Tumours. Pathology and genetics, head and neck tumours. Lyon: IARC Press. 2005
4. Vasudev P, Boutross-Tadross O, Radhi J. Basaloid squamous cell carcinoma: two case reports. Cases journal. 2009;2:9351
5. Ereno C, Gaafar A, Garmendia M, Etxezarraga C, Bilbao FJ, Lopez JI. Basaloid squamous cell carcinoma of the head and neck: a clinicopathological and follow-up study of 40 cases and review of the literature. Head and neck pathology. 2008;2:83-91
6. Paulino AF, Singh B, Shah JP, Huvos AG. Basaloid squamous cell carcinoma of the head and neck. The Laryngoscope. 2000;110:1479-82
7. Zbaren P, Nuyens M, Stauffer E. Basaloid squamous cell carcinoma of the hypopharynx. ORL; journal for oto-rhino-laryngology and its related specialties. 2003;65:332-40
8. Erisen LM, Coskun H, Ozuysal S, Basut O, Onart S, Hizalan I. et al. Basaloid squamous cell carcinoma of the larynx: a report of four new cases. The Laryngoscope. 2004;114:1179-83
9. Thariat J, Badoual C, Faure C, Butori C, Marcy PY, Righini CA. Basaloid squamous cell carcinoma of the head and neck: role of HPV and implication in treatment and prognosis. Journal of clinical pathology. 2010;63:857-66
10. Surveillance Epidemiology, End Results (SEER) Program. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973-2013 varying) - Linked To County Attributes - Total U.S, 1969-2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission.
11. Patel PN, Mutalik VS, Rehani S, Radhakrishnan R. Basaloid squamous cell carcinoma of oral cavity with incongruent clinical course. BMJ case reports. 2013. 2013
12. Shen W, Sakamoto N, Yang L. Melanoma-specific mortality and competing mortality in patients with non-metastatic malignant melanoma: a population-based analysis. BMC cancer. 2016;16:413
13. Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141-54
14. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of competing risks in survival analysis. J Am Stat Assoc. 1999;94:496-509
15. Harrell F. Regression Modeling Strategies Springer; 2015.
16. Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555-61
17. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2016 URL https://www.R-project.org/
18. Gray B. cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.2-7. 2014 http://CRAN.R-project.org/package=cmprsk
19. Harrell FEJr. rms: Regression Modeling Strategies. 2015. R package version 4.3-1. http://CRAN.R-project.org/package=rms.
20. Ulla B. Mogensen HI, Thomas A. Gerds (2012). Evaluating Random Forests for Survival Analysis Using Prediction Error Curves. Journal of Statistical Software, 50(11): 1-23. URL http://www.jstatsoft.org/v50/i11/.
21. Thariat J, Ahamad A, El-Naggar AK, Williams MD, Holsinger FC, Glisson BS. et al. Outcomes after radiotherapy for basaloid squamous cell carcinoma of the head and neck: a case-control study. Cancer. 2008;112:2698-709
22. Linton OR, Moore MG, Brigance JS, Gordon CA, Summerlin DJ, McDonald MW. Prognostic significance of basaloid squamous cell carcinoma in head and neck cancer. JAMA otolaryngology- head & neck surgery. 2013;139:1306-11
23. Fritsch VA, Lentsch EJ. Basaloid squamous cell carcinoma of the oropharynx: an analysis of 650 cases. Otolaryngology-head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2013;148:611-8
24. Fritsch VA, Gerry DR, Lentsch EJ. Basaloid squamous cell carcinoma of the oral cavity: an analysis of 92 cases. The Laryngoscope. 2014;124:1573-8
25. Fritsch VA, Lentsch EJ. Basaloid squamous cell carcinoma of the larynx: analysis of 145 cases with comparison to conventional squamous cell carcinoma. Head & neck. 2014;36:164-70
26. Fritsch VA, Lentsch EJ. Basaloid squamous cell carcinoma of the head and neck: location means everything. Journal of surgical oncology. 2014;109:616-22
27. Cronin KA, Feuer EJ. Cumulative cause-specific mortality for cancer patients in the presence of other causes: a crude analogue of relative survival. Statistics in medicine. 2000;19:1729-40
28. Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. Journal of the National Cancer Institute. 2004;96:1311-21
29. Yang L, Shen W, Sakamoto N. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:468-74
30. Larner JM, Malcolm RH, Mills SE, Frierson HF Jr, Banks ER, Levine PA. Radiotherapy for basaloid squamous cell carcinoma of the head and neck. Head & neck. 1993;15:249-52
31. Shen W, Sakamoto N, Yang L. Cancer-specific mortality and competing mortality in patients with head and neck squamous cell carcinoma: a competing risk analysis. Annals of surgical oncology. 2015;22:264-71
32. Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ Jr, Yossepowitch O, Vickers AJ. et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:4300-5
33. Kattan MW, Heller G, Brennan MF. A competing-risks nomogram for sarcoma-specific death following local recurrence. Statistics in medicine. 2003;22:3515-25
34. Kutikov A, Egleston BL, Wong YN, Uzzo RG. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:311-7
35. Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, Rouzier R, Broglio KR, Hortobagyi GN. et al. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:4952-60
36. Shen W, Sakamoto N, Yang L. Prognostic models to predict overall and cause-specific survival for patients with middle ear cancer: a population-based analysis. BMC cancer. 2014;14:554
37. Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-Year Survival After Surgical Treatment for Kidney Cancer: A Population-Based Competing Risk Analysis. Cancer. 2007;109:1763-8
Author contact
![]() Corresponding author: Dr. Limin Yang, Medical Support Center for Japan Environment and Children's Study, National Center for Child Health and Development, 2-10-1 Okura, Setagaya-ku, Tokyo 157-8535, Japan. Tel.: +81-03-3416-0181 (ext. 7877); FAX: +81-03-3415-9260; E-mail: yo-rgo.jp
Corresponding author: Dr. Limin Yang, Medical Support Center for Japan Environment and Children's Study, National Center for Child Health and Development, 2-10-1 Okura, Setagaya-ku, Tokyo 157-8535, Japan. Tel.: +81-03-3416-0181 (ext. 7877); FAX: +81-03-3415-9260; E-mail: yo-rgo.jp

 Global reach, higher impact
Global reach, higher impact