Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(15):2603-2611. doi:10.7150/jca.24918 This issue Cite
Research Paper
microRNA-485-5p Functions as a Tumor Suppressor in Colorectal Cancer Cells by Targeting CD147
1. Medical School of Southeast University, Nanjing, Jiangsu, China
2. General Clinical Research Center, Nanjing First Hospital, Nanjing Medical University, Nanjing 210000, China
#These two authors contributed equally to this paper.
Received 2018-1-13; Accepted 2018-4-14; Published 2018-6-23
Abstract
Colorectal cancer(CRC) is a prevalent malignancy in the world. There is growing evidence that microRNAs (miRNAs) as crucial modulator are in connection with many tumor-related diseases including CRC. Though miR-485-5p has been reported as an anti-oncogene in certain cancers, it remains unclear in CRC. In this research, we found that miR-485-5p was at lower level expression in CRC tissues and cell lines compared to the paired paracancerous tissues and the normal colon epithelial cell line FHC, correspondingly. Furthermore, Experimental up-regulation miR-485-5p in DLD-1 and SW480 cells with mimic could inhibit the ability of proliferation, migration, invasion of CRC cell lines and facilitate cells apoptosis. Also, we confirmed that CD147 existed typically negative regulation by miR-485-5p through binding a conserved sequence specifically within the CD147 3′-untranslated region (3′UTR) and reintroduction of CD147 could rescue the phenotypic changes caused by miR-485-5p. The findings provide evidence to demonstrate the role of miR-485-5p/CD147 interaction in CRC and indicate that miR-485-5p might be exploited therapeutically in CRC.
Keywords: miR-485-5p, colorectal cancer, proliferation, migration, invasion, CD147
1. Introduction
Colorectal cancer (CRC) is a greatly prevalent malignancy [1]. It has high morbidity and mortality mainly since the majority of CRC patients are at late stage when they are diagnosed. For invariable manifestations of tumor recurrence and unfavorable prognosis of distant metastasis, though a great deal of progress has been made, CRC remains a problematic disease. The five-year survival rate of CRC patients is still not high [2].
Small and non-coding microRNAs (miRNAs) belong to one kind of evolutionarily conserved endogenous RNAs [3]. There are numerous reports that miRNAs as important regulators participate in tumor formation and progression including the pathological processes of CRC [4] through affecting cell proliferation, apoptosis, migration, invasion, et al. And they have become the primary starting point recent years. MiRNAs were certified as an oncogene or cancer suppressor gene in multiple cancers via binding conserved sequence specifically within the 3' UTR of their target messenger RNA(mRNA) molecules to interfere with transcription or inhibit translation [5]. It was suggested that some miRNAs can be used as specific diagnostic and prognostic biomarkers [6]. Moreover, they may be employed as potential therapeutic targets as well [7]. Thus, researches on miRNAs are highly meaningful to further our understanding of their regulatory systems and enhance the level of CRC treatment.
miR-485-5p lies in the human genomic region of chromosome 14q32. 31 and was reported as a antineoplastic gene in many human cancers, such as oral tongue squamous cell carcinoma, melanoma, lung adenocarcinoma, gastric cancer, breast cancer [8-12] et al. But the function of miR-485-5p in CRC remains no reported. Whether miR-485-5p could affect metastasis and its specific regulatory mechanism in CRC remains unclear. Thus, this research was designed to make an investigation that whether miR-485-5p affected CRC cells proliferation, migration, invasion, apoptosis and the potential mechanisms. As far as we know, this is the first functional study to elucidate the effect of miR-485-5p in CRC biological process.
2. Material and methods
2.1. Tissue samples
Forty-one pairs of tissues histologically confirmed CRC and matching adjacent normal tissues (ANT) were obtained from patients who underwent routine surgical treatment at the Department of Oncological Surgery, the Nanjing First Hospital Affiliated to Nanjing Medical University (Nanjing, China) between September 2014 and July 2017. All tissues were sharp-freeze after excision and then preserved in liquid nitrogen container until use. We obtained informed written consent from all participants involved in our study and this study was approved by the ethics committee of the Nanjing First Hospital Affiliated to Nanjing Medical University (Nanjing, China).
2.2. Cell Culture and Cell Transfection
293 cell line, normal colon epithelial cell line FHC and 2 kinds of human CRC cell lines (DLD-1, SW480) were acquired from Shanghai Cell Collection, Chinese Academy of Sciences. 293 and FHC cells were routinely cultured in Dulbecco's Modified Eagle's Medium (DMEM, Hyclone, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, USA) and incubated in a humidified cell incubator (37°C, 5% CO2). DLD-1, SW480 were incubated in the same environment except that the complete medium was RPMI-1640 medium containing 10% FBS. Cells transfection with miR-485-5p mimic or corresponding miRNA mimic negative control (miR-NC) synthesized by RiboBio (Guangzhou, China) was carried out with riboFECTTM CP Transfection Kit (RiboBio, China). Transfection efficiency was monitored by qRT-PCR. To construct CD147 expression vector, the CD147 DNA (828bp) were synthesized by Synbio Technologies (Suzhou, China), and then cloned into pcDNA3.1(+) in NheI and XhoI sites (Invitrogen), named pcDNA3.1-CD147. All constructs were further confirmed by sequencing.
2.3. qRT-PCR
SYBR green qRT-PCR assays were performed for the purpose of quantifying miR-485-5p in CRC tissue samples and cells. Total RNA was extracted with TRIzol reagent (Invitrogen, USA). To detect CD147, reverse transcription of total RNA was carried out using the PrimeScriptTM RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China). Then the CD147 mRNA expression was tested via the SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) (Takara, Dalian, China). β-actin as internal control was employed for normalization. Forward and reversed primer sequences synthesized by Sangon (Shanghai, China) were as follows:
CD147, 5'-CCATGCTGGTCTGCAAGTCAG-3' (forward), 5'-CCGTTCATGAGGGCCTTGTC-3' (reverse);
β-actin, 5'-CTGGAACGGTGAAGGTGACA-3' (forward), 5'-AAGGGACTTCCTGTAACAACGCA-3' (reverse). Specifically, the primers for miR-485-5p were purchased from RiboBio (Guangzhou, China) with the small nuclear RNA (snRNA) U6 as an endogenous control. Then miR-485-5p level was measured using the miDETECT A TrackTM miRNA qRT-PCR Starter Kit. All reactions were carried out on the Applied Biosystems 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The relative expression level of CD147 and miR-485-5p were analyzed by the 2-ΔΔCt method.
2.4. Cell proliferation assay
The ability of cell proliferation was appraised by Cell Counting Kit-8 (CCK-8, KeyGen Biotech, China). CRC cells were seeded into 96-well plates (about 2, 000 cells per well) and cultured with complete media (10% FBS) for 12h. The next day, miR-485-5p mimic or miR-NC was transfected into CRC cells using riboFECTTM CP Transfection Kit (RiboBio, Guangzhou, China) according to the manufacturer's instructions. Starting from 0h after transfection, each well was added CCK-8 solution every 24 hours and incubated for 2 h. At the indicated time-point, a microplate reader (Bio-Rad Laboratories, Richmond, CA, USA) was used to record the absorbance (A) at 450 nm.
2.5. Cell migration and invasion assays
The migratory and invasive capability of CRC cells which had been transfected with either miR-485-5p mimic or miR-NC were tested in transwell chambers. For invasion assays, CRC cells resuspended with serum-free medium were added to the upper part of 24-well transwell chambers (Corning, USA) containing a polycarbonate membrane filter (pore size: 8 μM) which was coated with Matrigel (BD Biosciences, USA) while the lower chambers were filed with complete media (10%FBS) as a chemoattractant. Cells on lower surface of the polycarbonate membrane were fixed in methanol and stained with crystal violet after 24 hours incubation. The migration assays were the same as the invasion assays except that the membranes were non-coated with matrigel and the incubation time was just 12h. The migration and invasion activities were quantified by the average number of cells under five high-power fields (HPFs, 200×) per chamber.
2.6. Cell apoptosis assay
Cell apoptosis was tested using annexin V-FITC/PI apoptosis kit (KeyGen Biotech, Nanjing, China) according to the manufacturer's instructions. Cells were washed and resuspended in the binding buffer. After that, the cells were maintained in the dark for 5-15 min after adding Annexin V-FITC and propidium iodide (PI). Finally, the apoptotic cells were detected using flow cytometer (BD Biosciences, CA).
2.7. Luciferase reporter assay
microRNA. org (http://www. microrna. org/) and miRDB (http://mirdb. org/) were applied to predict the binding sites of miR-485-5p. The wild type (Wt, including the predicted miR-485-5p target sites ) and mutant (Mut, eight nucleotides were replaced within the binding site of miR-485-5p) 3'UTR of target CD147 were synthesized by Synbio Technologies (Suzhou, China), and then inserted into PmirGLO plasmids later, named CD147-3'UTR-Wt and CD147-3'UTR-Mut. All constructs were sequenced for further validation. 293 cells were co-transfected with either miR 485-5p mimic or miR-NC and double luciferase reporter gene (CD147-3'UTR-NC (PmirGLO), CD147-3'UTR-Wt or Mut) using Lipofectamine 2000 (Invitrogen, USA). After transfection, the luciferase activities were detected at 48h in a luminometer (GloMax-20/20 Luminometer from Promega, USA) with the Dual-Glo Luciferase Assay System (Promega, Madison WI, USA). The results were the average of 3 duplicates and repeated three times.
2.8. Protein extraction and Western blotting
Total protein in the cultured cells was isolated using lysis buffer (KeyGen Biotech, Nanjing, China) at 48h after transfection, and a BCA protein assay kit (KeyGen Biotech, Nanjing, China) was used to quantify the concentration of total protein. After denatured, extracted protein was separated by the 10% SDS-PAGE gel and then transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were kept at 4 °C overnight with following specific antibodies: CD147 (cat. no. Ab108317, Abcam, 1:5000) and GAPDH (cat. no. ab181602, Abcam, 1:10000) after blocking the nonspecific binding sites with 5% skim milk for 1 h. Whereafter, secondary antibody, goat anti-rabbit IgG-HRP (cat. no. Ab6721, Abcam, 1:10000) was used to incubate the immunoreactive protein bands for 1h followed by visualization through an enhanced chemiluminescence system (ECL). The GAPDH served as an internal control.
2.9. Immunohistochemical staining
The expression of CD147 in the CRC and matching adjacent normal tissues were evaluated by immunohistochemical staining. Clinical tissue samples were fixed with formalin and embedded with paraffin. Then paraffin-embedded tissue sections (4 µm) were deparaffinized, rehydrated and kept in a solution of 0.3% methanol/H2O2 for 10-30 min at room temperature to quench endogenous peroxidases. After Washing sections 3 times for 5 minutes with PBS, sections were subjected to heat-mediated antigen retrieval and incubated with blocking serum for 10-30 min at room temperature. The sections were kept at 4 °C overnight with following specific antibodies: CD147 (cat. no. Ab108317, Abcam) after blocking the nonspecific binding sites and then incubated with HRP-conjugated anti-rabbit antibody(cat. no. Ab201237, Abcam) for 1 h at room temperature. After washing, the 3,3'-diaminobenzidine (DAB) substrate (Sigma) was added immediately to the tissue sections and waited for color change. The sections were counterstained with hematoxylin and estimated under a microscope.
2.10. Evaluation immunohistochemical of staining
For CD147 immunohistochemical staining assessment, semi-quantitative scoring was assessed which evaluated both the number of positively stained cells and color depth. The percentage of positive cells was scored as 0 (≤ 5%), 1 (6%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (>75%). Color depth of positive cells was graded as 0 (no coloration), 1 (light yellow), 2 (pale brown), and 3 (dark brown). After multiplying the 2 scores, we got a negative result (-) for 0-2 points, weakly positive (+) for 3-4 points, moderately positive (++) for 5-8 points, and strongly positive (+++) for 9-12 points. Tissue sections were reviewed and scored by two investigators concurrently.
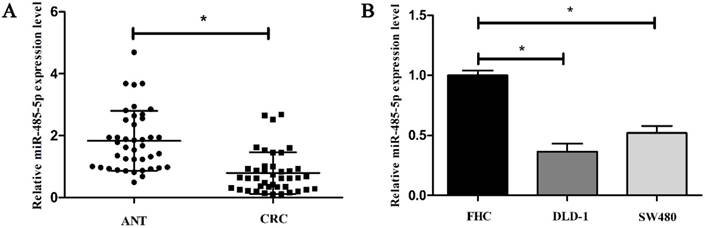
miR-485-5p is down-expression in CRC. qRT-PCR was performed to examine the expression level of miR-485-5p in CRC tissue samples compared with ANT (A, *P < 0.01) and different cell lines (B, *P < 0.001).
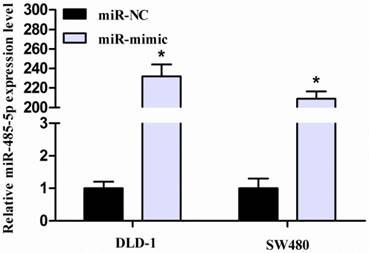
miR-485-5p is upregulated in CRC cells transfected with miR-485-5p mimic or miR-NC, *P < 0.001.
2.11. Statistical analysis
The data was expressed as mean ±standard deviation(SD). To analyze the difference between groups, student's t-test or one-way ANOVA were performed. It is considered statistically significant when P < 0.05. Statistical analysis and plotting were performed using the SPSS software version 19. 0 (IBM, Chicago, USA) and GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA) respectively.
3. Results
3.1. miR-485-5p is low expressed in CRC
To investigate the expression signature of miR-485-5p in CRC progression, we first performed qRT-PCR analysis in 41 clinical tumor samples and matched adjacent tissues. The data showed that miR-485-5p expression in CRC tissues obviously decreased compared to the paired adjacent tissues (P < 0.01, Fig. 1A). Subsequently, to explore the relation between miR-485-5p and CRC, we detected miR-485-5p of FHC, DLD-1, SW480 at the same time and found that miR-485-5p was also cut down in the invasive CRC cells compared to normal cells FHC (P < 0.001, Fig. 1B). These results demonstrate that miR-485-5p may be correlated with CRC.
3.2. miR-485-5p expression is up-regulated in transfected CRC cells
For elucidating the biological role of miR-485-5p in CRC progression, its mimic was transfected into both DLD-1 and SW480 cells. In addition, miR-NC was used as control group. After transfection for 48 hours, performing qRT-PCR monitored the efficiency of transfection. As expected, miR-485-5p expression was obviously enhanced in miR-485-5p mimic-transfected group compared with miR-NC-transfected group (P < 0.001, Fig. 2).
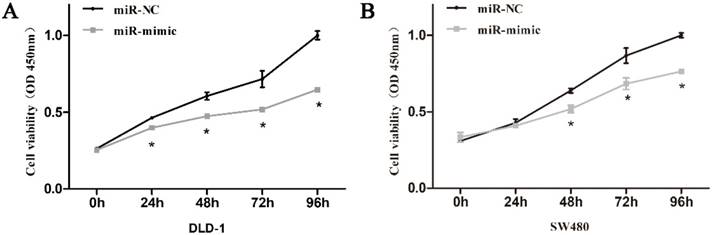
3.3. miR-485-5p inhibits DLD-1 and SW480 proliferation in vitro
In the process of culturing cells, we noticed that CRC cells in miR-485-5p mimic-transfected group proliferated slower than that in miR-NC-transfected group. Consequently, CCK-8 assays were used to assess the effects of miR-485-5p on the proliferation of CRC cell lines and the final results testified to the inhibitory effect of miR-485-5p in CRC cell proliferation as observed (Fig. 3A, Fig. 3B).
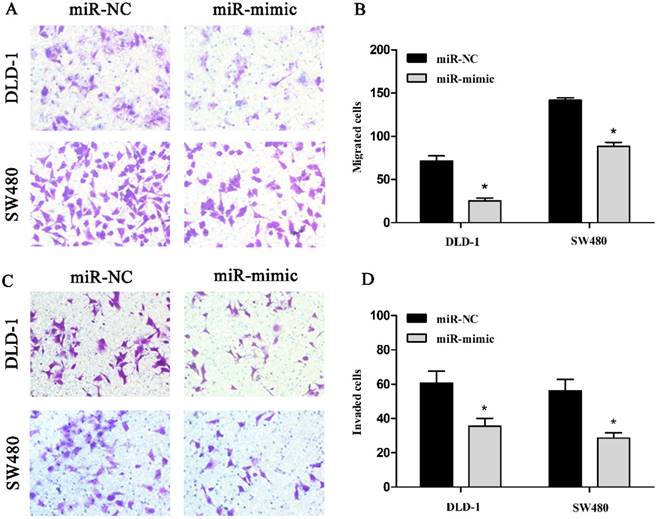
3.4. miR-485-5p over-expression inhibits CRC cells migration and invasion
Next, transwell assays were carried out to analyze the role of miR-485-5p in CRC cells migration and invasion. As shown in figures, the number of migrated DLD-1, SW480 cells (Fig. 4A, Fig. 4B) and the number of invaded DLD-1, SW480 (Fig. 4C, Fig. 4D) transfected with mimic were obviously decreased compared with their controls (p < 0.01), which suggested that experimental up-regulation of miR-485-5p inhibited the migratory ability and invasive ability of DLD-1 and SW480 cells.
Cell growth ability was analyzed using CCK-8 assays in DLD-1 (A) and SW480 (B) cells transfected with miR-485-5p mimic or miR-NC.
Representative images of migrated DLD-1 and SW480 were shown in A (200 ×). The number of migrated DLD-1 and SW480 was measured in B, respectively, *P < 0.01. Representative images of invaded DLD-1 and SW480 were shown in C (200 ×). The amount of invaded cells was measured in the D, respectively, *P < 0.01.
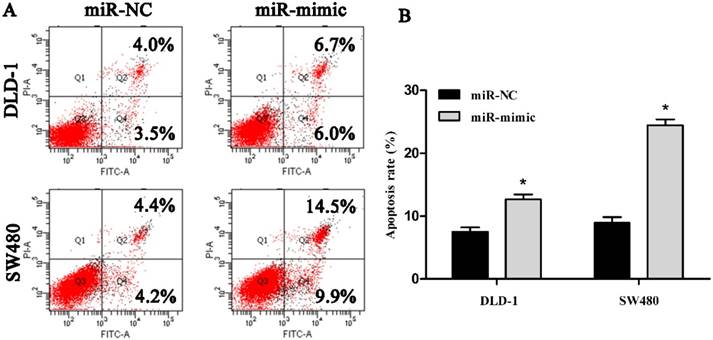
3.5. miR-485-5p facilitates DLD-1 and SW480 cell apoptosis
To address whether miR-485-5p regulated CRC cell apoptosis, we used flow cytometry to conduct cell apoptosis assay. Our findings revealed that the apoptosis rate of DLD-1 and SW480 cells with mimic-transfection was 12.7% and 24.4% at post-transfection 48 h respectively, while the apoptosis rate of DLD-1 and SW480 cells with miR-NC-transfection was 7.5% and 8.6% respectively (Fig. 5A, Fig. 5B), which suggested that up-regulated miR-485-5p obviously facilitated DLD-1 and SW480 cell apoptosis.
3.6. 3′UTR of CD147 is a direct target of miR-485-5p
MicroRNA. org (http://www. microrna. org/) and miRDB (http://mirdb. org/) were employed to predict target genes of miR-485-5p and found CD147 might be targeted. We used immunohistochemistry to examine the CD147 protein level in paraffin sections of CRC and matching ANT. Staining is weak or absent in matching ANT, whereas CD147 is strongly expressed in CRC tissue samples. The positive rates of CD147 in CRC tissues and ANT were 73.17%(30/41) and 26.83%(11/41), respectively (Fig. 6A, *P < 0.001).
Representative images of the apoptotic DLD-1 and SW480 were showed in A and the apoptosis rate of cells was measured in the B, respectively, *P < 0.01.
miR-485-5p targets CD147 in CRC cells (DLD-1 and SW480). (A) Representative images of CD147 protein expression in clinical CRC and matching adjacent normal tissue samples were measured by immunohistochemical staining (×400). (B) qRT-PCR analysis indicated that miR-485-5p inhibited CD147 mRNA expression, *P < 0.01. (C) Western blot results suggested that miR-485-5p overexpression decreased CD147 protein level in CRC cell lines. (D) We constructed pmirGLO-CD147-3′UTR-Wt and pmirGLO-CD147-3′UTR-Mut plasmids to perform a dual-luciferase reporter assay. (E) Dual-luciferase reporter assay indicated that miR-485-5p could bind to CD147 3′UTR directly. *P < 0.001.
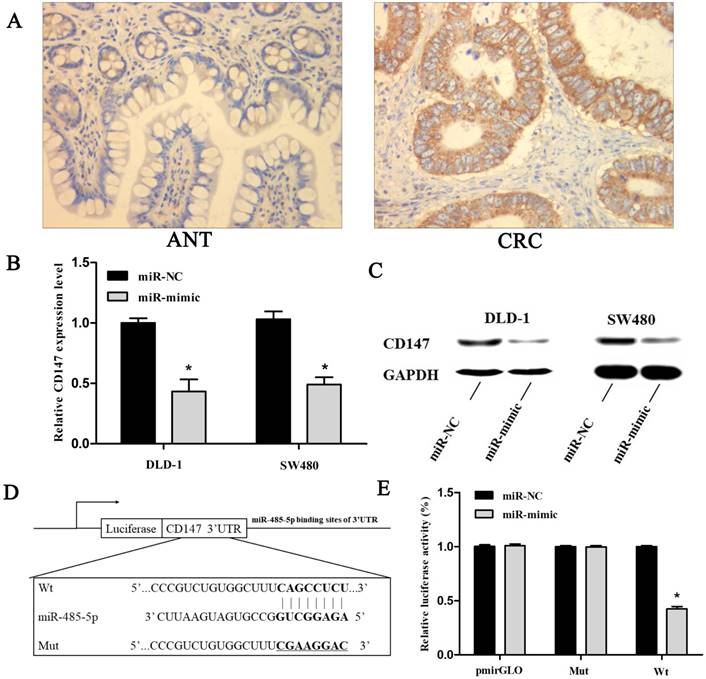
The qRT-PCR assays were applied to explore the relevance between miR-485-5p and CD147 in CRC. As shown in Fig. 2 and Fig. 6B, transfection significantly up-regulated miR-485-5p in DLD-1 and SW480 cells, whereas down-regulated CD147 was. At the same time, western blot analysis demonstrated that CD147 protein content was obviously reduced in CRC cells with miR-485-5p mimic transfection (Fig. 6C) which suggested that miR-485-5p inhibited CD147 protein expression. To verify whether miR-485-5p targeted CD147 directly, a dual-luciferase reporter assay was performed. The recombination plasmids (pmirGLO-CD147-3′-UTR-Wt or pmirGLO-CD147-3′-UTR-Mut (Fig. 6D)) were transfected into 293 cells along with mimic or mimic-NC respectively. As expected, mimic and pmirGLO-CD147 3′-UTR-Wt co-transfection resulted in a 42.3% decrease of the luciferase activity compared to the other controls (P < 0.001, Fig. 6E). These results indicated that miR-485-5p could be combined with the 3'UTR of CD147 to partly suppress the expression of CD147.
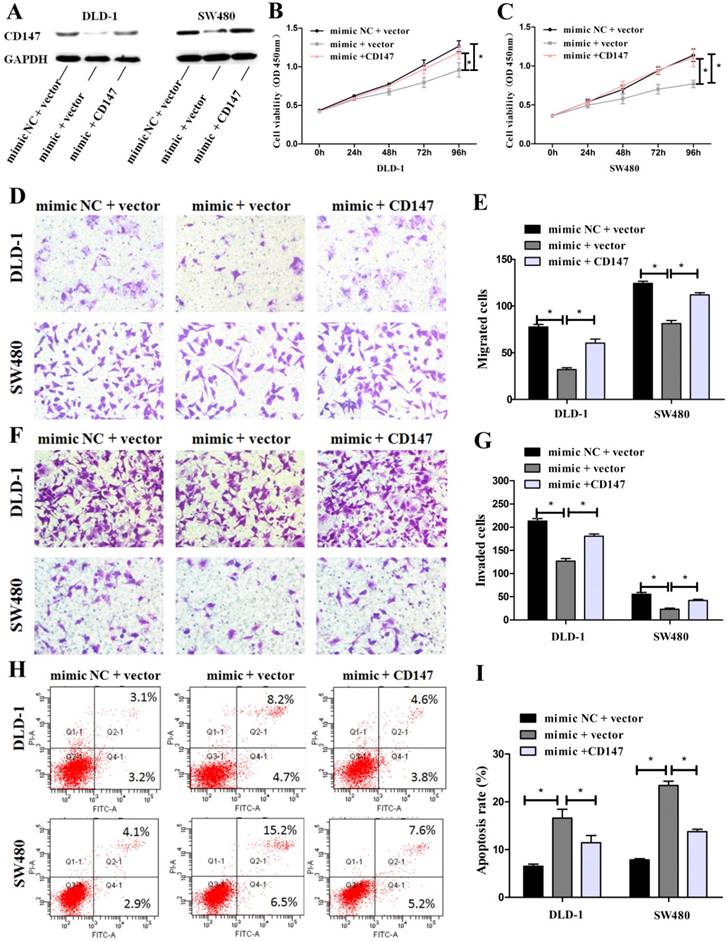
3.7. Restoration of CD147 counteracts the effects of miR-485-5p
To further elucidate whether CD147 was the target gene of miR-485-5p, rescue experiments were performed by constructing an overexpression plasmid of CD147 (pcDNA3.1-CD147) to transfect into DLD-1 and SW480 cells treated with miR-485-5p mimic. Western blot showed that overexpression CD147 could restored the protein level decreased by miR-485-5p mimic (Fig. 7A). Furthermore, the inhibitory effects on cell growth (Fig. 7B, Fig. 7C), migration (Fig. 7D, Fig. 7E), invasion (Fig. 7F, Fig. 7G), and the enhancement of apoptosis (Fig. 7H, Fig. 7I) caused by miR-485-5p mimic were partly abolished by overexpressing CD147.These results demonstrate that CD147 and miR-485-5p appear to play an opposite role to CRC cells phenotype and CD147 can rescue the phenotypic changes caused by miR-485-5p in human CRC cells.
4. Discussion
Accumulating evidences have suggested that abnormal expression of miRNAs is connected with CRC pathologies. They perform important roles in the pathogenesis of CRC as tumor suppressors or promoters depending on their target genes. Thus, identification of CRC-related miRNAs will obtain a deepening understanding of the molecular mechanism in CRC tumorigenesis and progression which may give a new perspective for the effective therapeutic strategies for this deadly cancer.
Studies have shown that miR-485-5p was low expressed in multiple tumor types and that it could inhibit the occurrence and development of tumors. Chen et al. indicated that miR-485-5p could target and regulate the expression of AT hook 2 (HMGA2) gene, and inhibit the invasion and metastasis of bladder cancer [13]. Kang et al. found that exogenous miR-485-5p could suppresses the growth of gastric cancer cells by directly and negatively regulating flotillin-1 (Flot-1) [14]. Guo et al. reported that miR-485-5p exerting its tumor-suppressive function, may regulated hepatocellular carcinoma cells proliferation and invasion. They also demonstrated that stanniocalcin 2 is a direct target of miR-485-5p [15]. The above researches indicate that miR-485-5p presents a tumor-suppressive function in the aggressiveness of cancers by down-regulating oncogenic target genes. Consistent with the previous reports, we found that the expression of miR-485-5p in 41 paired CRC tissues as well as 2 kinds of CRC cell lines was generally reduced when compared to that in paired adjacent tissues and the normal cells FHC respectively, indicating that down-regulating miR-485-5p may be associated with the CRC progression. On the basis of functional experiments, up-regulation of miR-485-5p revealed a distinct suppressive effect on cell proliferation, invasion and migration as well as facilitated CRC cells apoptosis in vitro. These findings suggested that miR-485-5p presented an inhibited function in CRC tumorigenesis and progression.
Subsequently, using predictive software, CD147 was suggested as a potential downstream mediator of miR-485-5p for further study. It has been reported that CD147 acting as a tumor related protein may contribute to the biological abnormity of cancers, promoting cells metastasis and invasion. Over-expression of CD147 has been found in a variety of tumors and exerts its roles in gastric cancer [16], hepatocellular carcinoma [17], lung cancer [18], prostate cancer [19] et al. Tao Xu et al. [20] showed that CD147 expressed higher in human CRC and increased abilities of cell invasiveness and epithelial-to-mesenchymal transition through activating MAPK/ERK pathway. Furthermore, our previous study has found that CD147 significantly contributes to tumor growth and metastasis in CRC [21]. However, the more molecular mechanisms via which CD147 expresses aberrantly are less explored.
In this study, miR-485-5p was significantly up-regulated in CRC cells after transfection with mimic, whereas down-regulated CD147 mRNA and protein detected through qRT-PCR and western blot assays. These findings indicated a remarkably negative relation between CD147 and miR-485-5p. Dual-luciferase reporter assay showed that miR-485-5p overexpression inhibited the luciferase activity of wild-type, but had no effect on mutant-type which confirmed that miR-485-5p could be combined with the 3' UTR of CD147 to partly suppress the expression of CD147. Furthermore, the data of rescue experiments, taken together, indicated that miR-485-5p and CD147 appear to play an opposite role to cell proliferation, invasion, migration and apoptosis in the CRC cell lines. Thus, we thought that miR-485-5p may directly target CD147, suppressing the growth and metastasis capability of CRC and facilitating cells apoptosis.
CD147 can rescue the phenotypic changes caused by miR-485-5p in human CRC cells. (A) CD147 protein expression was measured by western blot in CRC cells co-transfected with miR-485-5p mimic/miR-NC and with CD147 overexpression plasmid/empty vector. GAPDH was used as an internal control. (B-I) Cell proliferation, migration, invasion and apoptosis were determined in CRC cells co-transfected with miR-485-5p mimic/miR-NC and with CD147 overexpression plasmid/empty vector, all *P < 0.05.
But CD147 is not the only one that can be targeted by miR-485-5p. It is well known that a single miRNA may target numerous mRNAs while individual mRNAs can also be regulated by multiple miRNA. MiR-485-5p and its targeting of CD147 is only one element in a complex regulatory network. It is essential to recognize other genes targeted by miR-485-5p or other miRNAs regulating CD147, which will be able to give a deeper understanding about the molecular pathogenesis of CRC.
Overall, as the first investigation on miR-485-5p in CRC, this study demonstrates a potential relation between abnormal expressions of miR-485-5p and the pathological processes of CRC. A significant down-regulation of miR-485-5p was observed in CRC, and the exogenetic miR-485-5p inhibited cells proliferation, invasion, migration as well as facilitated cells apoptosis partially via regulating CD147 expression. On the one hand these findings give a new perspective that this novel miR-485-5p/CD147 axis might be helpful to develop some new and valuable methods for CRC prevention and treatment. On the other hand, the accurate mechanisms leading to the suppression of miR-485-5p and via which miR-485-5p promoted the occurrence and development of CRC are needed to be further studied.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81501820, 81472027) to YQP and SKW, the Jiangsu Youth Medical Talents Training Project to Y.P.(QNRC2016074) and Key Project of Science and Technology Development of Nanjing Medicine (ZDX16005).
Abbreviations
miRNAs, microRNAs; CD147, cluster of differentiation 147; CRC, colorectal cancer; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; CCK-8, cell counting kit-8 assay; 3′UTR, 3′-untranslated region; mRNA, messenger RNA; ANT, adjacent non-tumor tissues; FBS, fetal bovine serum; DMEM, Dulbecco's Modified Eagle's Medium; miR-NC, miRNA mimic negative control; PI, propidium iodide; Wt, wild type; Mut, mutant.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Fedewa SA. et al. Colorectal cancer statistics, 2017. Ca Cancer J Clin. 2017;67:104-17
2. Mcquade RM, Stojanovska V, Bornstein JC. et al. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Current Medicinal Chemistry. 2017;24:1537-57
3. Han YN, Xia SQ, Zhang YY. et al. Circular RNAs: A novel type of biomarker and genetic tools in cancer. Oncotarget. 2017;8:64551-63
4. Mohammadi A, Mansoori B, Baradaran B. The role of microRNAs in colorectal cancer. Biomedicine & Pharmacotherapy. 2016;84:705
5. Oliveto S, Mancino M, Manfrini N. et al. Role of microRNAs in translation regulation and cancer. World Journal of Biological Chemistry. 2017;8:45-56
6. Zhu M, Huang Z, Zhu D. et al. A panel of microRNA signature in serum for colorectal cancer diagnosis. Oncotarget. 2017;8:17081
7. Xu P, Zhu Y, Sun B. et al. Colorectal cancer characterization and therapeutic target prediction based on microRNA expression profile. Scientific Reports. 2016;6:20616
8. Lin XJ, He CL, Sun T. et al. hsa-miR-485-5p reverses epithelial to mesenchymal transition and promotes cisplatin-induced cell death by targeting PAK1 in oral tongue squamous cell carcinoma. International Journal of Molecular Medicine. 2017;40:83-9
9. Wu J, Li J, Ren J. et al. MicroRNA-485-5p represses melanoma cell invasion and proliferation by suppressing Frizzled7. Biomedicine & Pharmacotherapy. 2017;90:303-10
10. Lou C, Xiao M, Cheng S. et al. MiR-485-3p and miR-485-5p suppress breast cancer cell metastasis by inhibiting PGC-1|[alpha]| expression. Cell Death & Disease. 2016;7:e2159
11. Mou X, Liu S. MiR-485 inhibits metastasis and EMT of lung adenocarcinoma by targeting Flot2. Biochemical & Biophysical Research Communications. 2016;477:521-6
12. Jing LL, Mo XM. Reduced miR-485-5p expression predicts poor prognosis in patients with gastric cancer. Eur Rev Med Pharmacol Sci. 2016;20:1516-20
13. Chen Z, Li Q, Wang S. et al. miR-485-5p inhibits bladder cancer metastasis by targeting HMGA2. International Journal of Molecular Medicine. 2015;36:1136-42
14. Kang M, Ren MP, Zhao L. et al. miR-485-5p acts as a negative regulator in gastric cancer progression by targeting flotillin-1. American Journal of Translational Research. 2015;7:2212
15. Guo GX, Li QY, Ma WL. et al. MicroRNA-485-5p suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting stanniocalcin 2. International Journal of Clinical & Experimental Pathology. 2015;8:12292
16. Hu C, Dong X, Wu J. et al. CD147 overexpression may serve as a promising diagnostic and prognostic marker for gastric cancer: evidence from original research and literature. Oncotarget. 2017;8:30888
17. Ding P, Xin Z, Jin S. et al. CD147 functions as the signaling receptor for extracellular divalent copper in hepatocellular carcinoma cells. Oncotarget. 2017;8:51151-63
18. Zhang X, Tian T, Zhang X. et al. Elevated CD147 expression is associated with shorter overall survival in non-small cell lung cancer. Oncotarget. 2017:8
19. Mori A, Watanabe M, Sadahira T. et al. The Downregulation of the Expression of CD147 by Tumor Suppressor REIC/Dkk-3, and Its Implication in Human Prostate Cancer Cell Growth Inhibition. Acta Medica Okayama. 2017;71:135
20. Xu T, Zhou M, Peng L. et al. Upregulation of CD147 promotes cell invasion, epithelial-to-mesenchymal transition and activates MAPK/ERK signaling pathway in colorectal cancer. Int J Clin Exp Pathol. 2014;7:7432-41
21. Pan Y, He B, Chen J. et al. Gene therapy for colorectal cancer by adenovirus-mediated siRNA targeting CD147 based on loss of the IGF2 imprinting system. International Journal of Oncology. 2015;47:1881-9
Author contact
![]() Corresponding authors: S.-K. Wang, Y.-Q. Pan, Central Laboratory of Nanjing First Hospital, 68 Changle Road, 210006, Nanjing, Jiangsu, China. Fax/Tel: +86 025 52887003; E-mail: sk_wangedu.cn
Corresponding authors: S.-K. Wang, Y.-Q. Pan, Central Laboratory of Nanjing First Hospital, 68 Changle Road, 210006, Nanjing, Jiangsu, China. Fax/Tel: +86 025 52887003; E-mail: sk_wangedu.cn

 Global reach, higher impact
Global reach, higher impact