Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(10):1765-1772. doi:10.7150/jca.24573 This issue Cite
Research Paper
The prognosis of neck residue nasopharyngeal carcinoma (NPC) patients: results from a case-cohort study
1. Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine
2. Department of Nasopharyngeal Carcinoma; Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University
3. Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University
4. Department of Radiation Oncology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University
#These authors contributed equally to this article.
*The senior authors contributed equally to this work.
Received 2017-12-26; Accepted 2018-3-3; Published 2018-4-19
Abstract
Background: To assess the prognosis of neck residue nasopharyngeal carcinoma (NPC) patients and the efficacy of neck dissection in the treatment of these patients.
Methods: We recruited 68 neck residue NPC patients. For each neck residue patient we had three matched NPC patients without neck residue as controls (n = 204). The primary endpoint was progression-free survival (PFS). The Cox proportional hazards model was used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs), and multivariable analysis was used to test the independent statistical significance of NPC patients.
Results: Compared to controls, the neck residue patients showed significantly lower 3-year PFS (46.7% vs. 87.6%; P < 0.001). Multivariable analysis showed that neck residue was an independent prognostic factor for PFS.
Conclusions: NPC patients who had pathologically proven neck residue are associated with poor prognosis. Management with neck dissection alone seems not to be sufficient for these patients.
Keywords: nasopharyngeal carcinoma, neck residue, prognosis, neck dissection, case-cohort study
Background
Nasopharyngeal carcinoma (NPC) differs from malignant tumors arising from other head and neck mucosal sites with regard to its epidemiology, pathological types, and therapeutic management[1]. NPC has a distinct ethnic and geographical distribution in Guangdong, Southern China, where environmental factors, genetic predisposition, and Epstein-Barr virus (EBV) infection play an important role in its pathogenesis[2]. Radiotherapy (RT) is the primary treatment modality for NPC patients. Several prospective randomized trials[3-6] and meta-analyses have demonstrated that concurrent chemoradiotherapy (CCRT) with or without adjuvant chemotherapy is superior to RT alone for the treatment of NPC[7-9]. Currently, intensity-modulated radiotherapy (IMRT) is the preferred radiation technique for NPC patients. It provides excellent locoregional control[10], but there is still a small proportion of patients who have residual lymph nodes after complete treatment, i.e., neck lymph nodes that have not regressed completely by 3 months (i.e., neck lymph nodes larger than 1cm in diameter shown in MRI) after definitive radiotherapy with or without chemotherapy[11]. The management of these patients is challenging. According to our previous study[12], the incidence of patients having presumed neck residue after IMRT is about 3%, a finding that was consistent with the results of Leung's study[13]. The National Comprehensive Cancer Network (NCCN) guidelines advocate neck dissection for these patients[14]. Although many previous studies have reported the safety of neck dissection in the treatment of neck residue NPC patients, there is little literature on the prognosis of these patients following neck dissection, as compared with that of NPC patients without neck residue. Most previous studies grouped neck residue patients and patients presenting with recurrent nodal disease together and had control groups that did not comprise matched patients without neck residue[15-18]. Therefore, it is still unclear whether treatment with neck dissection provides a survival benefit for patients with neck residue.
We conducted this case-cohort study to examine the prognosis of neck residue NPC patients and the efficacy of neck dissection in the treatment of these patients.
Methods
Design, setting, and participants
For this retrospective case-cohort study we recruited 68 NPC patients who had been diagnosed with residual cervical lymphadenopathy after completion of radical radiotherapy with or without chemotherapy. The study was approved by the Institutional Review Board of the Sun Yat-sen University Cancer Center. Patients were eligible for this study if they fulfilled all of the following criteria: (1) had neck nodes that had not regressed completely by 3 months (i.e., neck lymph nodes larger than 1cm in diameter shown in MRI) after completion of therapy; (2) had undergone neck dissection in the Department of Head and Neck Surgery, Sun Yat-sen University Cancer Center; (3) had malignant cells found in their final neck specimen; (4) were newly diagnosed cases of NPC without metastasis; (5) had biopsy-proven World Health Organization types II-III NPC[19]; (6) had no history of previous anticancer therapy; and (7) had completed radical RT with or without chemotherapy. NPC patients with neck recurrence (i.e., reappearance of lymphadenopathy after initial complete regression of nodal disease)[15] or neck residue patients having received chemotherapy or salvage re-irradiation were excluded. Clinical, pathological, and radiological data of the eligible patients were reviewed and retrospectively reclassified. All patients were restaged according to the 7th AJCC/UICC staging system.
From January 2006 to December 2014, 292 NPC patients underwent neck dissection in the Department of Head and Neck Surgery at Sun Yat-sen University Cancer Center. Of them, 91 were NPC neck residue patients. Histopathological examination of the specimens from these patients showed 77 (84.6%) to be positive for malignant cells and 14 (15.4%) to be negative. During the follow-up period, 1 of the 14 patients who had clinically presumed but pathologically negative neck disease had disease progression. Bone metastasis was seen in 1/14 (7.1%) patients. No death and locoregional recurrence was seen in all during the follow-up period. As shown in supplement Figure 1, both of the 3-year PFS and 3-year DMFS of patients who had clinically presumed but pathologically negative neck diseases were 92.3% in our sample. As mentioned, the favorable outcomes of 14 patients who had clinically presumed but pathologically negative neck disease were also roughly the same as the NPC patients without neck residue. For this reason, we focus on the clinical characteristics and outcomes of the patients with positive histological findings. Among the 77 patients with positive histological findings, clinical data of initial treatment were not available for 9 patients who had completed radical RT with or without chemotherapy at other hospitals, and these patients were therefore excluded. The remaining 68 patients who met all the eligibility criteria comprised the cases (the neck residue group). Each patient in the neck residue group was matched with three other NPC patients who had been treated at Sun Yat-sen University Cancer Center between January 2006 and October 2014; these patients (n = 204) were selected from the institutional database and comprised the control group. Cases and controls were matched for the following: age (± 5 years), gender, pathological type, TNM stage, RT technique, type of treatment, and time period of therapy (±18 months) from the same institute. When an exact match was not available, a patient with more favorable characteristics was selected from the institutional database to prevent the results from being biased in favor of the study group. Table 1 and table 2 have summarized the matched characteristics and other characteristics of the patients in the two groups, respectively.
Clinical assessment
All patients were evaluated by a complete physical examination, fiberoptic nasopharyngoscopy, magnetic resonance imaging (MRI) or computed tomography of the head and neck, chest radiography, abdominal ultrasonography, electrocardiography, bone scan by emission computed tomography, complete blood count with differential count, biochemical profile, and Epstein-Barr virus serology. All patients diagnosed with NPC were treated with standard-course conventional radiotherapy or IMRT, whether chemotherapy was administered depended on age and the stage of disease.
Follow-up
Patients were assessed at the time of treatment completion, at least once every 3 months over the next 3 years, and at least once every 6 months thereafter. The patient evaluation at follow-up included clinical examination, nasopharyngeal endoscopy, MRI of the nasopharynx and the neck area, chest radiograph, and abdominal ultrasonography. Patient status was determined by reviewing the medical records of patients as well as the follow-up requests from the treating physicians. All neck residue patients underwent neck dissection.
Statistical analysis
The progression-free survival (PFS) rate was the primary endpoint of this study; the secondary endpoints included overall survival (OS), distant metastasis-free survival (DMFS), and locoregional relapse-free survival (LRRFS). PFS was defined as the duration from the date of first treatment to the date of disease progression or patient censoring at the date of the last follow-up. OS was calculated from the date of the first NPC treatment to the date of death from any cause or patient censoring at the date of the last follow-up. DMFS was defined as the duration from the date of first treatment to the date of diagnosis of distant metastasis or patient censoring at the date of the last follow-up. LRRFS was determined from the date of first treatment to the date of diagnosis of locoregional (nasopharynx, local/regional lymph nodes) recurrence of disease or patient censoring at the date of the last follow-up. Kaplan-Meier survival curves were used to analyze the time-to-event endpoints, and the log-rank test was used to compare the differences between the two groups. The hazard ratios (HRs) were calculated by the Cox proportional hazards model. Multivariable analyses were performed using the Cox proportional hazards model to test the independent statistical significance of treatment intervention. Potentially important prognostic factors considered in the modeling process included EBV DNA (>4000 copies/ml vs. ≤4000 copies/ml), VCA-IgA (>1:80 vs. ≤1:80), EA-IgA (>1:10 vs. ≤1:10), size of lymph node (>3 cm vs. ≤3 cm), bilateral cervical lymphadenopathy (yes vs. no), lymph node necrosis (yes vs. no), and neck residue (yes vs. no). Analyses were performed using SPSS 19.0 (SPSS, Chicago, IL). All statistical tests were two-sided, and P ≤ 0.05 indicated statistical significance. All data in our study have been recorded at Sun Yat-sen University Cancer Center for future reference (number RDDA2018000480).
Results
Patient characteristics
Among the 272 participants, there were 208 men and 64 women. The two groups were well balanced with respect to baseline demographic and disease characteristics (Table 1). Cases and controls were completely matched for gender, pathological type, and TNM stage. The percentages of patients matched for age, RT technique, time period of therapy, and type of treatment method were 90.1%, 96.7%, 94.1%, and 78.7%, respectively. The association between neck residue and the other clinicopathological characteristics of NPC patients was analyzed using the χ2 test (Table 2).
Patient matched characteristics.
| Neck residue group (%) | Control group (%) | P value | |
|---|---|---|---|
| n = 68 | n = 204 | ||
| Age (years) | 0.281 | ||
| Mean (Range) | 43.76 (21-77) | 43.5 (23-74) | |
| Sex | 1.000 | ||
| Male | 52 (76.5) | 156 (76.5) | |
| Female | 16 (23.5) | 48 (23.5) | |
| Pathological type | 1.000 | ||
| WHO type II | 2 (2.9) | 6 (2.9) | |
| WHO type III | 66 (97.1) | 198 (97.1) | |
| T stage* | 1.000 | ||
| T2 | 24 (35.3) | 72 (35.3) | |
| T3 | 35 (51.5) | 105 (51.5) | |
| T4 | 9 (13.2) | 27 (13.2) | |
| N stage* | 1.000 | ||
| N1 | 13 (19.1) | 39 (19.1) | |
| N2 | 34 (50.0) | 102 (50.0) | |
| N3 | 21 (30.9) | 63 (30.9) | |
| Overall stage* | 1.000 | ||
| II | 7 (10.3) | 21 (10.3) | |
| III | 34 (50.0) | 102 (50.0) | |
| IV | 27 (39.7) | 81 (39.7) | |
| RT technique | 0.216 | ||
| 2D Conventional RT | 7 (10.3) | 12 (5.9) | |
| IMRT | 61 (89.7) | 192 (94.1) | |
| Type of treatment | 0.120 | ||
| RT | 4 (5.9) | 10 (4.9) | |
| CCRT | 22 (32.4) | 70 (34.3) | |
| NAC + CCRT | 38 (55.9) | 122 (59.8) | |
| CCRT + AC | 4 (5.9) | 2 (1) | |
| Radical neck dissection | <0.001 | ||
| 68 (100) | 0 (0) |
Abbreviations: WHO, World Health Organization; RT, radiotherapy; IMRT, intensity-modulated radiotherapy; CCRT, concurrent chemoradiotherapy; NAC, neoadjuvant chemotherapy; AC, adjuvant chemotherapy.
* The 7th AJCC/UICC staging system.
Patient other characteristics.
| Neck residue group (%) | Control group (%) | P value | |
|---|---|---|---|
| n = 68 | n = 204 | ||
| EBV DNA, copies/ml | |||
| ≤4000 | 23(33.8) | 99(48.5) | 0.035 |
| >4000 | 45(66.2) | 105(51.5) | |
| VCA-IgA | |||
| ≤1:80 | 15(22.1) | 54(26.5) | 0.469 |
| >1:80 | 53(77.9) | 150(73.5) | |
| EA-IgA | |||
| ≤1:10 | 27(39.7) | 84(41.2) | 0.831 |
| >1:10 | 41(60.3) | 120(58.8) | |
| Size of lymph node | |||
| ≤3 cm | 42(61.8) | 175(85.8) | <0.001 |
| >3 cm | 26(38.2) | 29(14.2) | |
| Bilateral cervical Lymphadenopathy | |||
| yes | 53(77.9) | 156(76.5) | 0.803 |
| no | 15(22.1) | 48(23.5) | |
| Lymph node invasion | |||
| yes | 4(5.9) | 4(2.0) | 0.097 |
| no | 64(94.1) | 200(98.0) | |
| Lymph node necrosis | |||
| yes | 16(23.5) | 21(10.3) | 0.006 |
| no | 52(76.5) | 183(89.7) | |
Abbreviations: EBV = Epstein-Barr virus.
Three-year PFS (progression-free survival), OS (overall survival), DMFS (distant metastasis-free survival), LRRFS (locoregional relapse-free survival), and HRs with 95% CIs
| Neck residue group (%) n = 68 | Control group (%) n = 204 | P value | |
|---|---|---|---|
| Progression-free survival | |||
| Failures | 36 (53%) | 27 (13%) | |
| Rate at 3 years | 47% (33-60) | 88% (83-92) | <0.001 |
| Overall survival | |||
| Deaths | 16 (23%) | 17 (8%) | |
| Rate at 3 years | 85% (76-95) | 93% (89-97) | <0.001 |
| Distant metastasis-free survival | |||
| Distant failures | 18 (26%) | 21 (10%) | |
| Rate at 3 years | 78% (66-90) | 90% (86-95) | <0.001 |
| Locoregional relapse-free survival | |||
| Locoregional failure | 28 (41%) | 7 (3%) | |
| Rate at 3 years | 54% (41-68) | 97% (95-100) | <0.001 |
Data are n (%) or rate (95% CI). *Hazard ratios were calculated with the unadjusted Cox proportional hazards model. P values were calculated with the unadjusted log-rank test.
χ2 test showed that neck residue was significantly associated with EBV DNA level (p=0.035), size of lymph node (p<0.001) and lymph node necrosis (p=0.006). However, no significant correlation was observed between neck residue and any other clinical features including VCA-IgA level, EA-IgA level, bilateral cervical lymphadenopathy and lymph node invasion.
Survival
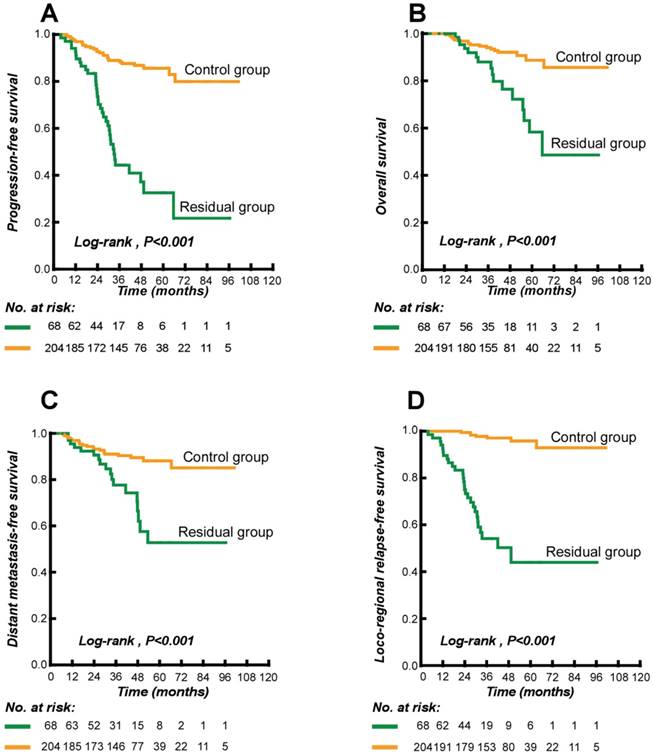
84.2% patients were followed regularly until June 2015. The median follow-up time was 44 months (range 6 - 101 months). During the follow-up period, 63 of the 272 patients had disease progression: 36/68 (53%) neck residue patients and 27/204 (13%) controls. Locoregional (i.e., nasopharynx or regional lymph nodes) recurrence was seen in 35/272 (12.9%) patients: 28/68 (41.2%) neck residue patients vs. 7/204 (3.4%) controls. We also found that nasopharynx recurrence was seen in 21/272 (7.7%) patients: 17/68 (25%) neck residue patients vs. 4/204 (2.0%) controls and regional lymph nodes recurrence was seen in 19/272 (7.0%) patients: 16/68 (23.5%) neck residue patients vs. 3/204 (1.5%) controls. Distant metastasis was seen in 39/272 (14.3%) patients: 18/68 (26.5%) neck residue patients vs. 21/204 (10.3 %) controls. There were 33 deaths in all (33/272; 12.1%) during the follow-up period: 16/68 (23.5%) in the neck residue group vs. 17/204 (8.3%) in the controls. Of these, 31 deaths (31/33; 93.9%) were disease-related: 15/68 (22.1%) in the neck residue group vs. 16/204 (7.8%) in the controls. One patient died of a comorbid illness and another in an accident. The 3-year PFS, OS, DMFS, and LRRFS were all significantly lower in the neck residue group than in the control group (Table 3). The Kaplan-Meier survival curves are shown in Figure 1. The log-rank test showed significant differences between cases and controls in all four endpoints (P < 0.0001).
In univariate analysis, high EBV DNA level, size of lymph nodes, lymph node invasion, and neck residue were all significantly associated with poor survival in NPC patients (Table 4). In multivariate analysis, after adjusting for other risk factors, neck residue remained a significant independent predictor for disease progression (HR = 5.58, 95% CI = 3.27 - 9.51); death (HR = 2.80, 95% CI = 1.34 - 5.87); metastasis (HR = 2.42, 95% CI = 1.24 - 4.75); and locoregional relapse (HR = 18.11, 95% CI = 7.66 - 42.82) (Table 5).
Discussion
This is the first case-cohort study to compare the prognosis of the neck residue NPC patients with that of patients without neck residue. Previous studies have indicated that for nonmetastatic NPC the 5-year PFS rate is 66.7% - 82.1% and the 5-year OS rate is 68.2% - 87.4%[10, 20, 21]. In our study the 3-year PFS and OS rates in neck residue NPC patients were only 46.7% and 85.3%, respectively. We also found that neck residue increased the risk of progression and death more than 5-fold and 2-fold, respectively, which is partially consistent with the results of Zhang et al.[18].
Previous research has reported 5-year DMFS rates of 82.6% - 87.6% for nonmetastatic NPC patients [10, 20, 21]. In our sample, the 3-year DMFS of neck residue patients was only 77.6%; these patients have increased 3-fold risk of metastasis compared with patients without neck residue. Earlier studies have shown that approximately 10% of NPC patients experience local or regional recurrence within 5 years[10, 22]. Our data have shown that neck residue patients had up increased risk both of nasopharynx and regional lymph node relapse. In our research, the 3-year LRRFS rate in the neck residue group was markedly lower at 54.1%. Furthermore, neck residue patients had up to 18-fold increased risk of locoregional relapse. It seems that neck residue patients can have locoregional relapse even after undergoing neck dissection, probably due to the persistence of microscopic remnants of tumor tissue.
Kaplan-Meier estimates of the survival of neck residue nasopharyngeal carcinoma patients and control patients. (A) Progression-free survival; (B) Overall survival; (C) Distant metastasis-free survival; and (D) Locoregional relapse-free survival.
The diagnosis and treatment of residual lymph nodes after curative radiotherapy or chemoradiotherapy for NPC can be challenging. The ideal course of treatment for neck residue NPC patients is still undecided. The NCCN guidelines recommend neck dissection. Although previous studies have reported the efficacy of neck dissection in NPC patients with cervical lymphadenopathy, most of these studies grouped neck residue patients and those with recurrent nodal disease together and had control groups that did not comprise NPC patients without neck residue[15-18, 23]. All 68 neck residue NPC patients in our study had undergone neck dissection, but despite receiving the recommended treatment they had poor prognosis. It seems that neck dissection alone is probably not sufficient for NPC patients who had pathologically proven neck residue, and maybe more intensive treatment methods are needed. Adding reirradiation or chemotherapy on the basis of neck dissection may be alternative to consider in this situation.
Univariate analysis of prognostic factors for PFS (progression-free survival), OS (overall survival), DMFS (distant metastasis-free survival), and LRRFS (locoregional relapse-free survival)
| Hazard ratio* (95% CI) | P value | |
|---|---|---|
| Progression-free survival | ||
| EBV DNA | 1.59 (1.21-2.09) | 0.001 |
| VCA-IgA | 1.02 (0.76-1.37) | 0.894 |
| EA-IgA | 1.13 (0.88-1.47) | 0.342 |
| Size of lymph node | 1.41 (1.09-1.85) | 0.010 |
| Bilateral cervical lymphadenopathy | 1.99 (0.99-3.97) | 0.051 |
| Lymph node invasion | 2.58 (1.03-6.45) | 0.043 |
| Lymph node necrosis | 1.75 (0.95-3.22) | 0.074 |
| Neck residue | 6.09 (3.66-10.14) | <0.001 |
| Overall survival | ||
| EBV DNA | 1.73 (1.16-2.58) | 0.007 |
| VCA-IgA | 1.42 (0.84-2.40) | 0.187 |
| EA-IgA | 1.23 (0.85-1.78) | 0.274 |
| Size of lymph node | 1.51 (1.06-2.16) | 0.022 |
| Bilateral cervical lymphadenopathy | 2.45 (0.88-6.89) | 0.088 |
| Lymph node invasion | 3.06 (1.06-8.89) | 0.039 |
| Lymph node necrosis | 1.88 (0.81-4.33) | 0.139 |
| Neck residue | 3.54 (1.78-7.04) | <0.001 |
| Distant metastasis-free survival | ||
| EBV DNA | 1.95 (1.32-2.87) | 0.001 |
| VCA-IgA | 1.16 (0.77-1.74) | 0.481 |
| EA-IgA | 1.31 (0.92-1.85) | 0.132 |
| Size of lymph node | 1.60 (1.16-2.20) | 0.005 |
| Bilateral cervical lymphadenopathy | 2.26 (0.90-5.67) | 0.084 |
| Lymph node invasion | 3.17 (1.12-8.96) | 0.003 |
| Lymph node necrosis | 2.11 (1.00-4.46) | 0.050 |
| Neck residue | 3.21 (1.70-6.05) | <0.001 |
| Locoregional relapse -free survival | ||
| EBV DNA | 2.11 (1.04-4.33) | 0.040 |
| VCA-IgA | 0.86 (0.60-1.24) | 0.417 |
| EA-IgA | 0.95 (0.68-1.32) | 0.740 |
| Size of lymph node | 1.61 (1.14-2.27) | 0.007 |
| Bilateral cervical lymphadenopathy | 1.97 (0.78-4.98) | 0.152 |
| Lymph node invasion | 1.90 (0.45-7.94) | 0.381 |
| Lymph node necrosis | 1.40 (0.58-3.37) | 0.456 |
| Neck residue | 18.93 (8.19-43.78) | <0.001 |
Abbreviations: CI = confidence interval; HR = hazard ratio.
A Cox proportional hazards regression model was used to detect variables one by one without adjustment. All variables were transformed into categorical variables. HRs were calculated for EBV DNA (>4000 copies/ml vs. ≤4000 copies/ml), VCA-IgA (>1:80 vs. ≤1:80), EA-IgA (>1:10 vs. ≤1:10), size of lymph node (>3 cm vs. ≤3 cm), bilateral cervical lymphadenopathy (yes vs. no), lymph node invasion (yes vs. no), lymph node necrosis (yes vs. no), and neck residue (yes vs. no).
Cox regression model of multivariable analysis for PFS (progression-free survival), OS (overall survival), DMFS (distant metastasis-free survival), and LRRFS (locoregional relapse-free survival)
| Hazard ratio* (95% CI) | P value | |
|---|---|---|
| Progression-free survival | ||
| EBV DNA | 1.46 (1.10-1.94) | 0.010 |
| Size of lymph node | 0.97 (0.73-1.29) | 0.832 |
| Lymph node invasion | 1.48 (0.58-3.77) | 0.411 |
| Neck residue | 5.58 (3.27-9.51) | <0.001 |
| Overall survival | ||
| EBV DNA | 1.53 (1.01-2.33) | 0.045 |
| Size of lymph node | 1.06 (0.71-1.59) | 0.766 |
| Lymph node invasion | 1.77 (0.59-5.37) | 0.311 |
| Neck residue | 2.80 (1.34-5.87) | 0.006 |
| Distant metastasis-free survival | ||
| EBV DNA | 1.73 (1.15-2.59) | 0.008 |
| Size of lymph node | 1.15 (0.80-1.64) | 0.453 |
| Lymph node invasion | 1.85 (0.64-5.36) | 0.259 |
| Neck residue | 2.42 (1.24-4.75) | 0.010 |
| Locoregional relapse-free survival | ||
| EBV DNA | 1.63 (0.78-3.45) | 0.197 |
| Size of lymph node | 1.02 (0.70-1.47) | 0.933 |
| Lymph node invasion | 0.85 (0.20-3.63) | 0.830 |
| Neck residue | 18.11 (7.66-42.82) | <0.001 |
Although NPC is a radiosensitive cancer, it appears that the residual lymph nodes may not be sensitive to radiotherapy. Radioresistance has been linked to increased likelihood of recurrence and distant metastasis[24, 25] and radioresistance can pose a major challenge in NPC treatment, but little is known about how it develops and further research is needed to clarify the mechanism of radioresistance of residual lymph nodes[26-28]. In addition, previous studies have showed that significant morbidity was also associated with the frequent radiation complications, including serious radiation fibrosis, radiation encephalopathy, cranial nerve injury, and cumulative dose of radiation complications after reirradiation[29, 30]. However, the chemotherapy after neck dissection can kill tumor cells that might have remained after macroscopic tumor removal and also can eliminate the micrometastasis. As a systemic treatment, chemotherapy is expected to reduce the occurrence of distant metastasis and provide an additional survival benefit over neck dissection in neck residue NPC patients. For these reasons, our group is intending to initiate a multicenter prospective randomized trial to compare neck dissection plus adjuvant chemotherapy with neck dissection alone to assess how adjuvant chemotherapy can benefit the management of neck residue NPC patients.
There are some limitations to our study. First, this was a retrospective study; second, our sample size was small because we were dealing with rare cases; and third, we were not able to match cases and controls completely for all factors, which may have introduced a selection bias.
Conclusion
NPC patients who had pathologically proven neck residue are associated with poor prognosis. Management with neck dissection alone seems not to be sufficient for these patients, and maybe more intensive treatment should be provided. Prospective randomized trials with large samples are needed to examine how other treatment methods can be combined with neck dissection to improve survival in NPC patients who had pathologically proven neck residue. Our group is intending to initiate a multicenter prospective randomized trial to compare neck dissection plus adjuvant chemotherapy with neck dissection alone to assess how adjuvant chemotherapy can benefit the management of NPC patients who had pathologically proven neck residue.
Abbreviations
NPC, nasopharyngeal carcinoma; RT, radiotherapy; PFS, progression-free survival; OS, overall survival; DMFS, distant metastasis-free survival; LRRFS, locoregional relapse-free survival; HRs, hazard ratios; CIs, confidence intervals; CCRT, concurrent chemoradiotherapy; IMRT, intensity-modulated radiotherapy; NCCN, National Comprehensive Cancer Network; MRI, magnetic resonance imaging; EBV, Epstein-Barr virus; WHO, World Health Organization; NP, nasopharynx; LN, lymph node; NAC, neoadjuvant chemotherapy; AC, adjuvant chemotherapy.
Supplementary Material
Supplementary figure S1.
Acknowledgements
We gratefully recognize the patients who participated in this study.
Funding
This work was partly supported by the Ministry of Science and Technology of China (No. 2011CB504300), the National Natural Science Foundation of China (Nos. 81201629, 81072226, 81372814, 81572848, 81772877, 81773103), the National High Technology Research and Development Program of China (863 Program) (No. 2012AA02A501), the National Key Basic Research Program of China (No. 2013CB910304), the Sci-Tech Project Foundation of Guangdong Province, China (Nos. 2011B080701034, 2011B031800161, 2012B031800255, 2014A020212528), the Sci-Tech Project Foundation of Guangzhou City, China (No. 2011J4300100), Guangzhou Science and Technology Planing Project, china(No. 2014J4100181), the Sun Yat-sen University Clinical Research 5010 Program and the Fundamental Research Funds for the Central Universities.
Availability of data and materials
The dataset supporting the conclusions of this article is included within additional file.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Sun Yat-sen University Cancer Center.
Authors' contributions
Study concepts: Ling-Guo, Hai-Qiang Mai. Study design: Sai-Lan Liu, Lin-Quan Tang. Data acquisition: Sai-Lan Liu, Lin-Quan Tang, Wen Wen, Qi Yang, Qian Zhu, Shan-Shan Guo, Li-Ting Liu, Yang Li, Hao-Jun Xie, Qing-Nan Tang, Xue-Song Sun, Yu-Jing Liang, Xiao-Yun Li, Jin-Jie Yan, Chao-Lin, Xiao-Wen Lan. Quality control of data and algorithms: Sai-Lan Liu, Lin-Quan Tang. Data analysis and interpretation: Sai-Lan Liu, Lin-Quan Tang. Statistical analysis: Sai-Lan Liu, Lin-Quan Tang. Manuscript preparation: Sai-Lan Liu, Lin-Quan Tang, Qiu-Yan Chen, Huan-Xin Lin. Manuscript editing: Sai-Lan Liu, Lin-Quan Tang. Manuscript review: Ling-Guo, Hai-Qiang Mai.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Seminars in cancer biology. 2002;12:421-9
2. Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chinese journal of cancer. 2011;30:114-9
3. Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY. et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. Journal of the National Cancer Institute. 2011;103:1761-70
4. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T. et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16:1310-7
5. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21:631-7
6. Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T. et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:6730-8
7. Huncharek M, Kupelnick B. Combined chemoradiation versus radiation therapy alone in locally advanced nasopharyngeal carcinoma: results of a meta-analysis of 1,528 patients from six randomized trials. American journal of clinical oncology. 2002;25:219-23
8. Langendijk JA, Leemans CR, Buter J, Berkhof J, Slotman BJ. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:4604-12
9. Huncharek M, Kupelnick B. In regards to Baujat et al.: Chemotherapy in locally advanced nasopharyngeal carcinoma: An individual patient data meta-analysis of eight randomized trials and 1753 patients (Int J Radiat Oncol Biol Phys 2006; 64:47-56). International journal of radiation oncology, biology, physics. 2006;65:958
10. Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ. et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? International journal of radiation oncology, biology, physics. 2011;80:661-8
11. Wei WI, Mok VW. The management of neck metastases in nasopharyngeal cancer. Current opinion in otolaryngology & head and neck surgery. 2007;15:99-102
12. Liu LT, Tang LQ, Chen QY, Zhang L, Guo SS, Guo L. et al. The Prognostic Value of Plasma Epstein-Barr Viral DNA and Tumor Response to Neoadjuvant Chemotherapy in Advanced-Stage Nasopharyngeal Carcinoma. International journal of radiation oncology, biology, physics. 2015;93:862-9
13. Leung TW, Tung SY, Sze WK, Wong FC, Yuen KK, Lui CM. et al. Treatment results of 1070 patients with nasopharyngeal carcinoma: an analysis of survival and failure patterns. Head & neck. 2005;27:555-65
14. Karantanos T, Theodoropoulos G, Gazouli M, Vaiopoulou A, Karantanou C, Lymberi M. et al. Expression of clock genes in patients with colorectal cancer. The International journal of biological markers. 2013;28:280-5
15. Ho CM, Wei WI, Sham JS, Lau SK, Lam KH. Radical neck dissection in nasopharyngeal carcinoma. The Australian and New Zealand journal of surgery. 1991;61:898-902
16. Yen KL, Hsu LP, Sheen TS, Chang YL, Hsu MH. Salvage neck dissection for cervical recurrence of nasopharyngeal carcinoma. Archives of otolaryngology-head & neck surgery. 1997;123:725-9
17. Wei WI, Lam KH, Ho CM, Sham JS, Lau SK. Efficacy of radical neck dissection for the control of cervical metastasis after radiotherapy for nasopharyngeal carcinoma. American journal of surgery. 1990;160:439-42
18. Zhang L, Zhu YX, Wang Y, Huang CP, Wu Y, Ji QH. Salvage surgery for neck residue or recurrence of nasopharyngeal carcinoma: a 10-year experience. Annals of surgical oncology. 2011;18:233-8
19. Shanmugaratnam K, Sobin LH. The World Health Organization histological classification of tumours of the upper respiratory tract and ear. A commentary on the second edition. Cancer. 1993;71:2689-97
20. Zhang MX, Li J, Shen GP, Zou X, Xu JJ, Jiang R. et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. European journal of cancer. 2015
21. Zhang W, Dou H, Lam C, Liu J, Zhou J, Liu Y. et al. Concurrent chemoradiotherapy with or without adjuvant chemotherapy in intermediate and locoregionally advanced nasopharyngeal carcinoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:1729-36
22. Yu KH, Leung SF, Tung SY, Zee B, Chua DT, Sze WM. et al. Survival outcome of patients with nasopharyngeal carcinoma with first local failure: a study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Head & neck. 2005;27:397-405
23. Wei WI, Ho WK, Cheng AC, Wu X, Li GK, Nicholls J. et al. Management of extensive cervical nodal metastasis in nasopharyngeal carcinoma after radiotherapy: a clinicopathological study. Archives of otolaryngology-head & neck surgery. 2001;127:1457-62
24. Lee AW, Poon YF, Foo W, Law SC, Cheung FK, Chan DK. et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: overall survival and patterns of failure. International journal of radiation oncology, biology, physics. 1992;23:261-70
25. Kristensen CA, Kjaer-Kristoffersen F, Sapru W, Berthelsen AK, Loft A, Specht L. Nasopharyngeal carcinoma. Treatment planning with IMRT and 3D conformal radiotherapy. Acta oncologica (Stockholm, Sweden). 2007;46:214-20
26. Qu JQ, Yi HM, Ye X, Zhu JF, Yi H, Li LN. et al. MiRNA-203 reduces nasopharyngeal carcinoma radioresistance by targeting IL-8/AKT signaling. Molecular cancer therapeutics. 2015
27. Sun Z, Pan X, Zou Z, Ding Q, Wu G, Peng G. Increased SHP-1 expression results in radioresistance, inhibition of cellular senescence, and cell cycle redistribution in nasopharyngeal carcinoma cells. Radiation oncology (London, England). 2015;10:152
28. Xu J, Ai Q, Cao H, Liu Q. MiR-185-3p and miR-324-3p Predict Radiosensitivity of Nasopharyngeal Carcinoma and Modulate Cancer Cell Growth and Apoptosis by Targeting SMAD7. Medical science monitor: international medical journal of experimental and clinical research. 2015;21:2828-36
29. Teo PM, Kwan WH, Chan AT, Lee WY, King WW, Mok CO. How successful is high-dose (> or = 60 Gy) reirradiation using mainly external beams in salvaging local failures of nasopharyngeal carcinoma? International journal of radiation oncology, biology, physics. 1998;40:897-913
30. Oksuz DC, Meral G, Uzel O, Cagatay P, Turkan S. Reirradiation for locally recurrent nasopharyngeal carcinoma: treatment results and prognostic factors. International journal of radiation oncology, biology, physics. 2004;60:388-94
Author contact
![]() Corresponding author: Ling-Guo, M.D., Ph.D.; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, P. R. China; E-mail: guolingorg.cn; Tel: +86-20-87343155; Fax: +86-20-87343392. Hai-Qiang Mai, M.D., Ph.D.; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, P. R. China; E-mail: maihqsysu.edu.cn; Tel: +86-20-87343380; Fax: +86-20-87343392.
Corresponding author: Ling-Guo, M.D., Ph.D.; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, P. R. China; E-mail: guolingorg.cn; Tel: +86-20-87343155; Fax: +86-20-87343392. Hai-Qiang Mai, M.D., Ph.D.; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, P. R. China; E-mail: maihqsysu.edu.cn; Tel: +86-20-87343380; Fax: +86-20-87343392.

 Global reach, higher impact
Global reach, higher impact