Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(7):1318-1328. doi:10.7150/jca.20150 This issue Cite
Research Paper
Downregulation of BANCR Promotes Aggressiveness in Papillary Thyroid Cancer via the MAPK and PI3K Pathways
1. Department of Thyroid and Breast Surgery, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, Hubei 430030, P.R. China
2. Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, P.R. China
*Equal contributors
Received 2017-3-19; Accepted 2017-12-2; Published 2018-3-26
Abstract
Recent evidence indicates that long non-coding RNAs play important roles in tumorigenesis and cancer progression. BRAF-activated non-protein coding RNA (BANCR) is a novel and potential regulator of cancer cell proliferation and migration. However, little is known regarding the role of BANCR in papillary thyroid cancer (PTC). The current study used quantitative PCR to demonstrate that BANCR was significantly downregulated in 60 paired PTC tissues compared with normal tissues. In addition, BANCR was significantly correlated with lymph node metastasis (p = 0.02). Furthermore, Cell Counting Kits and Transwell assays were used to demonstrate that knocking down BANCR with short hairpin RNA (shRNA) transfection significantly promoted the proliferation and invasion of PTC cell lines. The flow cytometric analysis of apoptosis and the cell cycle revealed that the overexpression of BANCR inhibited cancer cell proliferation and invasion, which was associated with the induction of cell-cycle G2/M phase arrest and increased apoptosis. Moreover, western blotting was used to show that the MAPK and PI3K-Akt pathways were aberrantly activated during BANCR-mediated PTC cell proliferation and migration. These findings revealed that BANCR functions as a tumor suppressor during thyroid carcinogenesis.
Keywords: papillary thyroid cancer, BRAF-activated non-protein coding RNA, MAPK signaling pathway, PI3K-Akt signaling pathway, cell cycle, apoptosis
Introduction
Thyroid cancer is a common endocrine malignancy that has rapidly increased in global incidence in recent decades [1-3]. In China, the incidence of thyroid cancer increased by 20.1% between 2003 and 2011, which was the highest increase among all cancers [4]. Follicular thyroid cell-derived tumors include papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), poorly differentiated thyroid cancer (PDTC), and anaplastic thyroid cancer (ATC) [5]. PTC is the primary component of the rapid rise in the incidence of thyroid cancer; it accounts for 80-90% of all thyroid cancers [6]. Although PTC is a highly curable and indolent cancer that has excellent prognosis, 10% of patients with aggressive tumors will develop progressive disease [7, 8]. Therefore, it is important to understand molecular mechanisms involved in the development and progression of thyroid cancer. Although several molecules contributing to tumor invasion and migration have been identified [5,9,10], the precise mechanisms underlying tumor pathogenesis remain to be fully elucidated.
Recently, thousands of long non-coding RNAs (lncRNAs) were uncovered by deep sequencing; they have a large variety of roles in both gene expression and remodeling of the eukaryotic genome [11]. LncRNAs are transcripts that are longer than 200 nucleotides, devoid of evident open reading frames, and often polyadenylated. Emerging evidence suggests that lncRNAs play a crucial role in gene regulation, which is strongly associated with cell fate determination and human disease, especially tumorigenesis [12-14]. For example, HOTAIR is highly expressed in ~10% of breast cancer patients [15, 16], MALAT1 is significantly associated with non-small cell lung cancer [17], and the aberrant expression of GAS5 is observed in prostate cancer [18]. Together, these findings suggest that lncRNAs are important for understanding the molecular biology of cancer progression, including thyroid cancer.
BRAF-activated non-protein coding RNA (BANCR) is a 693-base pair (bp) lncRNA originally identified using massively parallel cDNA sequencing in melanoma cells. It is highly expressed in melanoma and contributes to cell migration [19, 20]. Previous studies revealed that BANCR functions as both an oncogene and a tumor suppressor. Wang et al. demonstrated that BANCR expression was upregulated in six pairs of PTC and matching adjacent normal tissues and that BANCR increased PTC cell proliferation by activating autophagy [21]. Interestingly, Tian et al. reported that BANCR expression was downregulated in 68.5% (63/92) of PTC tissues compared with normal tissues [22]. However, the significance of BANCR in thyroid cancer is still unclear. In the present study, we assessed the expression of BANCR and performed in vitro studies to elucidate its role in PTC.
Materials and methods
Tissue samples
Tissue was obtained from patients (18 male and 42 female) who underwent surgery at the Department of Thyroid and Breast Surgery, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology in 2016. The study was carried out in accordance with the institutional ethical guidelines, and the use of human tissues was approved by the Medical Ethics Committee of Tongji Hospital (Institution Review Board Approval: TJ-C20141222). Each patient involved in the study was asked to sign a written informed consent form and the specimens were anonymized and handled according to accepted ethical and legal standards. The diagnosis of papillary thyroid cancer was confirmed histopathologically by an experienced pathologist. All samples were snap-frozen in RNAlater solution and stored at -80°C until use. Detailed patient descriptions are provided in Supplementary Table 1.
Cell line and culture conditions
Three human PTC cells lines NPA, BCPAP, TPC1 and normal thyroid tissue cell lines Nthy-ori3-1 were provided by The Institute of Interdisciplinary Research (Berkeley, CA, USA). The cell lines were authenticated at Shanghai Biowing Applied Biotechnology Co. Ltd using short tandem repeat (STR) DNA profiling (ABI 3730XL Genetic Analyzer, Life Technologies, Waltham, MA, USA). The cells were used in experiments within 25 passages. TPC1 and BCPAP cells were maintained in RPMI-1640 medium (Boster, Wuhan, China). NPA and Nthy-ori3-1 cells were maintained in DMEM medium (Boster), both supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), in a 37°C incubator with a humidified atmosphere of 5% CO2.
RNA interference using shRNA
BANCR was knocked down by transfecting cells with BANCR short hairpin RNA (shRNA, Genepharma, Shanghai, China) using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). The target sequences were as follows:
LV3‑BANCR ‑323 (shRNA1#), 5´-GGAGTGGCGACTATAGCAAAC-3´
LV3‑BANCR ‑540 (shRNA2#), 5´-GGACTCCATGGCAAACGTTGT-3´
BANCR shRNA stable transfectants were selected in medium containing 2 µg/mL puromycin (Goodbio, Wuhan, China). LV3-NC empty vector was used as the control. The specific silencing of BANCR expression was assessed using qRT-PCR.
Plasmid DNA transfection
The ectopic expression of BANCR was achieved by transfecting cells with pEX-2-BANCR (Genepharma, Shanghai, China) using Lipofectamine 3000. BANCR-overexpressing stable transfectants were selected in medium containing G418 (Goodbio, Wuhan, China; 800 µg/mL for NPA cells and 1100 µg/mL for TPC1 cells). pEX-2-NC empty vector was used as the control. The expression of BANCR was assessed using qRT-PCR.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from tissues or cells using Trizol reagent (Invitrogen). BANCR expression was then measured in triplicate using SYBR Green qPCR Mix (Toyobo, Shanghai, China). Primer sequences were as follows:
BANCR
Forward: 5´-ACAGGACTCCATGGCAAACG-3´
Reverse: 5´-ATGAAGAAAGCCTGGTGCAGT-3´
GAPDH
Forward: 5´- GGGAGCCAAAAGGGTCAT-3´
Reverse: 5´- GAGTCCTTCCACGATACCAA-3´
The data were processed using the 2-ΔΔ CT method and normalized to GAPDH expression.
Cell viability assays
Cell viability was monitored using Cell Counting Kit-8 (CCK-8, Dojindo, Kyushu, Japan) according to the manufacturer's instructions. NPA and TPC1 cells were seeded into six-well plates (Corning, New York, NY, USA) and incubated overnight. They were then transfected with BANCR shRNA1# and 2#, pEX-2-BANCR, or the respective negative control. The cells were maintained for 48 h and then seeded into 96-well plates (3000 cells per well). The cells were maintained for 4 h, and CCK-8 was then added to each well and incubated at 37°C for 3 h. The optical densities (ODs) were measured at a wavelength of 450 nm using a microplate reader. The results are represented as the mean of five different wells; wells were assessed in triplicate for each treatment group.
Transwell migration and invasion assays
For Transwell invasion assays, 3 × 104 NPA and TPC1 cells in serum-free medium were plated in the upper chamber that was coated with Matrigel (24‑well insert, 8‑mm pore size; Corning, NY, USA). For the Transwell migration assays the upper chamber was not coated with Matrigel. The media, which were supplemented with serum, served as a chemotactic agent in the lower chamber. The cells were incubated for 72 h and 48 h for invasion and migration assays, respectively. The cells that did not migrate through the pores were removed with a cotton swab, and the cells on the lower surface of the membrane were stained with crystal violet (Goodbio, Wuhan, China). The number of cells that penetrated the membrane was then counted in five random fields per chamber under a microscope (Olympus Corp. Tokyo, Japan). Each experiment was performed in triplicate.
Protein extraction and western blotting
Western blotting was performed as described previously [23]. The blots were probed with primary antibodies, including those against p-MEK, ERK1/2, p-ERK1/2, EGFR, p-EGFR, p-PI3K, AKT, p-AKT, p-FAK, p-STAT1, and p-STAT3 (all from Cell Signaling Technology, Danvers, MA, USA), followed by HRP-conjugated secondary antibodies. Signals were visualized using ECL reagent (Goodbio, Wuhan, China). The intensity of the bands was quantified using densitometry (Quantity One software; Bio-Rad, Berkeley, CA, USA); GAPDH was used as a control.
Flow-cytometric analysis of the cell cycle and apoptosis
pEX-2-BANCR, BANCR shRNA1# and 2#, and empty vector-transfected NPA and TPC1 cells were cultured in six-well plates for 48 h, washed with ice-cold phosphate-buffered saline (PBS), and fixed with 75% ethanol overnight. The cells were then collected by centrifugation (1000 rpm for 5 min) and resuspended in 1 ml of propidium iodide (PI) solution (50 mg/mL in PBS) containing 0.25 mg/mL of RNaseA. After incubation for 30 min in the dark at 4°C, the cells were analyzed by flow cytometry (FACScan; BD Biosciences, Franklin Lakes, NJ, USA) using an instrument equipped with Cell Quest software (BD Biosciences). The percentage of cells in G0-G1, S, and G2-M phase was counted and compared.
For apoptosis, analysis, pEX-2-BANCR, BANCR shRNA1# and 2#, and empty vector-transfected NPA and TPC1 cells were harvested 48 h after transfection. Floating and adherent cells were collected using 0.1% trypsin, washed twice with cold PBS, and suspended in 0.5 mL binding buffer (10 mmol/L HEPES buffer pH 7.4, 140 mmol/L NaCl, and 2.5 mmol/L CaCl2). The cells were then treated with an Annexin V-APC/7-AAD Apoptosis Detection Kit (Abnova, Atlanta, GA, USA) according to the manufacturer's instructions. Flow cytometry was carried out using FACScan (BD Biosciences). The percentage of early and late apoptotic cells were compared among groups in each experiment. This assay was repeated three times.
Fluorescence in situ hybridization (FISH)
Cells were plated onto coverslips, washed with phosphate buffered saline (PBS), fixed in 4% paraformaldehyde for 10 min, and permeabilized with 0.25% Triton-100 for 5 min Cy3-labeled lncRNA BANCR probes were obtained from RiboBio (Guangzhou, China). Slides of tissues were hybridized with probes overnight, washed as the above description. RNA FISH was performed using a FISH kit (Ribobio Co., Guangzhou, China) following the manufacturer's instructions. Then, the slides were counterstained with 4, 6-diamidino-2-phenylindole (DAPI) to mark the nuclei, U6 and 18S were used as cytoplasmic and nuclear positive control. Fluorescently labeled cells were visualized and imaged under a confocal laser scanning microscope (Olympus Corp. Tokyo, Japan).
Statistical analysis
Statistical analyses were performed using SPSS software version 21 (SPSS, Inc., Chicago, IL, USA) and GraphPad prism 5.0 for Windows (GraphPad Software, La Jolla, CA, USA). The Wilcoxon signed rank tests were applied to assess the expression of BANCR in cancer tissues compared with paired normal tissues. Chi-square tests or Fisher's exact test were used to examine the relationship between BANCR expression and clinicopathologic characteristics. The differences among groups in proliferation assays, western blotting, and PCR were estimated using Student's t-tests or one-way ANOVA. The results are reported as means ± SDs. Statistical significance was assigned as p < 0.05 (*) or p < 0.01 (**).
Correlation between BANCR expression and clinical pathological characteristics in papillary thyroid carcinoma
| Characteristics | n | High expression (%) | Low expression (%) | P |
|---|---|---|---|---|
| Gender | 60 | |||
| Female | 42 | 11(26.2) | 31(73.8) | 0.755 |
| Male | 18 | 6 (33.3) | 12(66.7) | |
| Age (y) | ||||
| <45 | 35 | 11(31.4) | 24(68.6) | 0.575 |
| ≥45 | 25 | 6 (24.0) | 19(76.0) | |
| Clinical stage | ||||
| Ⅰ-Ⅱ | 49 | 16(32.7) | 33(67.3) | 0.155 |
| Ⅲ-Ⅳ | 11 | 1 (9.1) | 10(90.9) | |
| Tumor depth | ||||
| T1-T2 | 55 | 17(30.9) | 38(69.1) | 0.309 |
| T3-T4 | 5 | 0 (0.0) | 5 (100) | |
| Tumor size | ||||
| <1cm | 16 | 2 (12.5) | 14(87.5) | 0.122 |
| 1-4cm | 27 | 11(40.7) | 16(59.3) | |
| >4cm | 17 | 4 (23.5) | 13(76.5) | |
| Lymph node metastasis | ||||
| Y | 36 | 6 (16.7) | 30(83.3) | 0.020* |
| N | 24 | 11(45.8) | 13(54.2) | |
| NIA | 20 | 4 (20.0) | 16(80.0) | 0.043* |
| NIB | 16 | 2 (12.5) | 14(87.5) |
Notes: BANCR, BRAF-activated non-protein coding RNA
P<0.05 on the parallelism test, indicating that the clinical and pathological characteristics are significantly correlated with BANCR expression.
Results
Expression of BANCR in PTC tissues and PTC cell lines
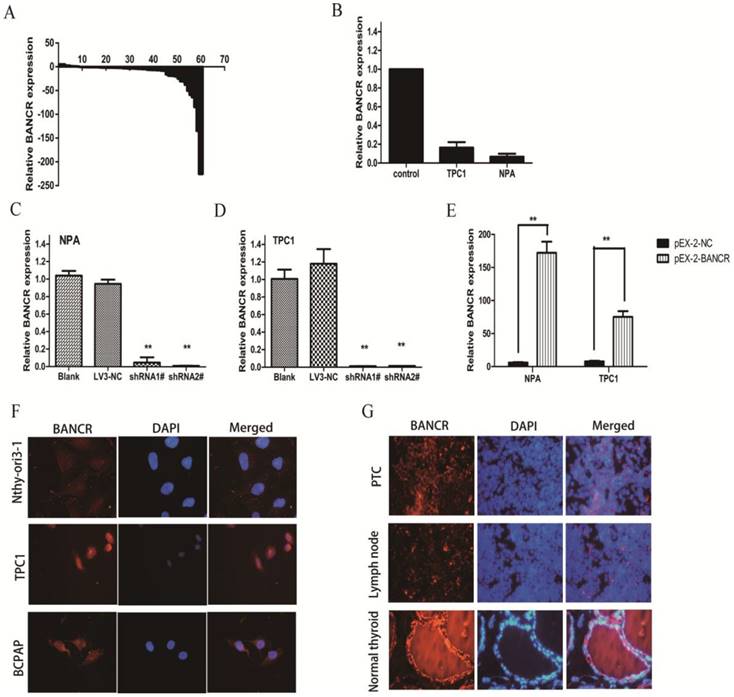
qRT-PCR was used to examine BANCR levels in 60 PTC tissues and matched normal thyroid tissues. The expression of BANCR was significantly downregulated (p < 0.01) in 71.7% (43/60) of cancerous tissues compared with normal tissues (Figure 1A). Furthermore, the correlation between BANCR expression and the patients' clinicopathological parameters (including gender, age, and TNM stage) was assessed to determine its clinical significance. BANCR expression in PTC was significantly correlated with lymph node metastasis (p = 0.02). BANCR expression was lower in 83.3% (30/36) of the patients with lymph node metastasis (Table 1). Two patients with advanced disease, one with invasion into subcutaneous soft tissues and the other with invasion into the larynx (Supplementary Table 1), both had lower BANCR expression. However, BANCR expression was not associated with other PTC parameters such as gender (p = 0.755), tumor stage (p = 0.155), and patient age (p = 0.575) (Table 1). The clinicopathological parameters for all patients were summarized in Supplementary Table 1. These analyses demonstrate that BANCR may be a good diagnostic biomarker for PTC.
BANCR expression was also detected in two human PTC cell lines (NPA and TPC1) using qRT-PCR. The two cell lines exhibited a notable downregulation in BANCR compared with control thyroid tissues (Figure 1B, p = 0.024). In the FISH experiment, which involved PTC cell lines (BCPAP and TPC1) and PTC patient specimens, we also confirmed the decreased BANCR expression, which further suggested that lncRNA BANCR was mainly localized in the cytoplasm of PTC cells and tissues (Figure 1F and 1G).
Modulating BANCR expression affects cell proliferation and motility
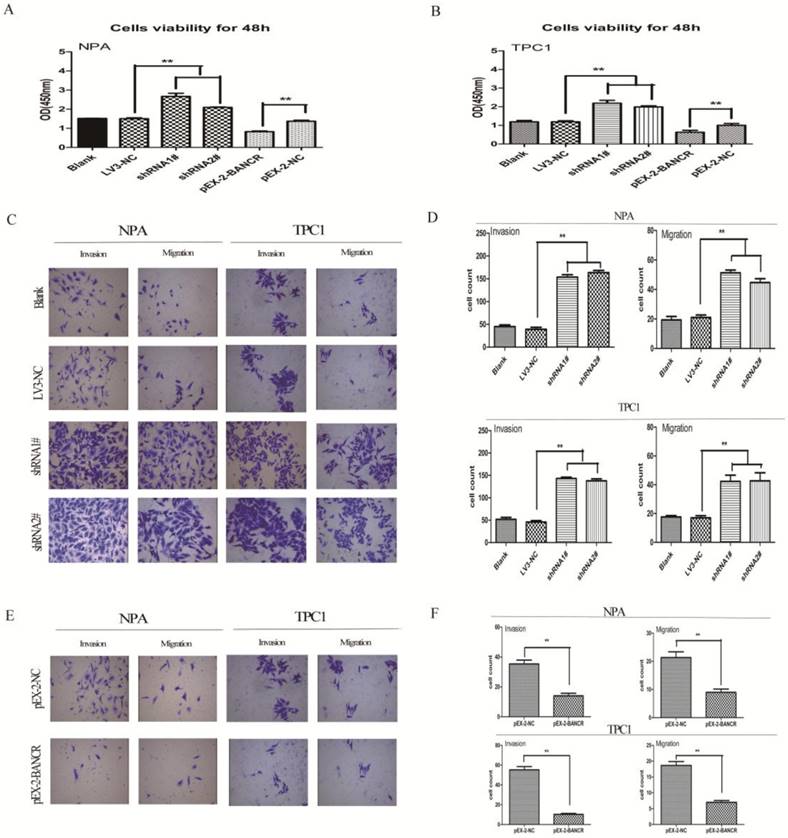
To clarify the role of BANCR in PTC cells, CCK-8 was used to assess the impact of BANCR overexpression or knockdown on NPA and TPC1 cells. Our clinical data indicated that BANCR expression was inversely correlated with tumor size and lymph node metastasis. Thus, the shRNAs LV3‑BANCR‑323 (shRNA1#) and LV3‑BANCR‑540 (shRNA2#) were used to downregulate endogenous BANCR expression in NPA and TPC1 cells. CCK-8 assays were then performed to monitor the effects of BANCR shRNA on cell proliferation. The results showed that the proliferation of BANCR shRNA-transfected was higher than control cells transfected with the empty vector at 48 h (p < 0.01; Figure 2A and 2B). Transwell migration and invasion assays showed that downregulating BANCR significantly promoted cell motility (p < 0.01; Figure 2C and 2D).
Consistent with these observations, the growth of NPA and TPC1 cells transfected with pEX-2-BANCR was impaired compared with control cells (p < 0.01; Figure 2A and 2B). To further examine the effects of BANCR on the motility of NPA and TPC1 cells, transwell migration and invasion assays were performed. BANCR overexpression impaired the migration and invasion of PTC cells (p < 0.01; Figure 2E and 2F), suggesting that BANCR is at least partially necessary for PTC cell motility in vitro. These observations suggest that BANCR contributes to PTC progression by enhancing cell proliferation and motility.
Relative expression of BANCR in papillary thyroid cancer tissues and cell lines. (A) Relative expression of BANCR in papillary thyroid cancer tissues (n = 60) compared with corresponding normal tissues (n = 60). BANCR expression was examined by qPCR and normalized to GAPDH expression. The results are presented as the fold-change in tumor tissues relative to normal tissues. (B) BANCR expression in NPA and TPC1 papillary thyroid cancer cell lines compared with control thyroid tissues pooled from three controls. (C and D) qPCR analysis of BANCR expression following the treatment of NPA and TPC1 cells with BANCRshRNA1#, shRNA2#, and empty vector. (E) qPCR analysis of BANCR expression following the treatment of NPA and TPC1 cells with pEX-2-BANCRand empty vector.(F) Fluorescence in situ hybridization in Nthy-ori3-1, TPC1 and BCPAP cells (G) Fluorescence in situ hybridization in papillary thyroid cancer, metastatic lymph node and normal thyroid tissues. **, p < 0.01. shRNA1#, shRNA2#, and LV3-NC, plasmid containing shRNA1# and shRNA2# for BANCR and negative control shRNA, respectively; pEX-2-BANCRand pEX-2-NC, pEX-2 plasmid containing human full-length BANCR cDNA and negative control, respectively; BANCR, BRAF-activated non-protein coding RNA; shRNA, short hairpin RNA; DAPI, 4',6-diamidino-2-phenylindole.
BANCR promotes G2/M arrest and causes apoptosis
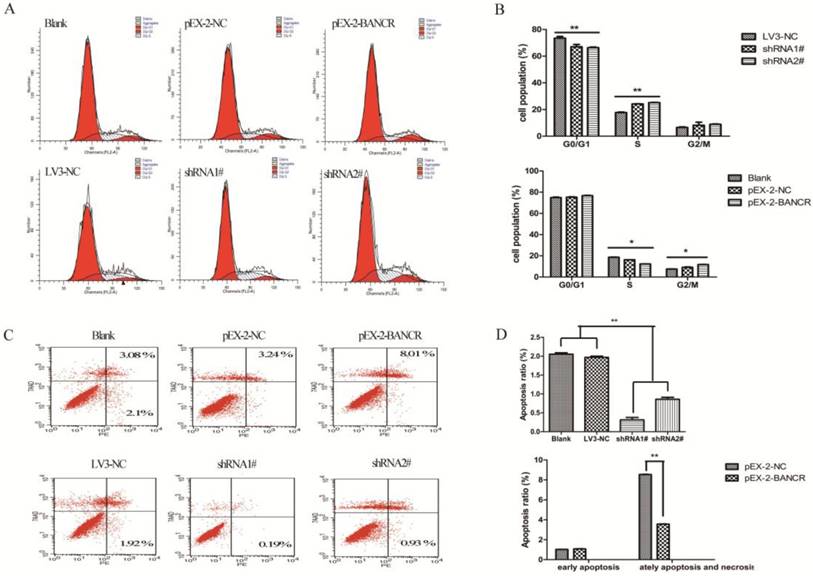
To determine whether the effects of BANCR on the proliferation and motility of PTC cells were mediated by inhibiting cell cycle progression, flow cytometry was used to assess the cell cycle in NPA cells (Figure 3A and 3B). BANCR overexpression led to the significant accumulation of cells in G2/M-phase and a significant decrease in cells in S-phase compared with control (both p < 0.05). Conversely, knocking down BANCR led to a significant decrease in the percent of cells in G1/G0- and G2/M-phases and a significant increase in S-phase compared with control (both p < 0.01). Next, the effects of BANCR on apoptosis were assessed in NPA cells (Figure 3C and 3D). The percentage of cells undergoing late apoptosis and necrosis was significantly increased in the BANCR overexpression group compared with control (p < 0.01). The percentage of early apoptotic cells was significantly decreased in the BANCR shRNA group compared with control (p < 0.01). Taken together, these data suggest that BANCR overexpression could induce G2/M phase arrest and enhance apoptosis in PTC cells, whereas BANCR downregulation promotes the entry of PTC cells into S phase and protects from early apoptosis.
BANCR regulates PTC proliferation and migration by inactivating the MAPK and PI3K-Akt pathways
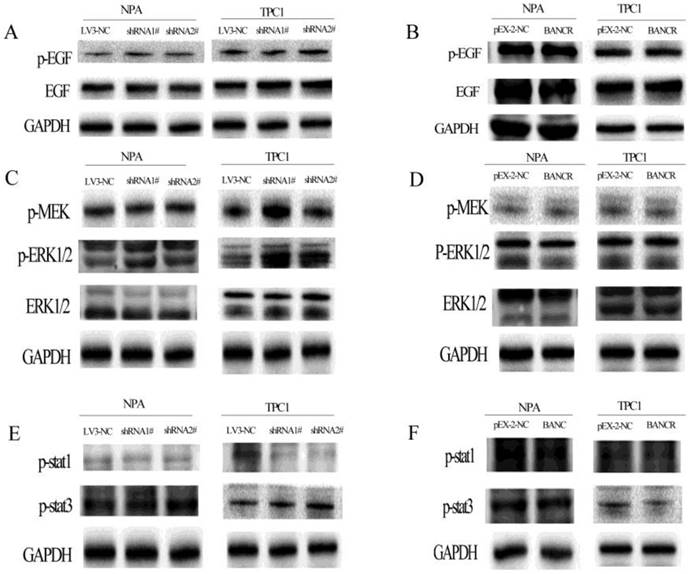
Next, the activation of the MAPK and PI3K-Akt pathways was assessed using western blotting to explore the potential mechanism underlying the role of BANCR in cell proliferation and migration. BANCR downregulation activated the MAPK (Figure 4) and PI3K-Akt (Figure 5) pathways in NPA and TPC1 cells. Interestingly, although the MAPK pathway was activated by BANCR knockdown, there was no effect on upstream EGFR or the classical activator MEK1/2; only ERK1/2 phosphorylation was upregulated (Figure 4A and 4C). There was also no difference in the phosphorylation of STAT-1 or STAT-3 (Figure 4E). When BANCR was upregulated, no significant differences were observed in MAPK pathway through either cell line (Fig 4B, 4D and 4F).
Effects of BANCR on papillary thyroid cancer cell proliferation and motility. (A and B) CCK-8 assays were performed to measure NPA and TPC1 cell proliferation. The data represent the means ± SDs of three independent experiments. (C and E) Transwell migration and invasion assays showed that knocking down BANCR promoted NPA and TPC1 cell motility. However, cell motility was reduced in BANCR-overexpressing NPA and TPC1 cells (crystal violet stain; magnification, ×200).(D and F) The bar graph represents at least three independent experiments and the bars indicate the number of migrated cells per field. **p<0.01. shRNA1#, shRNA2#, LV3-NC respective plasmid containing shRNA for BANCR and negative control;pEX-2-BANCRand pEX-2-NC,pEX-2plasmid containing human full-length BANCR cDNA and negative control, respectively; BANCR, BRAF-activated non-protein coding RNA; shRNA, short hairpin RNA.
The effects of BANCR on the cell cycle and apoptosis in papillary thyroid cancer cells in vitro. (A and B) The bar chart represents the percentage of cells in G0/G1, S, or G2/M phase. (C and D) The percentage of apoptotic cells was determined by flow cytometry. The data represent the means ± SDs of three independent experiments. *, p < 0.05; **, p<0.01. shRNA1#, shRNA2#, LV3-NC respective plasmid containing shRNA for BANCR and negative control; pEX-2 BANCR, pEX-2-NC respective plasmid containing human full length BANCR cDNA and negative control; BANCR, BRAF-activated non-protein coding RNA; shRNA, short hairpin RNA.
The PI3K-Akt pathway was activated by BANCR knockdown, as revealed by increased PI3K and Akt phosphorylation, but there was no effect on FAK (Figure 5A and 5C). In contrast, the PI3K-Akt pathway was inactivated when BANCR was upregulated in NPA cells. Although there were no significant differences in TPC1 cells, while was a similar a trend (Figure 5B and 5D).
Discussion
Genomic studies have demonstrated that <2% of the total human genome can be transcribed, and that noncoding RNAs likely account for the greater complexity transcriptome in eukaryotes [24, 25]. lncRNAs were once neglected and considered to be noise in cells for a long time. However, accumulating evidence has assigned new functions to lncRNAs, including roles in the transcriptional, epigenetic, and post-transcriptional regulation of gene expression [26-28]. It was reported that lncRNAs affect many cellular processes in tumorigenesis such as survival, proliferation, apoptosis, the cell cycle, and migration [29, 30]. Unlike microRNAs, lncRNAs are not currently well understood. BANCR , a recently found lncRNA, can regulate proliferation and migration in melanoma, colorectal cancer, and lung cancer [19,31,32]. In a recent meta-analysis [20], high BANCR expression was correlated with lymph node metastasis, tumor stage, and poor prognosis in gastrointestinal cancer patients, but not in other cancers. However, little is known about the role of BANCR in the development of papillary thyroid carcinoma; the mechanism underlying tumorigenesis and its clinical significance is still unclear. According to previous studies, the function of BANCR in PTC is controversial. Liao et al. demonstrated that BANCR expression was downregulated in 92 pairs of PTC tissues, as well as in the cell lines TPC-1, K1, and BCPAP. In other studies, Zheng et al. and Wang et al. demonstrated that BANCR is upregulated in human PTC tissues and IHH4 cells and that this promotes cell growth and proliferation [33]. In the current study, we investigated the clinical significance of BANCR and its function and molecular mechanism in PTC. Our results revealed that BANCR levels were significantly reduced in 60 pairs of PTC tissues from patients and PTC cell lines (TPC1 and NPA). The discrepancy with our results may stem from differences in the numbers of tissue samples tested (60 in the current study, 92 in Liao et al., 40 in Zheng et al., and six in Wang et al.), tumor heterogeneity (especially in Zheng et al., who had only 21 cases of classical PTC among 40 samples) and in the BANCR expression patterns in different cell lines (Zheng et al. and Wang et al. used IHH4 cells). In the current study, low BANCR expression levels were significantly correlated with lymph node metastasis, whereas Liao et al. reported that downregulated BANCR expression was correlated with tumor size, multifocal lesions, and an advanced pathological stage; this difference may stem from tumor heterogeneity. Overall, both studies confirm that low BANCR levels are associated with poor outcomes in PTC patients.
The phosphorylation of EGFR, MEK, ERK1/2, STAT-1, and STAT-3 in BANCR-silenced or -overexpressing NPA and TPC1 cells was detected by western blotting. The loss of BANCR induced the phosphorylation of ERK1/2 (C). However, there was no activation of upstream molecule including EGFR (A), MEK (C), MEK, STAT-1, and STAT-3 (E). BANCR overexpression had no effect on the phosphorylation of EGFR, MEK, ERK1/2, STAT-1, and STAT-3 in either cell line (B, D and F). GAPDH expression was used to normalize protein loading.
Liao et al. reported that the overexpression of BANCR promotes apoptosis in TPC1 and K1 cell lines. However, the current study reported that BANCR overexpression could induce G2/M phase arrest and enhance apoptosis in PTC cells whereas BANCR downregulation promoted the entry of PTC cells into S phase and protected from early apoptosis.
The MAPK and PI3K-Akt signaling pathways play important roles in complex cellular programs including proliferation, survival, and migration [34, 35]. The MAPK and PI3K-Akt pathways are involved in differentiated PTC, and the simultaneous activation of both pathways becomes more frequent as the grade of thyroid tumors increases [36-38]. Activation of the MAPK and PI3K-Akt pathways is an important mechanism that drives the progression of thyroid cancer. The current study investigated the activation of the MAPK and PI3K-Akt pathways in PTC cells by measuring the phosphorylation of proteins in the MAPK and PI3K-Akt pathways, including EGFR, MEK, ERK1/2, STAT-1, STAT-3, FAK, AKT, PI3K, in BANCR overexpressing and knockdown PTC cells. MAPK and PI3K-Akt were activated in BANCR knockdown PTC cells, but the phosphorylation of EGFR, MEK1/2, STAT-1, STAT-3, and FAK was not affected. The overexpression of BANCR by transfection with pEX-2-BANCR inhibited the activation of the PI3K-AKT pathway in NPA cells (which contain BRAFV600E), whereas there was no significant difference in the MAPK pathway. Liao et al., demonstrated BANCR overexpression inactivates ERK1/2 and p38 in K1 cells (which contain BRAFV600E and PI3K [E542K]). In contrast, the current study found that BANCR knock down increased p44 ERK1 and p42 ERK2 MAPK levels but did not affect the upstream molecules. In addition, the PI3K-Akt pathway was activated by BANCR knockdown and inactivated when BANCR was upregulated in NPA cells. The discrepancy between these observations and the current results may stem from the different cell lines used. Mammalian cells contain four well-characterized and widely studied MAPKs.
The phosphorylation of FAK, PI3K, and AKT in BANCR-silenced or overexpressing NPA and TPC1 cells was detected by western blotting. The loss of BANCR induced the phosphorylation of PI3K (A) and Akt (C). However, there was no activation of the upstream molecule FAK (A). In NPA cells, BANCR overexpression induced the inactivation of PI3K (B) and Akt (D), but there was no activation of the upstream molecule FAK. However, in TPC1 cells BANCR overexpression had no effect on the phosphorylation of FAK, PI3K, and Akt. (B, D) GAPDH expression was used to normalize protein loading.
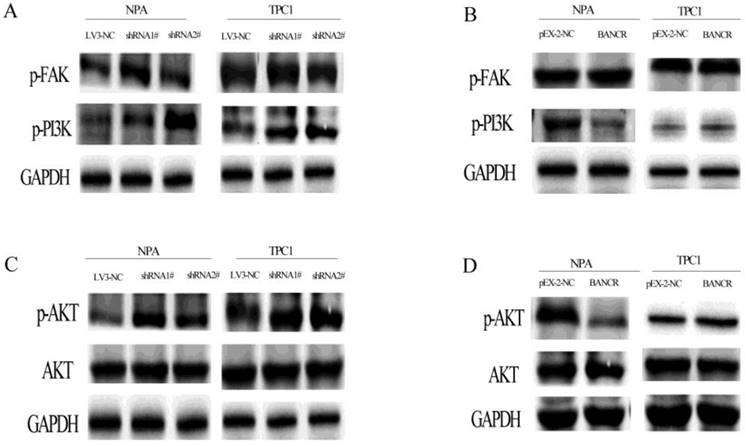
The terminal serine/threonine kinases (MAPKs) include ERK1/2, c-Jun amino-terminal kinases (JNK-12/3; also called SAPKs), p38 kinases (p38a/b/g/d), and ERK5. In the current study, we were most concerned with the EGFR-Ras-Raf-MEK-ERK MAPK signaling pathway because previous studies revealed that BRAF mutation activate MAPK signaling pathway in thyroid cancer. When BANCR was knocked down with shRNA, only p44 ERK1 and p42 ERK2 MAPKs were increased; the upstream molecules were not affected. When BANCR was overexpressed, the MAPK signaling pathway was not inactivated, as previous studies show that BANCR can recruit zeste homolog 2 (EZH2) to enhance thyroid stimulating hormone receptor which can activate MAPK pathway, we speculate may be this affect the final activation of this pathway. The PI3K-Akt pathway was activated by BANCR knockdown, as represented by increased PI3K and Akt phosphorylation. In contrast, the PI3K-Akt pathway was inactivated when BANCR was upregulated in NPA but not TPC1 cells. TPC1 cells are a thyroid cancer cell line that contain a RET/PTC rearrangement that can activate the PI3K-Akt pathway; therefore, these complex observations may stem from the different gene expression profiles in the two cancer cell lines. Therefore, we concluded that the effects of BANCR on the proliferation and migration of PTC cells were exerted via the ERK MAPK and PI3K-Akt activations pathways.
Clinical studies performed in BRAF-mutant metastatic melanoma patients treated with selective BRAFV600E inhibitors treatment revealed that some patients eventually relapse and succumb to acquire chemoresistance via mechanisms involving the reactivation of MAPK signaling [39-41]. Because BANCR was upregulated by the BRAFV600E mutation, the effects of BANCR on the MAPK pathway provide a novel mechanism behind the regulation of BRAFV600E mutations in PTC. These findings will help us fully understand the oncogenesis of PTC.
In summary, the current study demonstrated that BANCR is downregulated in PTC tissues. BANCR expression in PTC was significantly correlated lymph node metastasis. The effects of BANCR on cell proliferation and migration were mediated by inducing cell cycle arrest in G2/M phase and inducing apoptosis. Finally, BANCR regulated cell proliferation by activating the ERK-MAPK and PI3K-Akt pathways. Taken together, these findings suggest that BANCR plays a role in the oncogenic process; it may be both a new potential target and prognostic factor for PTC. Further investigations are required to investigate this in more detail.
Abbreviations
BANCR, BRAF-activated non-protein coding RNA; PTC, papillary thyroid cancer, lncRNAs, long non-coding RNAs; shRNA, short hairpin RNA; FTC, follicular thyroid cancer; PDTC, poorly differentiated thyroid cancer; ATC, anaplastic thyroid cancer; HOTAIR, HOX transcript antisense RNA; MALAT1, metastasis associated lung adenocarcinoma transcript 1; GAS5, growth arrest specific 5; STR, short tandem repeat; MAPK, mitogen activated protein kinase; PI3K, phosphatidylinositol 3'-kinase.
Supplementary Material
Supplementary figure and table.
Acknowledgements
This research was supported by Clinical Research Physician Program of Tongji Medical College, HUST (5001540018).
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
1. DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, Jemal A. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA: A Cancer Journal for Clinicians. 2016;66(4):290-308
2. Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer. 2016;23(4):313-322
3. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212
4. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians. 2016;66(2):115-132
5. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. NAT REV CANCER. 2013;13(3):184-199
6. Howlader N, Yu M. 2015 SEER Cancer Statistics Review, National Cancer Institute. Bethesda, MD. 2015:1975-2012 http://seer.cancer.gov/csr/1975-2012/. 2015
7. Brown RL, de Souza JA, Cohen EE. Thyroid cancer: burden of illness and management of disease. J CANCER. 2011;2:193-199
8. Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, Ehya H, Farrar WB, Haddad RI, Kandeel F, Kloos RT, Kopp P, Lamonica DM, Loree TR, Lydiatt WM, McCaffrey JC. et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8(11):1228-1274
9. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. LANCET. 2013;381(9871):1058-1069
10. Agrawal N, Akbani R, Aksoy BA, Ally A, Arachchi H. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. CELL. 2014;159(3):676-690
11. Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE. et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. NATURE. 2009;458(7235):223-227
12. Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, Yang X, Amit I, Meissner A, Regev A, Rinn JL, Root DE. et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. NATURE. 2011;477(7364):295-300
13. Wapinski O, Chang HY. Long noncoding RNAs and human disease. TRENDS CELL BIOL. 2011;21(6):354-361
14. Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. NATURE. 2012;482(7385):339-346
15. Wan Y, Chang HY. HOTAIR: Flight of noncoding RNAs in cancer metastasis. CELL CYCLE. 2010;9(17):3391-3392
16. Gokmen-Polar Y, Vladislav IT, Neelamraju Y, Janga SC, Badve S. Prognostic impact of HOTAIR expression is restricted to ER-negative breast cancers. Sci Rep. 2015;5:8765
17. Wu Y, Huang C, Meng X, Li J. Long Noncoding RNA MALAT1: Insights into its Biogenesis and Implications in Human Disease. Curr Pharm Des. 2015;21(34):5017-5028
18. Yacqub-Usman K, Pickard MR, Williams GT. Reciprocal regulation of GAS5 lncRNA levels and mTOR inhibitor action in prostate cancer cells. PROSTATE. 2015;75(7):693-705
19. Flockhart RJ, Webster DE, Qu K, Mascarenhas N, Kovalski J, Kretz M, Khavari PA. BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. GENOME RES. 2012;22(6):1006-1014
20. Fan YH, Ye MH, Wu L, Wu MJ, Lu SG, Zhu XG. BRAF-activated lncRNA predicts gastrointestinal cancer patient prognosis: a meta-analysis. ONCOTARGET. 2017;8(4):6295-6303
21. Wang Y, Guo Q, Zhao Y, Chen J, Wang S, Jun HU, Sun Y. BRAF-activated long non-coding RNA contributes to cell proliferation and activates autophagy in papillary thyroid carcinoma. ONCOL LETT. 2014;8(5):1947-1952
22. Liao T, Qu N, Shi RL, Guo K, Ma B, Cao YM, Xiang J, Lu ZW, Zhu YX, Li DS, Ji QH. BRAF-activated LncRNA functions as a tumor suppressor in papillary thyroid cancer. ONCOTARGET. 2017;8(1):238-247
23. Sun M, Liu XH, Wang KM, Nie FQ, Kong R, Yang JS, Xia R, Xu TP, Jin FY, Liu ZJ, Chen JF, Zhang EB, De W, Wang ZX. Downregulation of BRAF activated non-coding RNA is associated with poor prognosis for non-small cell lung cancer and promotes metastasis by affecting epithelial-mesenchymal transition. MOL CANCER. 2014;13:68
24. Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A. et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. NATURE. 2007;447(7146):799-816
25. Mattick JS. The central role of RNA in human development and cognition. FEBS LETT. 2011;585(11):1600-1616
26. Lee JT. Epigenetic regulation by long noncoding RNAs. SCIENCE. 2012;338(6113):1435-1439
27. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. CELL. 2009;136(4):629-641
28. Eddy SR. Non-coding RNA genes and the modern RNA world. NAT REV GENET. 2001;2(12):919-929
29. Rossi MN, Antonangeli F. LncRNAs: New Players in Apoptosis Control. Int J Cell Biol. 2014;2014:473857
30. Qiu MT, Hu JW, Yin R, Xu L. Long noncoding RNA: an emerging paradigm of cancer research. Tumour Biol. 2013;34(2):613-620
31. Jiang W, Zhang D, Xu B, Wu Z, Liu S, Zhang L, Tian Y, Han X, Tian D. Long non-coding RNA BANCR promotes proliferation and migration of lung carcinoma via MAPK pathways. BIOMED PHARMACOTHER. 2015;69:90-95
32. Guo Q, Zhao Y, Chen J, Hu J, Wang S, Zhang D, Sun Y. BRAF-activated long non-coding RNA contributes to colorectal cancer migration by inducing epithelial-mesenchymal transition. ONCOL LETT. 2014;8(2):869-875
33. Wang Y, Guo Q, Zhao Y, Chen J, Wang S, Hu J, Sun Y. BRAF-activated long non-coding RNA contributes to cell proliferation and activates autophagy in papillary thyroid carcinoma. ONCOL LETT. 2014;8(5):1947-1952
34. Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. ONCOGENE. 2007;26(22):3291-3310
35. Sathe A, Guerth F, Cronauer MV, Heck MM, Thalgott M, Gschwend JE, Retz M, Nawroth R. Mutant PIK3CA controls DUSP1-dependent ERK 1/2 activity to confer response to AKT target therapy. Br J Cancer. 2014;111(11):2103-2113
36. Hou P, Liu D, Shan Y, Hu S, Studeman K, Condouris S, Wang Y, Trink A, El-Naggar AK, Tallini G, Vasko V, Xing M. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. CLIN CANCER RES. 2007;13(4):1161-1170
37. Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, Vasko V, El-Naggar AK, Xing M. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93(8):3106-3116
38. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. NAT REV CANCER. 2013;13(3):184-199
39. Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. NATURE. 2010;468(7326):973-977
40. Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M. et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. NATURE. 2010;468(7326):968-972
41. Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE, Hahn WC, Meyerson M, Garraway LA. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J CLIN ONCOL. 2011;29(22):3085-3096
Author contact
![]() Corresponding author: Xingrui Li, M.D., Ph. D., Department of Thyroid and Breast Surgery, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, Hubei 430030, P.R. China. Telephone: +86-27-83665317; E-mail: lixingruitjmu.edu.cn
Corresponding author: Xingrui Li, M.D., Ph. D., Department of Thyroid and Breast Surgery, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, Hubei 430030, P.R. China. Telephone: +86-27-83665317; E-mail: lixingruitjmu.edu.cn

 Global reach, higher impact
Global reach, higher impact