Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(4):690-701. doi:10.7150/jca.22365 This issue Cite
Research Paper
Efficacy and safety of different interventions in castration resistant prostate cancer progressing after docetaxel-based chemotherapy: Bayesian network analysis of randomized controlled trials
1. Department of Urology, Sun Yat-Sen Memorial Hospital of Sun Yat-sen University, Guangzhou, China;
2. Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-sen Memorial Hospital of Sun Yat-sen University, Guangzhou, China;
3. Department of Interventional oncology, First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China.
4. Academic Urology Unit, University of Aberdeen, Cornhill Road, Aberdeen, UK.
*These authors (Changhao Chen, Hao Huang and Yue Zhao) contributed equally to this study.
Received 2017-8-14; Accepted 2017-12-8; Published 2018-1-11
Abstract
Background: Most patients receiving docetaxel-based chemotherapy for castration resistant prostate cancer (CRPC) will eventually progress, and the optimal interventions for these patients are controversial. The objective of our study is to evaluate the clinical efficacy and safety of pharmacological interventions for CRPC patients progressing after docetaxel-based chemotherapy.
Methods: A systematic review and Bayesian network meta-analysis of the literature was carried out according to standard methods. Major electronic databases including PubMed, Web of Science and Embase were searched until Jan 2017. Hazard ratios (HRs) and odds ratios (ORs) with corresponding 95% credible intervals (CrIs) were used to estimate the association.
Results: 17 Randomized Controlled Trials (RCTs) comprising 14 different interventions with 12347 patients were enrolled. Compared with control arms, Abiraterone Acetate (HR: 0.70, 95%CrI: 0.63-0.79), Cabazitaxel (HR: 0.70, 95%CrI: 0.51-0.95) and Enzalutamide (HR: 0.63, 95%CrI: 0.53-0.75) presented similar benefits in term of OS. Enzalutamide showed superiority over PFS and PSA response with a highest probability to rank 1. Moreover, sensitivity analysis showed that Abiraterone Acetate (HR: 0.71, 95%CrI: 0.63-0.78) exhibited the most efficacious intervention of being rank 1 in term of OS compared with control arms, followed by Cabazitaxel and Cetuximab. On the other hand, Abiraterone Acetate (OR: 0.86, 95%CrI: 0.35-2.03) presented no significant toxicities compared with control arms.
Conclusions: Our results demonstrated that Abiraterone Acetate might be the optimal intervention for CRPC patients after docetaxel failure with acceptable tolerability. Future well-designed RCTs and systematic reviews are needed to validate these findings.
Keywords: Castration resistant prostate cancer, Pharmacological Interventions, Docetaxel-based Chemotherapy, Bayesian network meta-analysis, Abiraterone Acetate, Enzalutamide
Introduction
Prostate cancer (PCa) is the second most commonly diagnosed cancer in the world, with 161,360 estimated new cases of PCa in 20171 2. Less than 5% of patients present with metastatic disease, up to 40% of detected cases will eventually develop metastasis 3. Androgen deprivation therapy (ADT) has been the standard of care for metastatic PCa 4-6. However, most patients eventually stop responding to ADT and are categorized as castration resistant prostate cancer (CRPC)7, which is defined as either biochemically or clinically progressive metastatic disease despite castrate serum levels of testosterone (<50 ng/dL; <1.7nmol/L)8. After developing mCRPC, it is dismal with a median survival of 12 to 18 months9.
Docetaxel is a standard first-line chemotherapy in men with CRPC based on improvements in overall survival (OS) and progression free survival (PFS), compared with Mitoxantrone plus prednisone 10 11. However, most of patients who receive docetaxel-based chemotherapy for mCRPC will eventually progress, and no consensus exist for the optimal interventions after docetaxel failure. The decision to initiate therapy demand the available high-level evidence of efficacy and tolerability in the post-docetaxel CRPC setting. Treatment options include Abiraterone with prednisone 12, Enzalutamide 13, Radium-22314, Cabazitaxel15 and so on. However, there are few randomized controlled trials (RCT) comparing different treatment strategies to inform patients regarding the comparative effectiveness of those interventions16-18. Therefore, pair-wise meta-analysis couldn't generate clear hierarchies among available treatments in this case, because they provide only partial information, and hence, do not optimally inform decision-making.
Network meta-analysis has been recently demonstrated to compare different interventions and integrate evidence from direct comparisons and indirect comparisons across a network of RCTs19 20. Moreover, Bayesian network meta-analysis which synthesizes all evidence on the relative treatment effects, enables unified and coherent analysis of relevant RCTs21. Therefore, we applied the established methodology in the comprehensive network involving post-docetaxel treatments evaluating the clinical efficacy and tolerability of interventions for CRPC patients.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines 22 23.
Search strategy and selection criteria
We identified all relevant RCTs published from inception up to January 30, 2017 for assessing the clinical significance of currently available interventions for CRPC patients after docetaxel failure from the following databases, including PubMed, EMBASE, MEDLINE, Web of Science, the Cochrane Library, Central Register of Controlled Trials. The MeSH terms were correctly adjusted in different database. The search strategy of PubMed is as follows: (("prostatic neoplasms"[MeSH Terms] OR ("prostatic" [All Fields] AND "neoplasms"[All Fields]) OR "prostatic neoplasms"[All Fields] OR ("prostate"[All Fields] AND "cancer"[All Fields]) OR "PCa"[All Fields]) AND ("orchiectomy"[MeSH Terms] OR "orchiectomy"[All Fields] OR "castration"[All Fields] OR "castration"[MeSH Terms]) AND resistant[All Fields] AND ("docetaxel"[MeSH Terms] OR "docetaxel-based chemotherapy" [All Fields] OR "taxane-based chemotherapy" [All Fields]) AND ("resistant" [All Fields] OR "failure" [All Fields] OR "refractory" [All Fields]) AND ("clinical trial" [Publication Type] OR "clinical trials as topic"[MeSH Terms] OR "clinical trial"[All Fields]). No language restrictions were applied. We also contacted the corresponding authors to acquire information if more information was needed.
Eligible studies in this network meta-analysis were RCTs that met the following inclusion criteria: (a) Patients: Histologically confirmed prostate cancer aged ≥18 years with castrate levels of serum testosterone (<50 ng/dL) were eligible if they had failed previous docetaxel-containing chemotherapy; documented progression was based on PSAWG criteria or radiographic progression in soft tissue or bone; (b) Intervention: established therapies for management of CRPC patients after docetaxel failure including chemotherapy, immunotherapy, androgen receptor targeting etc.; (c) Comparator: another active agent, Prednisone plus placebo, placebo, or no intervention; (d) Outcome: OS, PFS, PSA response and adverse events. We excluded observational studies, and trials comparing different doses of the same medication without an alternative intervention/comparator arm.
Data Extraction and quality assessment
Two authors (C.H. Chen and Y. Zhao) screened all the titles and abstracts identified by the search strategy independently, the results were assessed for eligibility. Disagreement was resolved by consensus between two authors or by a senior author (J. Huang). Data collection form was designed to collect information from these publications including first author, year of publication, follow-up, study type, participants, intervention and outcomes. We considered the OS, PFS and adverse events (Grade 3-4) for our primary analyses. Secondary outcome included PSA response (proportion of patients achieving ≥50% PSA decline according to PSAWG criteria). Since randomized trials used low-dose oral daily corticosteroids in combination with mitoxantrone 24, they have been employed in combination with taxanes, with the rationale being to maintain balance between the arms 15 25. However, the impact of prednisone on survival, as a single agent or in combination, remains unclear. Recently, comprehensive studies estimating the efficacy and safety with the use of daily prednisone indicated that Prednisone plus placebo arm showed similar benefit compared with placebo 26 27. Thus, the reference standard was regarded as Prednisone plus placebo in the present study. For studies presenting the same RCT, we extracted the updated data for our meta-analysis, such as TROPIC trial reported by Bahl et al (2013)28 and de Bono et al (2010)15. In order to evaluate the quality of including studies in this network meta-analysis, we assessed the risk of bias using the Cochrane Collaboration's Risk of Bias tool29.
Statistical Analysis
We fitted a Bayesian network meta-analysis model for each outcome separately, combining direct evidence for each comparison with indirect evidence, for all pair-wise comparisons simultaneously. We evaluated inconsistency by comparing the estimates from direct comparisons and those from indirect comparisons for magnitude and direction of the point estimates. We estimated treatment effects by posterior means with corresponding 95% credible intervals (CrIs) and adjusted for different arms. Both the fixed and random effects models were applied. The differences between the two models were that the latter considers between-study variance, thereby producing wider CrIs, and was preferred in the presence of heterogeneity. Bayesian deviance information criterion (DIC) statistics were utilized to compare the two models. The DIC statistics provide a model fit measure which penalizes model complexity with lower values. We updated Markov chain Monte Carlo model with 100,000 simulated draws after a burn in of 10,000 iterations. The probability of each treatment being the best, second best, third best and so on, from the rank orderings of the treatments at each iteration of the Markov chain were recorded30. The network meta-analyses were built in WinBUGS (MRC Biostatistics Unit, Cambridge, UK) 20.
Pair-wise meta-analyses were conducted on endpoints. The traditional direct meta-analysis, two or more studies that compared two interventions of interest were statistically combined. The survival endpoints were expressed as hazard ratio (HR). Estimated survival curves for OS and PFS were plotted using the method described by Parmar et al31. The estimated hazard ratios of OS32-35 and PFS32 33 35 were obtained by using the above method. Dichotomous variables for PSA response and adverse events were presented as odds ratio (OR) with 95% confidence intervals (CIs). A two-sided p value of less than 0.05 was considered significant. The pair-wise meta-analysis was performed using Stata 13.0 (StataCorp, College Station, TX, USA).
Sensitivity analyses of the primary outcome would be applied in the RCTs which presented high-quality with control arm of Prednisone plus placebo. The threshold for good quality studies for sensitivity analysis was based on the Cochrane assessment tool for assessing the risk of bias. The studies with low risk of selection and flow bias were included. Publication bias was assessed by examining funnel plot symmetry and Egger's regression test 36.
Results
Search Results
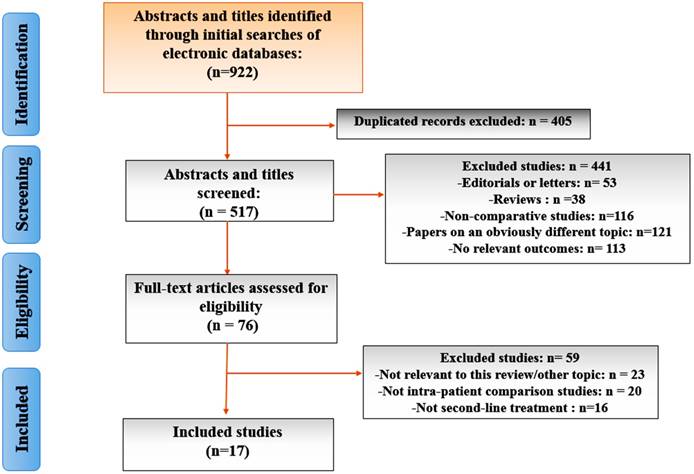
From a total of 922 unique studies comparing all dfferent interventions identified using the search strategy, 17 RCTs composing of 14 interventions met the eligibility criteria for the current study. A flow chart of trial selection was shown in Figure 1. Eight RCTs of CRPC therapies were excluded because of unclear primary outcome assessment 37-41 and comparison of different dosing regimens without a common comparator group 42-44. One study45 presenting Mitoxantrone plus prednisone was excluded because it did not meet our inclusion criterion for data integration of control arms.
Table 1 summarized the main characteristics of RCTs included in the network meta-analysis. Overall, 12347 mCRPC patients with docetaxel failure were randomly enrolled to included studies. Median follow-up was 21 (range 12.8-36 months) and the sample size ranged from 82 to 1199 people, with a median sample size of 755. Based on the interventions under comparison, the included trials were classified into the following five categories, 1) Chemotherapy: Satraplatin, Cabazitaxel, Ixabepilone, Mitoxantrone; 2) Hormonal Strategies: Abiraterone acetate, Enzalutamide, Orteronel; 3) Target therapies: Sunitinib, Cetuximab, Rilotumumab, Cabozantinib; 4) Immune strategies: Siltuximab; 5) Bone-targeting agents: Ra-223. 17 trials provided OS, of which 11 trials reported PFS and 15 trials presented adverse events as primary endpoints for comparisons. 14 trials showed PSA response as secondary endpoint for comparisons.
Characteristics of the studies included in the network meta-analysis
| Study, Year of Publication; Identifier | Country; Inclusion Period | Follow-up median month, (95% CI) | Study type | Participants | Intervention | Outcomes |
|---|---|---|---|---|---|---|
| Bahl et al (2013) | Multi-center, Jan 2007-September 2009 | 25.5(20.7-30.0) | Phase III, Open label, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) docetaxel failure | Experimental: Cabazitaxel + Prednisone Control: Mitoxantrone + Prednisone | OS, |
| de Bono et al (2010) | Multi-center, Jan 2007-Oct 2008 | 12.8(7.8-16.9) | Phase III, Open label, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) docetaxel failure | Experimental: Cabazitaxel + Prednisone Control: Mitoxantrone + Prednisone | OS, PFS, PSA Response, Adverse event |
| de Bono et al (2011) | Multi-center, May 2008-Oct 2012 | 12.8 | Phase III, Double blind, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Metastasis; 5) docetaxel failure | Experimental: Abiraterone acetate +Prednisone Control: Prednisone + Placebo | OS, PFS, PSA Response, Adverse event |
| Fizazi et al (1) (2012) | Multi-center, Nov 2006-Nov 2008 | NA | Phase II, Open label, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Metastasis; 5) docetaxel failure | Experimental: Siltuximab + Mitoxantrone + Prednisone Control: Mitoxantrone + Prednisone | OS, PFS, PSA Response, Adverse event |
| Fizazi et al (2) (2012) | Multi-center, May 2008-Oct 2012 | 20.2(18.4-22.1) | Phase III, Double blind, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Metastasis; 5) docetaxel failure | Experimental: Abiraterone acetate +Prednisone Control: Prednisone + Placebo | OS, PSA response, Adverse event |
| Fizazi et al (2015) | Multi-center, Nov 2010-Sep 2014 | NA | Phase III, Open label, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Metastasis; 5) docetaxel failure | Experimental: Orteronel + Prednisone Control: Prednisone + Placebo | OS, PFS, PSA Response, Adverse event |
| Fleming et al (2012) | Multi-center, May 2008-June 2011 | 30 | Phase II, Open label, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Metastasis; 5) docetaxel failure | Experimental: Cetuximab + Mitoxantrone + Prednisone Control: Mitoxantrone + Prednisone | PFS, OS, PSA Response Adverse event |
| Hoskin et al (2014) | Multi-center, June 2008- Feb 2014 | NA | Phase III, Double blind, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Bone metastasis; 5) docetaxel failure | Experimental: Radium-223 Control: Placebo | OS, Adverse event |
| Kantoff et al (1999) | Multi-center, Oct 1992-Sep 1995 | NA | RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) docetaxel failure | Experimental: Mitoxantrone + Prednisone Control: Prednisone | OS, PFS, PSA Response, Adverse event |
| Michaelson et al (2014) | Multi-center, Jul 2008-Aug 2010 | NA | Phase III, Double blind, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Metastasis; 5) docetaxel failure | Experimental: Sunitinib + Prednisone Control: Prednisone +Placebo | OS, PFS, PSA Response, Adverse event |
| Parker et al (2013) | Multi-center, Jan 2008-Feb 2011 | 36 | Phase III, Double blind, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Bone metastasis; 5) docetaxel failure | Experimental: Radium-223 Control: Placebo | OS, Adverse event |
| Rosenberg et al (2007) | Multi-center, Feb 2003-Jun 2005 | NA | Phase II, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) docetaxel failure | Experimental: Ixabepilone Control: Mitoxantrone + Prednisone | OS, PSA Response, Adverse event |
| Ryan et al (2012) | Multi-center, Mar 2009-Dec 2009 | 21 | Phase II, Double blind, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Metastasis; 5) docetaxel failure | Experimental One: Rilotumumab (15 mg/kg) + Mitoxantrone + Prednisone Experimental Two: Rilotumumab (7.5mg/kg) + Mitoxantrone + Prednisone Control: Mitoxantrone + Prednisone + Placebo | OS, PFS, PSA response, Adverse event |
| Scher et al (2012) | Multi-center, Sep 2009-Nov 2010 | NA | Phase III, Double blind, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) docetaxel failure | Experimental: Enzalutamide Control: Placebo | OS, PFS, PSA Response, Adverse event |
| Smith et al (2016) | Multi-center, Jul 2012-Nov 2014 | NA | Phase III, Double blind, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Bone metastasis; 5) docetaxel failure | Experimental: cabozantinib Control: Prednisone | OS, PFS PSA Response |
| Sternberg et al (2009) | Multi-center, Sep 2003-Jan 2006 | NA | Phase III, Double blind, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) Bone metastasis; 5) docetaxel failure | Experimental: Satraplatin + Prednisone Control: Prednisone + Placebo | OS, PFS, PSA response, Adverse event |
| Sun et al (2016) | Multi-center, Aug 2012-June 2014 | NA | Phase III, Double blind, RCT | 1) Pathologically proven prostate cancer; 2) Surgical or hormone-induced castration; 3) Disease progression; 4) docetaxel failure | Experimental: Abiraterone acetate + prednisone Control: Prednisone + Placebo | OS, PSA Response, Adverse event |
NA: not applicable; RCT: randomized controlled trial; OS: overall survival; PFS: progression free survival
Network meta-analysis outcomes
We established a network meta-analysis to compare the OS, PFS, PSA response and adverse event of different interventions. The respective sets of HRs and ORs with the corresponding 95% CrIs from the fixed effect model and random effect model had good consistency. The DIC values were lower in fixed effect model compared with random effect model for endpoints (range 5.36-10.07 vs range 5.57-88.68), indicating the fixed effect model had a substantially better fit than random effect model. Therefore, we applied the fixed effects model for the rest of the study. Supplementary Table 1 showed the summary of primary and secondary endpoints findings.
Efficacy results
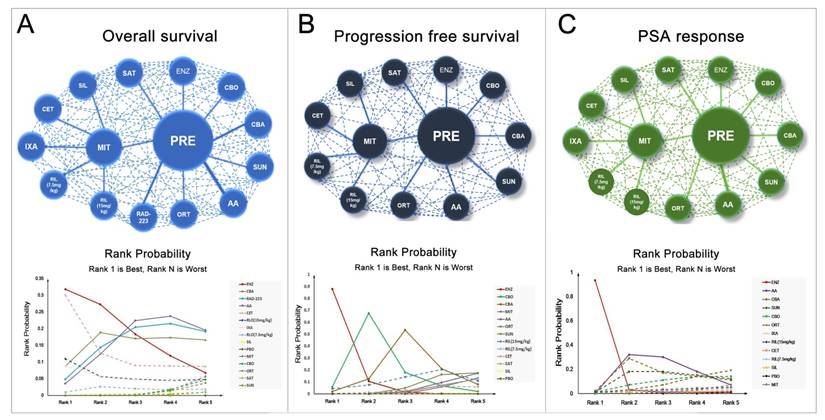
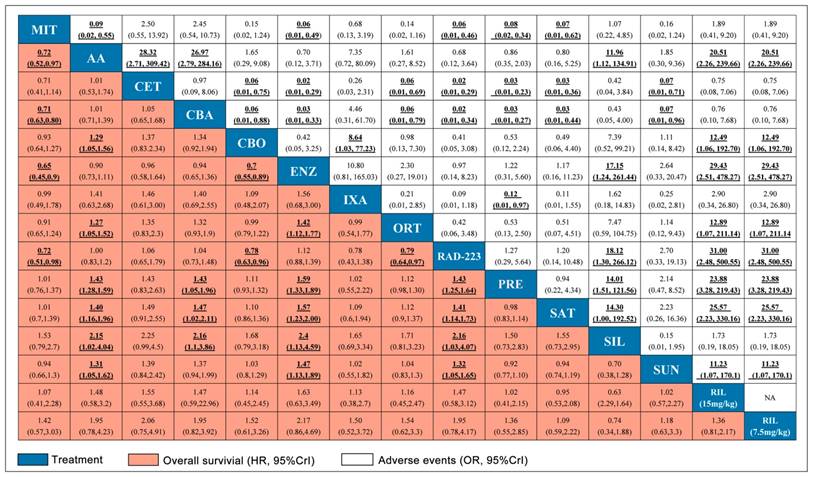
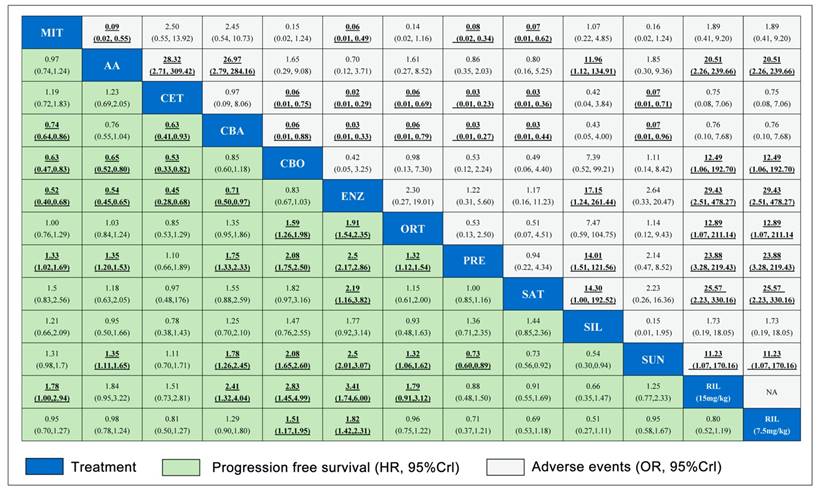
A total number of 17 studies reported information of OS and 11 studies reported PFS were included in the present study. Figure 2 showed the full network diagram of eligible comparisons for OS, PFS and PSA response. In terms of OS, Abiraterone Acetate (HR: 0.70, 95%CrI: 0.63-0.79), Enzalutamide (HR: 0.63, 95%CrI: 0.53-0.75) and Cabazitaxel (HR: 0.70, 95%CrI: 0.51-0.95) were superior to control arms. Moreover, as hormonal strategies, both Abiraterone Acetate and Enzalutamide were significantly increasing OS than other interventions (Figure 3). In terms of PFS, Enzalutamide (HR: 0.40, 95%CrI: 0.35-0.46) was the most efficacious intervention with 87.9% cumulative probabilities of being rank 1 compared with control, followed by Cabozantinib and Abiraterone Acetate (Figure 4). In regards to PSA response, Abiraterone Acetate (OR: 6.25, 95%CrI: 2.27-20.0) and Enzalutamide (OR: 50.0, 95%CrI: 12.5-100.0) demonstrated significant benefits in terms of PSA response efficacy (Supplementary Figure 1).
Safety results
The network meta-analysis for adverse events (grade 3-4) was shown in Figure 3 and 4. Abiraterone Acetate (OR: 0.86, 95%CrI: 0.35-2.03) and Enzalutamide (OR: 1.22, 95%CrI: 0.31-5.60) presented no significant toxicities compared with control arms, whereas Mitoxantrone, Cabazitaxel, Ixabepilone, Cetuximab, Siltuximab and Rilotumumab were not as well tolerated compared with control arms for adverse events (grade 3-4).
Pair-wise meta-analysis outcomes
Results from pair-wise meta-analysis of including interventions were shown in Supplementary Figure 2, which were consistent with that of network meta-analysis. Pooled data showed that Abiraterone Acetate (HR: 0.70, 95%CI: 0.62-0.77), Enzalutamide (HR: 0.63, 95%CI: 0.52-0.74), Cabazitaxel (HR: 0.71, 95%CI: 0.63-0.79) presented benefits compared with control in term of OS. Moreover, Abiraterone Acetate, Enzalutamide, Cabazitaxel and Orteronel were associated with longer PFS and higher PSA response than control arms.
The PRISMA flow chart of included studies in network meta-analysis.
Network and rank probability of comparisons included in the analysis. Evidence network of different interventions for OS, PFS and PSA response for CRPC patients after docetaxel failure. The thickness of the connection line corresponds to the numbers of studies between comparators. Probabilities of each intervention ranking best, second, third, fourth and fifth best based on the fixed effects model. Full lines stand for agents with significant difference, while dash lines stand for agents without significant difference in comparisons.
Pooled relative HRs for OS (pink region) and ORs for grade 3-4 adverse events (white region) based on mixed direct and indirect evidence from Bayesian network meta-analysis through fixed effects model with different pharmacological interventions in CRPC patients after docetaxel failure. The OS and safety estimates are located at the intersection of the column intervention and the row treatment (i.e., column intervention is reference for each comparison). To obtain HRs or ORs for comparisons in opposing direction, reciprocals should be applied. Results with statistic significant are in bold and underlined. Numbers in parentheses indicate 95% CrIs for network meta-analysis.
Pooled relative HRs for PFS (green region) and ORs for grade 3-4 adverse events (white region) based on mixed direct and indirect evidence from Bayesian network meta-analysis through fixed effects model with different pharmacological interventions in CRPC patients after docetaxel failure. The PFS and safety estimates are located at the intersection of the column intervention and the row treatment (i.e., column intervention is reference for each comparison). To obtain HRs or ORs for comparisons in opposing direction, reciprocals should be applied. Results with statistic significant are in bold and underlined. Numbers in parentheses indicate 95% CrIs for network meta-analysis.
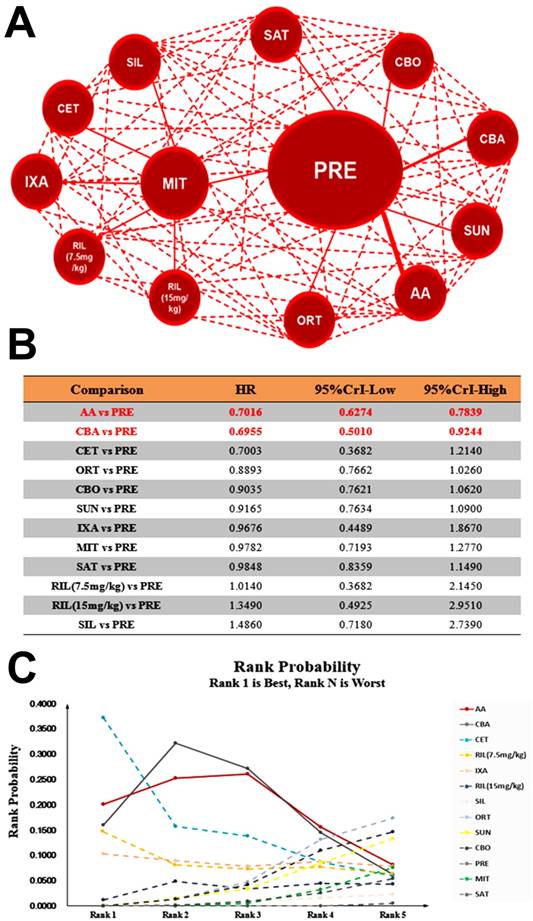
The network meta-analysis outcomes of eligible comparisons of OS excluding studies with control arm of placebo: network diagram (A), relative HRs (B) and rank probability (C) based on mixed direct and indirect evidence from Bayesian network meta-analysis through fixed effects model with different pharmacological interventions in CRPC patients after docetaxel failure.
Sensitivity analysis
Theoretically daily low dose corticosteroids appear to have modest antitumor activity and may avert adverse effects of other antitumor agents12, we performed further pooled analysis including studies with control arm of Prednisone plus placebo. Our results showed that Abiraterone Acetate (HR: 0.71, 95%CrI: 0.63-0.78) was the most efficacious intervention of being rank 1 in OS compared with Prednisone plus placebo followed by Cabazitaxel and Cetuximab, when Enzalutamide and Radium-223 were excluded for control arm of placebo (Figure 5). In terms of PFS, Abiraterone Acetate (HR: 0.74, 95%CrI: 0.66-0.83) also presented the most efficacious intervention of being rank 1 (Supplementary Figure 3). In regards to PSA response, Abiraterone Acetate showed significant superiority over control arm (OR: 6.17, 95%CrI: 2.83-13.59) (Supplementary Figure 4). Together, our results suggest that Abiraterone Acetate might be the efficacious intervention compared with control arm in terms of OS, PFS and PSA Response.
Risk of bias assessment
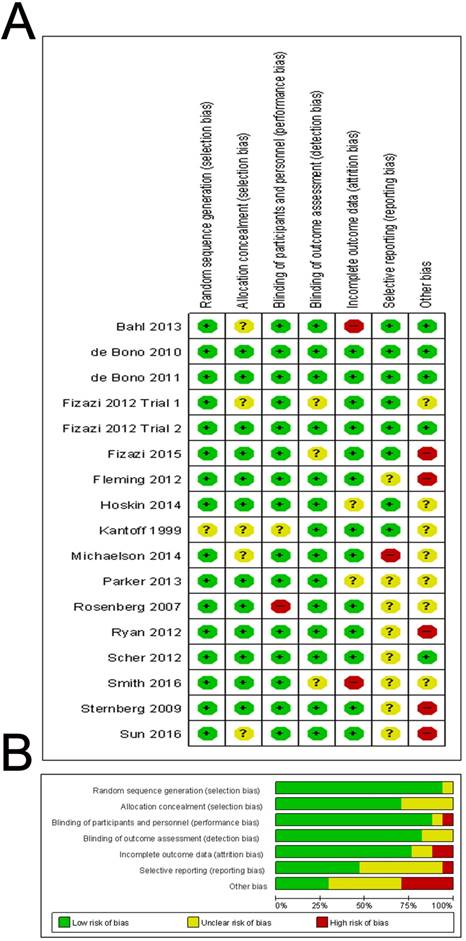
The risk of bias of included studies was reported in Figure 6. In fact, none of the trials were thought to have a high risk of bias for any of the methodological quality items assessed. In summary, 13 (76%) of the 17 trials reported an adequate method of allocation concealment and 14 (82%) of the 17 trials reported low risk method for blinding of outcomes. We did not find evidence of publication bias in OS, PSA response and adverse event, based on funnel plot asymmetry or quantitatively (Egger's regression test p=0.266, 0.178 and 0.084), while Egger's regression test in PFS showed statistical significance (p=0.038) (Supplementary Figure 5).
Discussion
This systematic review and network meta-analysis represents the most comprehensive synthesis of data for currently available pharmacological interventions for patients with mCRPC who had progressed after docetaxel-based chemotherapy. 17 RCTs with 12347 enrolled patients were included in this network meta-analysis. The main new finding is that Abiraterone Acetate, Cabazitaxel and Enzalutamide presented better benefits in term of OS compared with control arms. Enzalutamide demonstrated superiority over PFS and PSA response with a highest probability to rank 1. Moreover, sensitivity analysis showed that Abiraterone Acetate exhibited the most efficacious intervention of being rank 1 in term of OS compared with control, followed by Cabazitaxel and Cetuximab. In regards to PFS, Abiraterone Acetate also presented the most efficacious intervention of being rank 1. Together, our results suggested that Abiraterone Acetate might be the efficacious intervention compared with control arms for mCRPC patients progressing after docetaxel-based chemotherapy.
Previously, post-docetaxel treatment options for mCRPC were limited, with few benefits observed in terms of OS. Since 2010, we have witnessed unprecedented therapeutic advances in treatment after prior docetaxel-based chemotherapy for men with mCRPC, including Cabazitaxel (tubulin-binding taxane)15 28 approved by the FDA in 2010, Abiraterone Acetate (androgen biosynthesis inhibitor)12 46 47 approved by the FDA in 2011 and Enzalutamide (androgen receptor antagonist)13 38 48-50 approved by the FDA in 2012. All of the regimens have been demonstrated to improve efficacy and have become parts of the therapeutic arsenals mCRPC after docetaxel failure. An important finding within the present study is that, Abiraterone Acetate appears to be efficacious treatment option for mCRPC patients after docetaxel failure because of the higher efficacy and lower adverse events. Abiraterone Acetate, a steroidal drug, inhibits CYP17A1, blocks androgen synthesis decreasing the intracellular testosterone level and prolong survival before or after docetaxel chemotherapy, recent study has indicated the metabolites of Abiraterone Acetate showed antagonistic effect on androgen receptor 12 47 51. It might partially explain the outcomes in our study.
Entering this new therapeutic era in oncology, numbers of mechanistic drug classifications have produced a more diverse range of potential toxicities. CTCAE is a standard evaluating system designed to assess symptomatic toxicities and provide additional tolerability data 52. The interventions included in our study composed of chemotherapy, hormonal strategies, antiangiogenic therapies, immune strategies and bone-targeting agents, and the assessments of severe adverse events (Grade 3-4) could better inform us the tolerability information of the therapies. Our results indicated reported adverse events of hormone strategies were less severe than cytotoxic agents, and were manageable by appropriate patient monitoring. The novel androgen receptor targeting agents (Abiraterone Acetate and Enzalutamide) presented similar severe adverse events with control arms, which could be better tolerated than cytotoxic therapies.
To the best of our knowledge, this study is the first network meta-analysis to assess the efficacy and safety of different interventions for post-docetaxel therapy in mCRPC patients. We overcame the difficulties of different measures of survival across studies and synthesized all available studies within a single network meta-analysis, avoiding potential selection bias in the meantime. This network meta-analysis provides new insights into controversies on this issue with important implications in clinical care and future research. However, this study also has limitation. We extracted all information from published data rather than original individual patient data, which may lead to publication and reporting bias and missing information on certain endpoints might affected our analysis without access to individual patient data. We minimized the risk of bias through searching and reviewing the publications comprehensively, and extracting and evaluating the data systematically, we also performed further pooled analysis excluding agents with control arm of placebo for theoretical reasons. Moreover, our findings are the results of direct and indirect comparisons in a network meta-analysis. Although this method is widely accepted, it does not substitute results from RCTs. Future systematic reviews are needed to assess the efficacy and safety of these interventions by identifying those patients who most benefit.
Quality assessment of included studies. The Overall (A) and Study-level distribution plot for risk of bias using Cochrane's risk of bias assessment tool. Studies are deemed to be at high, low or unclear risk of bias for each risk of bias entry. The review authors' judgments about each risk of bias entry are presented as percentages across all included studies.
Conclusions
In conclusion, our network meta-analysis demonstrated that Abiraterone Acetate, Cabazitaxel and Enzalutamide were associated with favorable OS when compared with control arms. Abiraterone Acetate appears to be efficacious treatment option for mCRPC patients after docetaxel failure because of the higher efficacy and lower adverse events. Similar observations were also noted for PFS and PSA response. Further sensitivity analysis indicated Abiraterone Acetate showed significant benefit in prolonging survival. Future well-designed RCTs and systematic reviews are awaited to confirm the findings of this study.
Clinical Practice Points
- The consensus is not existing of optimal strategies for CRPC patients after docetaxel failure. Several randomized trials were conducted to investigate efficacy and safety of interventions.
- This network meta-analysis showed for the first time that Abiraterone Acetate (HR: 0.71, 95%CrI: 0.63-0.78) exhibited the most efficacious intervention of being rank 1 in term of OS compared with control arms.
- The analysis of 17 randomized trials provides evidence in favor of Abiraterone Acetate for CRPC patients after docetaxel failure with acceptable tolerability and good performance status.
Abbreviations
PCa: Prostate cancer; ADT: Androgen deprivation therapy; CRPC: Castration Resistant Prostate Cancer; OS: Overall Survival; PFS: Progression Fee Survival; RCT: Randomized Controlled Trials; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses; HR: Hazard Ratio; OR: Odds Ratio; CrIs: Credible Intervals; DIC: Deviance Information Criterion; CTCAE: Common Terminology Criteria for Adverse Events; EAU: European Association of Urology; NCCN: National Comprehensive Cancer Network; MIT: Mitoxantrone; CBA: Cabazitaxel; PRE: Prednisone plus Placebo; AA: Abiraterone acetate; SIL: Siltuximab; ORT: Orteronel; CET: Cetuximab; RAD: Radium-223; SUN: Sunitinib; IXA: Ixabepilone; RIL: Rilotumumab; ENZ: Enzalutamide; CBO: Cabozantinib; SAT: Satraplatin.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant No. 81572514, U1301221, 81472384, 81402106, 81372729, 81272808, 81172431), National Natural Science Foundation of Guangdong (Grant No. 2016A030313321, 2015A030311011, 2015A030310122, S2013020012671), Science and Technology Program of Guangzhou (Grant No. 201604020156, 201604020177), “Three Big Constructions” funds of Sun Yat-sen University (for Jian Huang and Tianxin Lin), Specialized Research Fund for the Doctoral Program of Higher Education (for Tianxin Lin, 20130171110073), the Fundamental Research Funds for the Central Universities (for Jian Huang), Project Supported by Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme (for Tianxin Lin) , Elite Young Scholars Program of Sun Yat-Sen Memorial Hospital (for Tianxin Lin, J201401) and National Clinical Key Specialty Construcion Project for Department of Urology and Department of Oncology. Grant KLB09001 from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes, Sun-Yat-Sen University. Grant [2013]163 from Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ferlay J, Soerjomataram I, Dikshit R. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-86 doi: 10.1002/ijc.29210 [published Online First: 2014/09/16]
2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7-30 doi: 10.3322/caac.21387
3. Beltran H, Beer TM, Carducci MA. et al. New therapies for castration-resistant prostate cancer: efficacy and safety. European urology. 2011;60(2):279-90 doi: 10.1016/j.eururo.2011.04.038
4. Heidenreich A, Bastian PJ, Bellmunt J. et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. European urology. 2014;65(2):467-79 doi: 10.1016/j.eururo.2013.11.002
5. Loblaw DA, Virgo KS, Nam R. et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(12):1596-605 doi: 10.1200/JCO.2006.10.1949
6. Pagliarulo V, Bracarda S, Eisenberger MA. et al. Contemporary role of androgen deprivation therapy for prostate cancer. European urology. 2012;61(1):11-25 doi: 10.1016/j.eururo.2011.08.026
7. Chowdhury S, Burbridge S, Harper PG. Chemotherapy for the treatment of hormone-refractory prostate cancer. International journal of clinical practice. 2007;61(12):2064-70 doi: 10.1111/j.1742-1241.2007.01551.x
8. Parker C, Gillessen S, Heidenreich A. et al. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26(Suppl 5):v69-77 doi: 10.1093/annonc/mdv222
9. Lam JS, Leppert JT, Vemulapalli SN. et al. Secondary hormonal therapy for advanced prostate cancer. The Journal of urology. 2006;175(1):27-34 doi: 10.1016/S0022-5347(05)00034-0
10. Mohler JL, Armstrong AJ, Bahnson RR. et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14(1):19-30
11. Berthold DR, Pond GR, Soban F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(2):242-5 doi: 10.1200/JCO.2007.12.4008
12. de Bono JS, Logothetis CJ, Molina A. et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364(21):1995-2005 doi: 10.1056/NEJMoa1014618
13. Scher HI, Fizazi K, Saad F. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-97 doi: 10.1056/NEJMoa1207506
14. Parker C, Nilsson S, Heinrich D. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-23 doi: 10.1056/NEJMoa1213755
15. de Bono JS, Oudard S, Ozguroglu M. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-54 doi: 10.1016/S0140-6736(10)61389-X
16. Loriot Y, Bianchini D, Ileana E. et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24(7):1807-12 doi: 10.1093/annonc/mdt136
17. Noonan KL, North S, Bitting RL. et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24(7):1802-7 doi: 10.1093/annonc/mdt138
18. Bianchini D, Lorente D, Rodriguez-Vida A. et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer. 2014;50(1):78-84 doi: 10.1016/j.ejca.2013.08.020
19. Cipriani A, Higgins JP, Geddes JR. et al. Conceptual and technical challenges in network meta-analysis. Annals of internal medicine. 2013;159(2):130-7 doi: 10.7326/0003-4819-159-2-201307160-00008
20. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in medicine. 2004;23(20):3105-24 doi: 10.1002/sim.1875
21. Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Bmj. 2005;331(7521):897-900 doi: 10.1136/bmj.331.7521.897
22. Liberati A, Altman DG, Tetzlaff J. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10):e1-34 doi: 10.1016/j.jclinepi.2009.06.006
23. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62(10):1006-12 doi: 10.1016/j.jclinepi.2009.06.005
24. Tannock IF, Osoba D, Stockler MR. et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14(6):1756-64 doi: 10.1200/JCO.1996.14.6.1756
25. Tannock IF, de Wit R, Berry WR. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-12 doi: 10.1056/NEJMoa040720
26. Morgan CJ, Oh WK, Naik G. et al. Impact of prednisone on toxicities and survival in metastatic castration-resistant prostate cancer: A systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2014;90(3):253-61 doi: 10.1016/j.critrevonc.2013.12.001
27. Sonpavde G, Pond GR, Templeton AJ. et al. Impact of single-agent daily prednisone on outcomes in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2017;20(1):67-71 doi: 10.1038/pcan.2016.44
28. Bahl A, Oudard S, Tombal B. et al. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol. 2013;24(9):2402-8 doi: 10.1093/annonc/mdt194
29. Higgins JP, Altman DG, Gotzsche PC. et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928
30. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of clinical epidemiology. 2011;64(2):163-71 doi: 10.1016/j.jclinepi.2010.03.016
31. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in medicine. 1998;17(24):2815-34
32. Fleming MT, Sonpavde G, Kolodziej M. et al. Association of rash with outcomes in a randomized phase II trial evaluating cetuximab in combination with mitoxantrone plus prednisone after docetaxel for metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2012;10(1):6-14 doi: 10.1016/j.clgc.2011.11.003
33. Kantoff PW, Halabi S, Conaway M. et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17(8):2506-13 doi: 10.1200/JCO.1999.17.8.2506
34. Rosenberg JE, Weinberg VK, Kelly WK. et al. Activity of second-line chemotherapy in docetaxel-refractory hormone-refractory prostate cancer patients: randomized phase 2 study of ixabepilone or mitoxantrone and prednisone. Cancer. 2007;110(3):556-63 doi: 10.1002/cncr.22811
35. Ryan CJ, Rosenthal M, Ng S. et al. Targeted MET inhibition in castration-resistant prostate cancer: a randomized phase II study and biomarker analysis with rilotumumab plus mitoxantrone and prednisone. Clin Cancer Res. 2013;19(1):215-24 doi: 10.1158/1078-0432.CCR-12-2605
36. Egger M, Davey Smith G, Schneider M. et al. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629-34
37. Cella D, Ivanescu C, Holmstrom S. et al. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26(1):179-85 doi: 10.1093/annonc/mdu510
38. Fizazi K, Scher HI, Miller K. et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15(10):1147-56 doi: 10.1016/S1470-2045(14)70303-1
39. Logothetis CJ, Basch E, Molina A. et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13(12):1210-7 doi: 10.1016/S1470-2045(12)70473-4
40. Sartor O, Coleman R, Nilsson S. et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15(7):738-46 doi: 10.1016/S1470-2045(14)70183-4
41. Saad F, Hotte S, North S. et al. Randomized phase II trial of Custirsen (OGX-011) in combination with docetaxel or mitoxantrone as second-line therapy in patients with metastatic castrate-resistant prostate cancer progressing after first-line docetaxel: CUOG trial P-06c. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(17):5765-73 doi: 10.1158/1078-0432.CCR-11-0859
42. Meulenbeld HJ, Bleuse JP, Vinci EM. et al. Randomized phase II study of danusertib in patients with metastatic castration-resistant prostate cancer after docetaxel failure. BJU international. 2013;111(1):44-52 doi: 10.1111/j.1464-410X.2012.11404.x
43. Droz JP, Medioni J, Chevreau C. et al. Randomized phase II study of nintedanib in metastatic castration-resistant prostate cancer postdocetaxel. Anticancer Drugs. 2014;25(9):1081-8 doi: 10.1097/CAD.0000000000000131
44. Hussain M, Rathkopf D, Liu G. et al. A randomised non-comparative phase II trial of cixutumumab (IMC-A12) or ramucirumab (IMC-1121B) plus mitoxantrone and prednisone in men with metastatic docetaxel-pretreated castration-resistant prostate cancer. Eur J Cancer. 2015;51(13):1714-24 doi: 10.1016/j.ejca.2015.05.019
45. Green AK, Corty RW, Wood WA. et al. Comparative effectiveness of mitoxantrone plus prednisone versus prednisone alone in metastatic castrate-resistant prostate cancer after docetaxel failure. Oncologist. 2015;20(5):516-22 doi: 10.1634/theoncologist.2014-0432
46. Fizazi K, Scher HI, Molina A. et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983-92 doi: 10.1016/S1470-2045(12)70379-0 [published Online First: 2012/09/22]
47. Ryan CJ, Smith MR, de Bono JS. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. The New England journal of medicine. 2013;368(2):138-48 doi: 10.1056/NEJMoa1209096
48. Clark MJ, Harris N, Griebsch I. et al. Patient-reported outcome labeling claims and measurement approach for metastatic castration-resistant prostate cancer treatments in the United States and European Union. Health Qual Life Outcomes. 2014;12:104. doi: 10.1186/s12955-014-0104-5
49. Beer TM, Armstrong AJ, Rathkopf DE. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-33 doi: 10.1056/NEJMoa1405095
50. Beer TM, Armstrong AJ, Rathkopf D. et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol. 2017;71(2):151-54 doi: 10.1016/j.eururo.2016.07.032
51. Li Z, Alyamani M, Li J. et al. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533(7604):547-51 doi: 10.1038/nature17954 [published Online First: 2016/05/27]
52. Basch E, Reeve BB, Mitchell SA. et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014:106 (9) doi: 10.1093/jnci/dju244
Author contact
![]() Corresponding authors: Jian Huang MD, PhD. Department of Urology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, 107 Yan-Jiang Xi Road, Guangzhou, 510120, China. Tel. +86 20 81332603; Fax: +86 20 81332853. E-mail address: changhaochen526com and Tianxin Lin MD, PhD. Department of Urology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, 107 Yan-Jiang Xi Road, Guangzhou, 510120, China. Tel. +86 20 81332603; Fax: +86 20 81332853. E-mail address: tianxinlcom
Corresponding authors: Jian Huang MD, PhD. Department of Urology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, 107 Yan-Jiang Xi Road, Guangzhou, 510120, China. Tel. +86 20 81332603; Fax: +86 20 81332853. E-mail address: changhaochen526com and Tianxin Lin MD, PhD. Department of Urology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, 107 Yan-Jiang Xi Road, Guangzhou, 510120, China. Tel. +86 20 81332603; Fax: +86 20 81332853. E-mail address: tianxinlcom

 Global reach, higher impact
Global reach, higher impact